Effects of Canada Goldenrod Invasion on Soil Extracellular Enzyme Activities and Ecoenzymatic Stoichiometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Soil Sample Collection and Preparation
2.3. Measurement of Soil Physicochemical and Microbial Biomass Properties
2.4. Measurement of Soil EEA
2.5. Statistical Analyses
3. Results
3.1. Soil Physicochemical and Microbial Biomass Properties
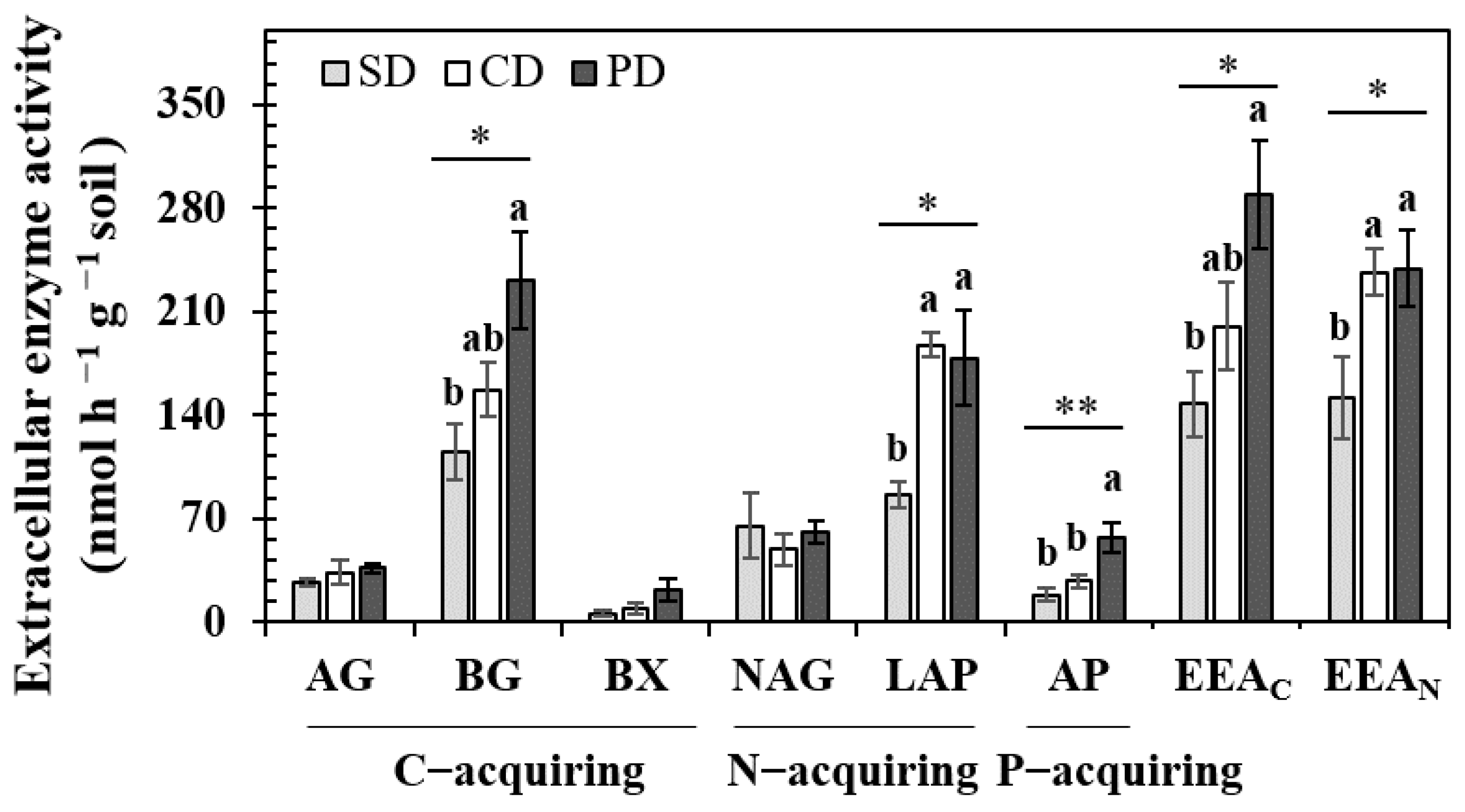
3.2. Soil EEAs and Microbial Metabolic Limitation Indicators
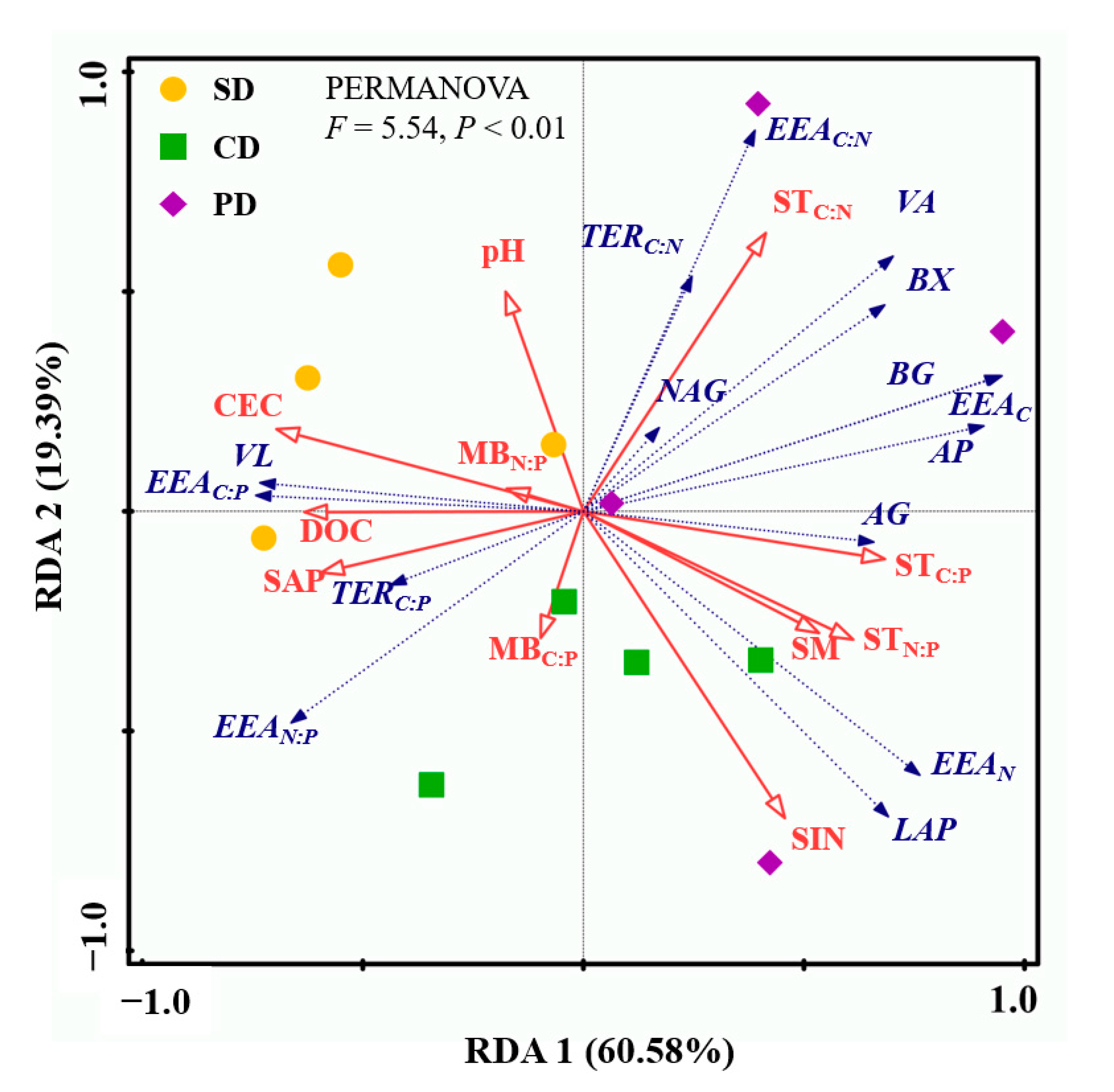
3.3. Relationships of Soil EEAs, Soil Microbial Metabolic Limitation Indicators, and Soil Properties
4. Discussion
4.1. The Effects of S. canadensis Invasion on Soil EEAs
4.2. The Effects of S. canadensis Invasion on Soil Microbial Metabolic Limitations
4.3. Implication of S. canadensis Invasion Effects on Soil EEAs and Microbial Metabolic Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, Z.C.; Fu, W.; Qi, S.S.; Zhai, D.L.; Chen, S.C.; Wan, L.Y.; Huang, H.; Du, D.L. Different responses of an invasive clonal plant Wedelia trilobata and its native congener to gibberellin: Implications for biological invasion. J. Chen. Ecol. 2016, 42, 85–94. [Google Scholar] [CrossRef]
- Mamik, S.; Sharma, A.D. Protective role of boiling stable antioxidant enzymes in invasive alien species of Lantana exposed to natural abiotic stress like conditions. Russ. J. Biol. Invasions 2017, 8, 75–86. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, D.; Kim, Y. Potential Distribution of Goldenrod (Solidago altissima L.) during Climate Change in South Korea. Sustainability 2020, 12, 6710. [Google Scholar] [CrossRef]
- Boscutti, F.; Pellegrini, E.; Casolo, V.; Nobili, M.; Buccheri, M.; Alberti, G.; Rapson, G. Cascading effects from plant to soil elucidate how the invasive Amorpha fruticosa L. impacts dry grasslands. J. Veg. Sci. 2020, 31, 667–677. [Google Scholar] [CrossRef]
- Wei, H.; Yan, W.B.; Quan, G.M.; Zhang, J.E.; Liang, K.M. Soil microbial carbon utilization, enzyme activities and nutrient availability responses to Bidens pilosa and a non-invasive congener under different irradiances. Sci. Rep. 2017, 7, 11309. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.; Gioria, M. Plant invasions. J. Plant Ecol. 2018, 11, 1–3. [Google Scholar] [CrossRef]
- Belnap, J.; Phillips, S.L.; Sherrod, S.K.; Moldenke, A. Soil Biota Can Change after Exotic Plant Invasion: Does This Affect Ecosystem Processes? Ecology 2005, 86, 3007–3017. [Google Scholar] [CrossRef]
- Vila, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarosik, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pysek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Staver, A.C. Enhanced activity of soil nutrient-releasing enzymes after plant invasion: A meta-analysis. Ecology 2019, 100, e02830. [Google Scholar] [CrossRef]
- Wan, L.Y.; Qi, S.S.; Zou, C.B.; Dai, Z.C.; Du, D.L. Phosphorus addition reduces the competitive ability of the invasive weed Solidago canadensis under high nitrogen conditions. Flora 2018, 240, 68–75. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.; Megonigal, J.P.; Kang, H.; Seo, J.; Ding, W. Impacts of phragmites australis invasion on soil enzyme activities and microbial abundance of tidal marshes. Microb. Ecol. 2018, 76, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Goncalves, P.; Copeland, E.; Qi, S.S.; Dao, Z.C.; Li, G.L.; Wang, C.Y.; Du, D.L.; Thomas, T. Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol. Biochem. 2020, 143, 107739. [Google Scholar] [CrossRef]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Vitti, S.; Pellegrini, E.; Casolo, V.; Trotta, G.; Boscutti, F.; Zhu, B. Contrasting responses of native and alien plant species to soil properties shed new light on the invasion of dune systems. J. Plant Ecol. 2020, 13, 667–675. [Google Scholar] [CrossRef]
- Adomako, M.O.; Xue, W.; Tang, M.; Du, D.L.; Yu, F.H. Synergistic effects of soil microbes on Solidago canadensis depend on water and nutrient availability. Microb. Ecol. 2020, 80, 837–845. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Q.; Wang, L.; Liu, W.; Christie, P. Response of soil enzymes and microbial communities to root extracts of the alien Alternanthera philoxeroides. Arch. Agron. Soil Sci. 2018, 64, 708–717. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Zeglin, L.H. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Torres, Y.; Elser, J.J.; Souza, V.; García-Oliva, F. Ecoenzymatic stoichiometry at the extremes: How microbes cope in an ultra-oligotrophic desert soil. Soil Biol. Biochem. 2015, 87, 34–42. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Deng, L.; Guo, X.; Zhang, X. Patterns of soil microbial nutrient limitations and their roles in the variation of soil organic carbon across a precipitation gradient in an arid and semi-arid region. Sci. Total Environ. 2019, 658, 1440–1451. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Bing, H.; Zhou, J.; Wang, J. Vegetation type rather than climate modulates the variation in soil enzyme activities and stoichiometry in subalpine forests in the eastern Tibetan Plateau. Geoderma 2020, 37, 114424. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, F. Canada Goldenrod Solidago canadensis L. In Biological Invasions and Its Management in China; Springer: Singapore, 2017; pp. 143–151. [Google Scholar] [CrossRef]
- Ren, G.; He, M.; Li, G.; Anandkumar, A.; Dai, Z.; Zou, C.B.; Hu, Z.; Ran, Q.; Du, D.L. Effects of Solidago canadensis invasion and climate warming on soil net N mineralization. Pol. J. Environ. Stud. 2020, 29, 3285–3294. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zou, J.; Siemann, E. Decomposition of phragmites australis litter retarded by invasive Solidago canadensis in mixtures: An antagonistic non-additive effect. Sci. Rep. 2014, 4, 5488. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, K.; Zhou, J.; Wu, B. Solidago canadensis invasion affects soil N-fixing bacterial communities in heterogeneous landscapes in urban ecosystems in east China. Sci. Total Environ. 2018, 631–632, 702–713. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Jiang, K. Allelopathic effects of Canada goldenrod leaf extracts on the seed germination and seedling growth of lettuce reinforced under salt stress. Ecotoxicology 2019, 28, 103–116. [Google Scholar] [CrossRef]
- Ren, G.Q.; Zou, C.B.; Wan, L.Y.; Johnson, H.J.; Li, J.; Zhu, L.; Qi, S.S.; Dai, Z.C.; Zhang, H.Y.; Du, D.L. Interactive effect of climate warming and nitrogen deposition may shift the dynamics of native and invasive species. J. Plant Ecol. 2020, rtaa071. [Google Scholar] [CrossRef]
- Baležentienė, L. Secondary metabolite accumulation and phytotoxicity of invasive species Solidago canadensis L. during the growth period. Allelopath. J. 2015, 35, 217–226. [Google Scholar]
- Zhenjiang Meteorological Administration. Available online: http://js.cma.gov.cn/dsjwz/zjs/ (accessed on 20 December 2020).
- Brown, I.C. A rapid method of determining exchangeable hydrogen and total exchangeable bases of soils. Soil Sci. 1943, 56, 353–358. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Duan, C.; Wang, X.; Zhang, X.; Ju, W.; Chen, H.; Yue, S.; Wang, Y.; Li, S.; et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Till. Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Gu, Y.; Guo, L.; Zhu, P. Ecoenzymatic stoichiometry and microbial nutrient limitations in rhizosphere soil along the hailuogou glacier forefield chronosequence. Sci. Total Environ. 2020, 704, 135413. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen-inorganic Forms. In Methods of Soil Analysis. Part 3-chemical Methods; SSSA and ASA: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Murphy, J.A.M.E.S.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorous. In Methods of Soil Analysis, Part 2, Chemical and Microbial Properties; SSSA and ASA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- DeForest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and LDOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Kandeler, E.; Boddy, L. Interactive effects of temperature and soil moisture on fungal-mediated wood decomposition and extracellular enzyme activity. Soil Biol. Biochem. 2014, 70, 151–158. [Google Scholar] [CrossRef]
- Fanin, N.; Moorhead, D.; Bertrand, I. Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient. Biogeochemistry 2016, 129, 21–36. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Turner, B.L.; Talbot, J.M.; Warning, B. Stoichiometry of microbial carbon use efficiency in soils. Ecol. Monogr. 2016, 86, 172–189. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, Y.; Liu, Y.; Chen, J.; Qin, H. Pyrolysis temperature of biochar affects ecoenzymatic stoichiometry and microbial nutrient-use efficiency in a bamboo forest soil. Geoderma 2020, 363, 114162. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Vienna, Austria, 2013; Available online: https://www.R-project.org (accessed on 20 December 2020).
- Chapuis-Lardy, L.; Vanderhoeven, S.; Dassonville, N.; Koutika, L.S.; Meerts, P. Effect of the exotic invasive plant Solidago gigantea on soil phosphorus status. Biol. Fertil. Soils 2006, 42, 481–489. [Google Scholar] [CrossRef]
- Mallerman, J.; Itria, R.; Alarcón-Gutiérrez, E.; Hernández, C.; Saparrat, M. Exotic litter of the invasive plant Ligustrum lucidum alters enzymatic production and lignin degradation by selected saprotrophic fungi. Can. J. For. Res. 2018, 48, 709–720. [Google Scholar] [CrossRef]
- Aragón, R.; Sardans, J.; Peñuelas, J. Soil enzymes associated with carbon and nitrogen cycling in invaded and native secondary forests of northwestern argentina. Plant Soil 2014, 384, 169–183. [Google Scholar] [CrossRef]
- Hernández, D.L.; Hobbie, S.E. The effects of substrate composition, quantity, and diversity on microbial activity. Plant Soil 2010, 335, 397–411. [Google Scholar] [CrossRef]
- Hu, W.J. Study on the Litter Decomposition Dynamics and Effects of Solidago canadensis L. under Different Invasion Levels. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2020. [Google Scholar]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, S.; Wu, B.; Cheng, H.; Wang, C. Combined allelopathy of Canada goldenrod and horseweed on the seed germination and seedling growth performance of lettuce. Landsc. Ecol. Eng. 2020, 16, 299–306. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Zhang, Y.; Li, P.; Zhang, X. Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern loess plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Duval, B.D.; Curtsinger, H.D.; Hands, A.; Martin, J.; McLaren, J.R.; Cadol, D.D. Greenhouse gas emissions and extracellular enzyme activity variability during decomposition of native versus invasive riparian tree litter. Plant Ecol. 2020, 221, 177–189. [Google Scholar] [CrossRef]


| Parameters | F | p | Invasion Gradient | ||
|---|---|---|---|---|---|
| SD | CD | PD | |||
| SM (w/w %) | 4.42 | * | 19.36 ± 0.57b | 24.46 ± 2.01a | 22.91 ± 0.51ab |
| pH | 3.07 | ns | 8.30 ± 0.01 | 8.20 ± 0.05 | 8.27 ± 0.02 |
| CEC (cmolc kg−1) | 9.48 | ** | 10.55 ± 0.02a | 10.38 ± 0.07b | 10.28 ± 0.03b |
| SOM (mg g−1 soil) | 1.16 | ns | 13.04 ± 0.92 | 16.81 ± 2.85 | 14.78 ± 0.49 |
| DOC (×10−1 mg C g−1 soil) | 13.12 | ** | 2.86 ± 0.04a | 2.66 ± 0.09b | 2.41 ± 0.03c |
| SOC (mg C g−1 soil) | 1.16 | ns | 7.56 ± 0.53 | 9.75 ± 1.65 | 8.57 ± 0.29 |
| STC (mg C g−1 soil) | 1.41 | ns | 10.82 ± 0.52 | 14.19 ± 2.21 | 13.17 ± 1.09 |
| SIN (×10−3 mg N g−1 soil) | 1.31 | ns | 2.24 ± 0.79 | 6.85 ± 0.78 | 7.67 ± 4.28 |
| STN (mg N g−1 soil) | 1.56 | ns | 0.89 ± 0.04 | 1.15 ± 0.17 | 1.06 ± 0.03 |
| SAP (×10−2 mg P g−1 soil) | 6.03 | * | 2.40 ± 0.16a | 2.69 ± 0.27a | 1.79 ± 0.09b |
| STP (×10−1 mg P g−1 soil) | 7.9 | * | 8.23 ± 0.24a | 7.15 ± 0.32b | 6.85 ± 0.20b |
| STC:N | 0.03 | ns | 12.21 ± 0.23 | 12.35 ± 0.22 | 12.34 ± 0.65 |
| STC:P | 4.57 | * | 13.12 ± 0.25b | 19.72 ± 2.74a | 19.15 ± 1.10a |
| STN:P | 5.05 | * | 1.08 ± 0.03b | 1.60 ± 0.22a | 1.55 ± 0.02a |
| MBC (×10−1 mg C g−1 soil) | 1.16 | ns | 6.40 ± 2.30 | 3.65 ± 1.86 | 8.84 ± 2.95 |
| MBN (×10−2 mg N g−1 soil) | 1.31 | ns | 2.45 ± 0.44 | 1.28 ± 0.44 | 1.84 ± 0.62 |
| MBP (×10−2 mg P g−1 soil) | 1.09 | ns | 1.36 ± 0.27 | 1.56 ± 0.41 | 2.12 ± 0.43 |
| MBC:N | 0.89 | ns | 27.49 ± 9.81 | 32.25 ± 9.02 | 58.79 ± 28.04 |
| MBC:P | 0.7 | ns | 50.70 ± 14.71 | 23.82 ± 7.20 | 53.94 ± 29.96 |
| MBN:P | 0.62 | ns | 2.58 ± 1.28 | 1.51 ± 1.00 | 1.08 ± 0.47 |
| Parameters | F | p | Invasion Gradient | ||
|---|---|---|---|---|---|
| SD | CD | PD | |||
| EEAC:N | 1.63 | ns | 1.02 ± 0.14 | 0.84 ± 0.09 | 1.27 ± 0.24 |
| EEAC:P | 5.07 | * | 8.66 ± 1.01a | 7.36 ± 0.50ab | 5.26 ± 0.69b |
| EEAN:P | 2.93 | ns | 9.30 ± 2.13 | 9.29 ± 1.72 | 4.45 ± 0.73 |
| VL | 4.81 | * | 8.73 ± 1.00a | 7.41 ± 0.49ab | 5.42 ± 0.70b |
| VA (°) | 5.97 | * | 7.12 ± 1.54b | 6.64 ± 0.90b | 13.63 ± 2.11a |
| TERC:N | 1.16 | ns | 1.80 ± 0.66 | 1.74 ± 0.47 | 5.99 ± 3.84 |
| TERC:P | 1.14 | ns | 4.34 ± 1.48 | 1.74 ± 0.62 | 2.69 ± 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Li, J.; Shi, K.; Ren, G.; Dai, Z.; Sun, J.; Zheng, X.; Zhou, Y.; Zhang, J.; Li, G.; et al. Effects of Canada Goldenrod Invasion on Soil Extracellular Enzyme Activities and Ecoenzymatic Stoichiometry. Sustainability 2021, 13, 3768. https://doi.org/10.3390/su13073768
Hu Z, Li J, Shi K, Ren G, Dai Z, Sun J, Zheng X, Zhou Y, Zhang J, Li G, et al. Effects of Canada Goldenrod Invasion on Soil Extracellular Enzyme Activities and Ecoenzymatic Stoichiometry. Sustainability. 2021; 13(7):3768. https://doi.org/10.3390/su13073768
Chicago/Turabian StyleHu, Zhiyuan, Jiating Li, Kangwei Shi, Guangqian Ren, Zhicong Dai, Jianfan Sun, Xiaojun Zheng, Yiwen Zhou, Jiaqi Zhang, Guanlin Li, and et al. 2021. "Effects of Canada Goldenrod Invasion on Soil Extracellular Enzyme Activities and Ecoenzymatic Stoichiometry" Sustainability 13, no. 7: 3768. https://doi.org/10.3390/su13073768
APA StyleHu, Z., Li, J., Shi, K., Ren, G., Dai, Z., Sun, J., Zheng, X., Zhou, Y., Zhang, J., Li, G., & Du, D. (2021). Effects of Canada Goldenrod Invasion on Soil Extracellular Enzyme Activities and Ecoenzymatic Stoichiometry. Sustainability, 13(7), 3768. https://doi.org/10.3390/su13073768









