The Influence of Seasonal Water Level Fluctuations on the Soil Nutrients in a Typical Wetland Reserve in Poyang Lake, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling, Preparation and Analysis
2.3. Evaluation of Soil Fertility
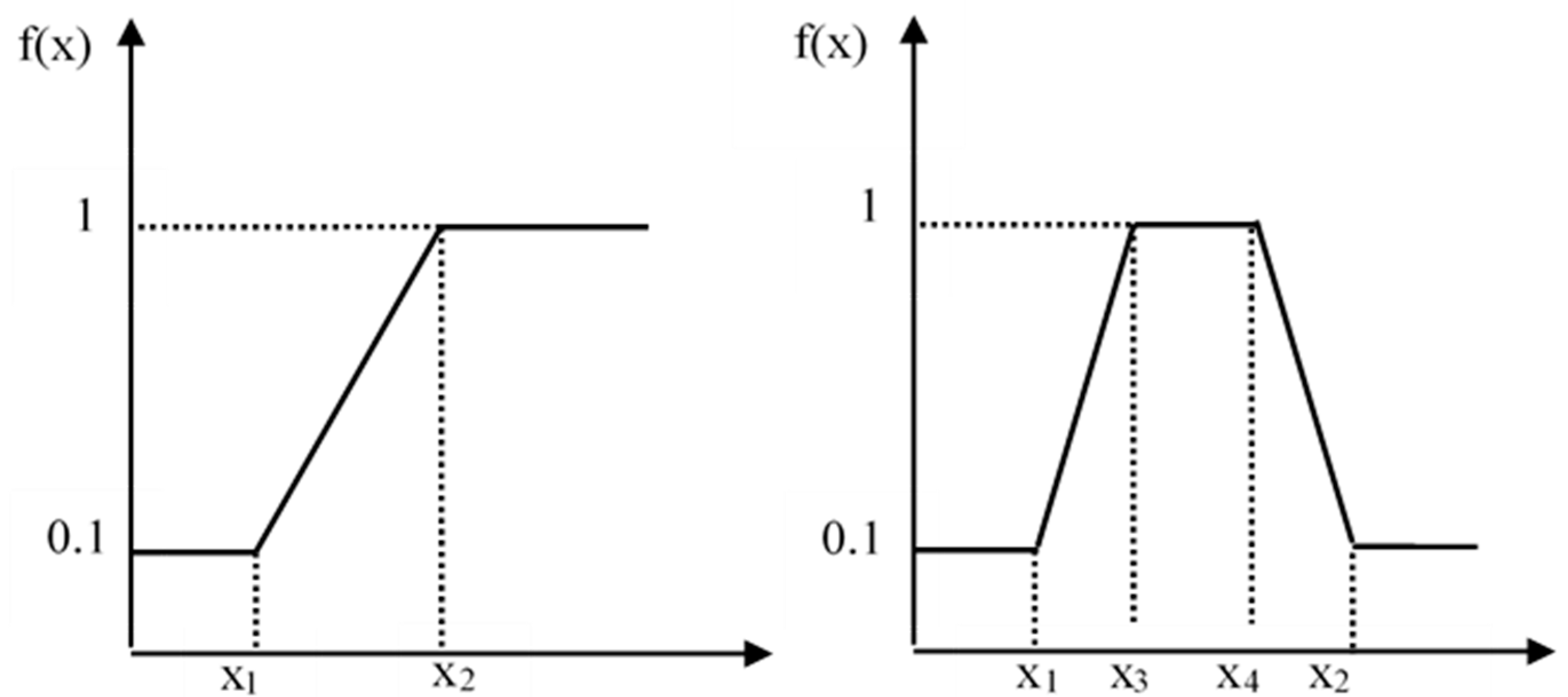
2.4. Application of Single Factor Standard Index Method
3. Result and Discussion
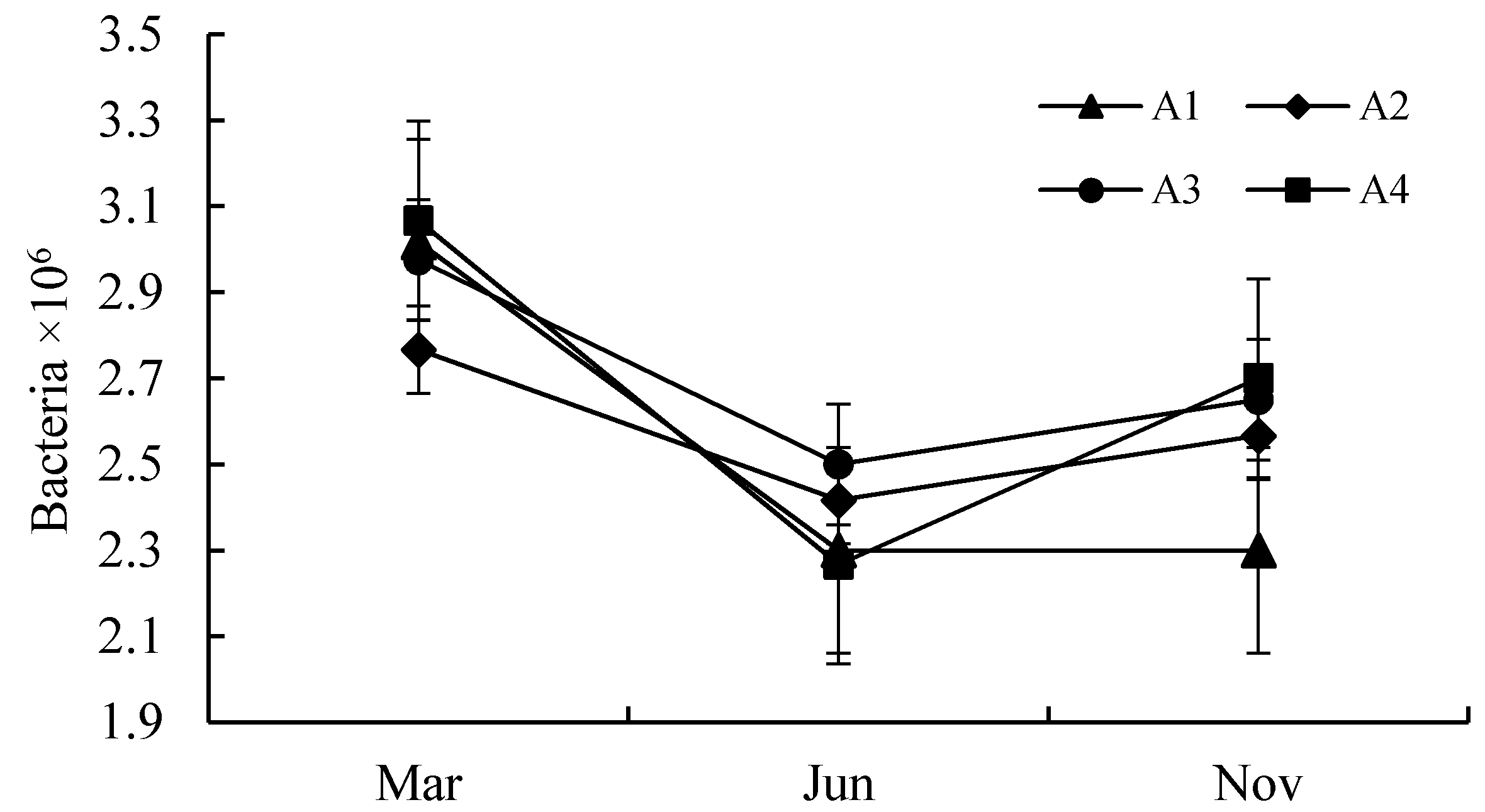
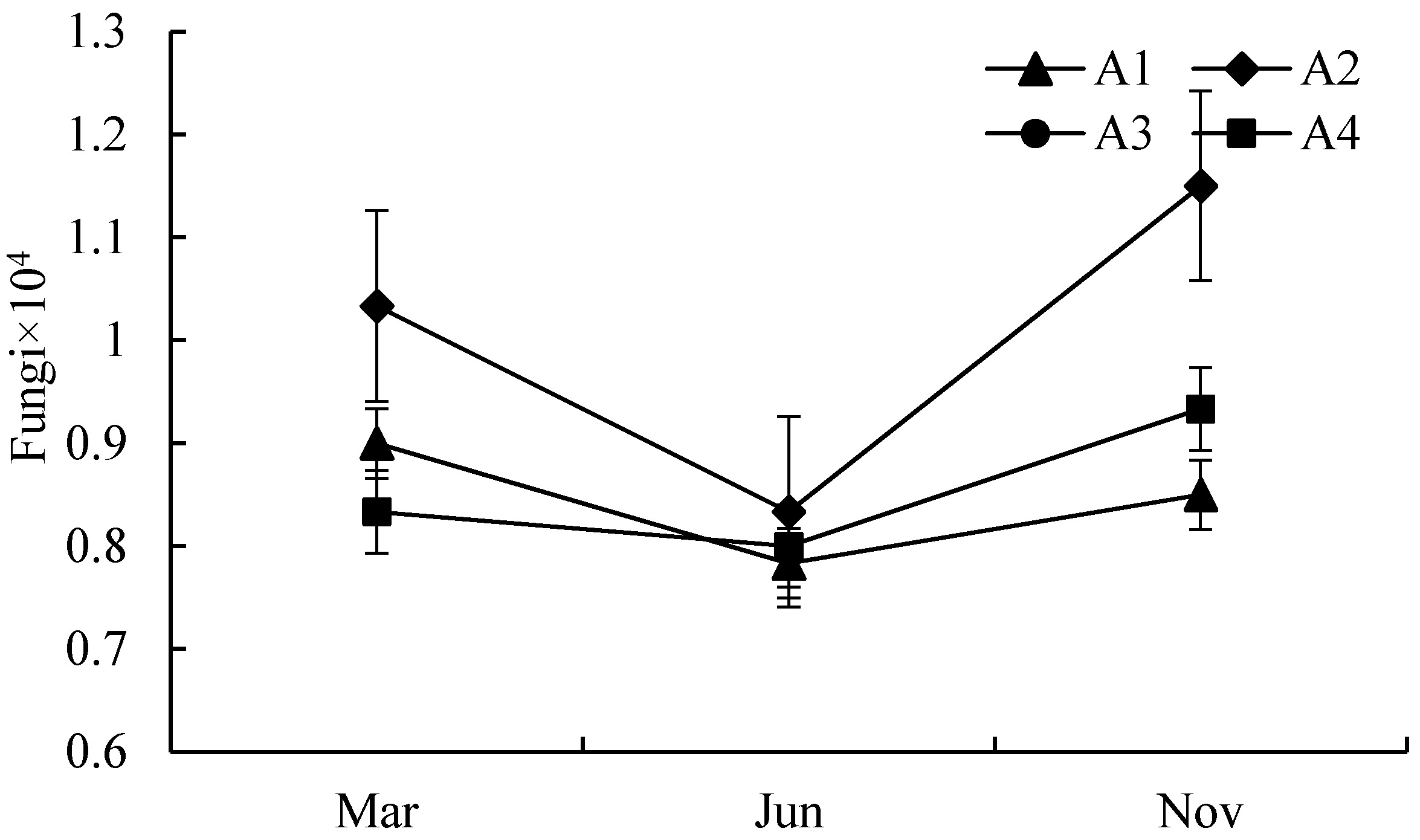
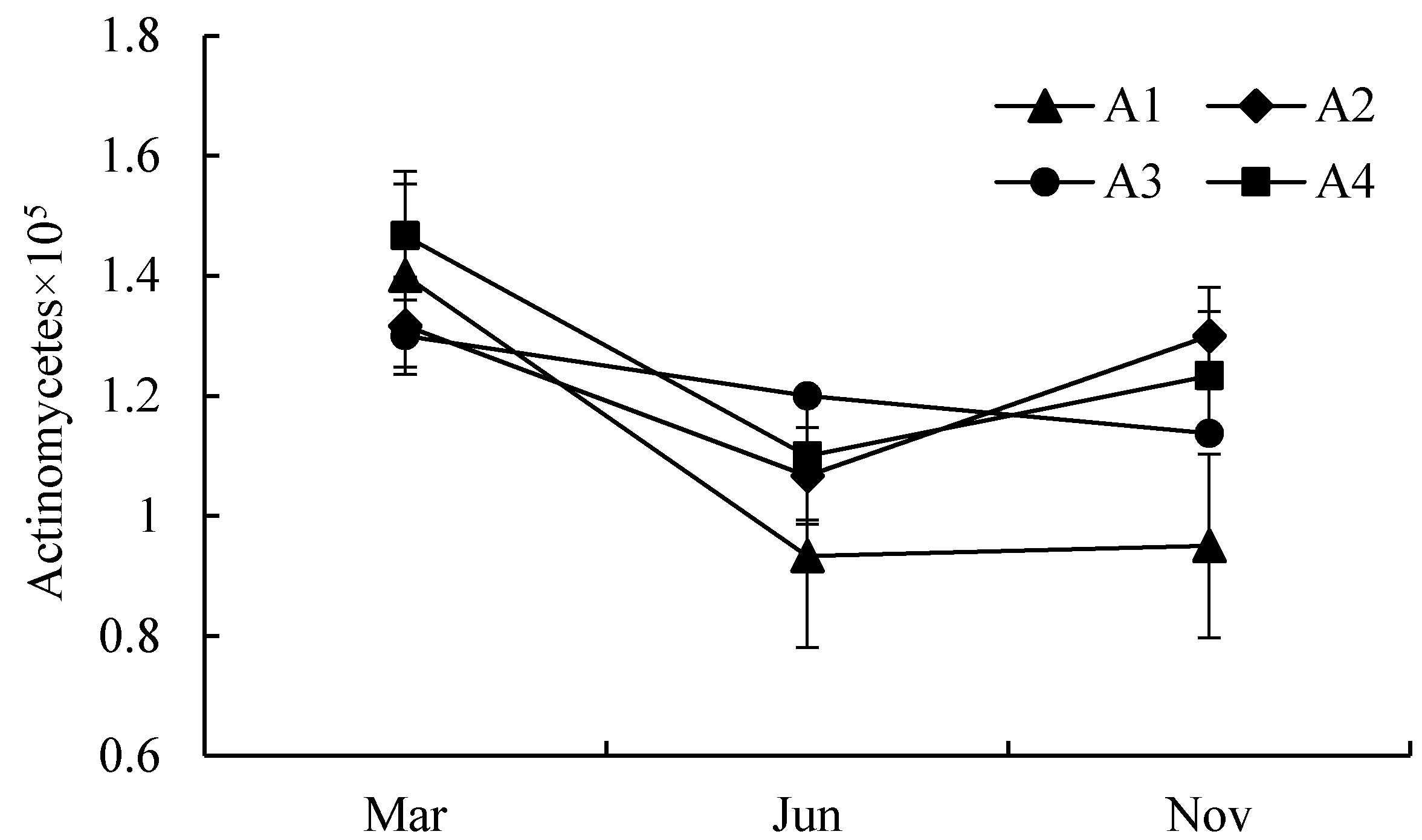
3.1. Quantitative Analysis of Biological Properties
3.2. The Spatial and Temporal Distribution of the Soil Organic Matter (SOM) and Nutrients
3.3. Correlation Analysis for Statistics
3.4. Evaluation of Soil Fertility Using the IFI
3.5. Potential Risk Assessment of Eutrophication
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borin, M.; Malagoli, M. Ecology, functioning and management of wetland systems. Environ. Sci. Pollut. Res. 2015, 22, 2357–2359. [Google Scholar] [CrossRef] [PubMed]
- Macci, C.; Peruzzi, C.; Doni, S.; Iannelli, E.; Masciandaro, G. Ornamental plants for micropollutant removal in wet-land sys-tems. Environ. Sci. Pollut. Res. 2015, 22, 2406–2415. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, H.; Zuo, J.; Wang, P.; Zhao, D.; An, S. Decreasing but still significant facilitation effect of cold-season macro-phytes on wetlands purification function during cold winter. Sci. Rep. 2016, 6, 27011. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Duan, M.; Li, Y.; Nwankwegu, A.S.; Ji, Y.; Zhang, J. Concentration and pollution assessment of heavy metals within surface sediments of the Raohe Basin, China. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, R.H.; MacArthur, J.W. On Bird Species Diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Lefebvre, G.; Poulin, B.; Tscharntke, T. Reed cutting affects arthropod communities, potentially reducing food for passerine birds. Biol. Conserv. 2004, 121, 157–166. [Google Scholar] [CrossRef]
- Wu, X.X.; Lv, M.; Jin, Z.Y.; Michishita, R.; Chen, J. Normalized difference vegetation index dynamic and spatiotem-poral distribution of migratory birds in the Poyang Lake wetland, China. Ecol. Indic. 2014, 47, 219–230. [Google Scholar] [CrossRef]
- You, H.; Xu, L.; Liu, G.; Wang, X.; Wu, Y.; Jiang, J. Effects of Inter-Annual Water Level Fluctuations on Vegetation Evolution in Typical Wetlands of Poyang Lake, China. Wetlands 2015, 35, 931–943. [Google Scholar] [CrossRef]
- Dai, X.; Wan, R.; Yang, G.; Wang, X.; Xu, L. Responses of wetland vegetation in Poyang Lake, China to water-level fluctua-tions. Hydrobiologia 2016, 773, 35–47. [Google Scholar] [CrossRef]
- Myungsyun, S.; Seongyoul, C.; Sangkun, P.; Ohkeun, K. Growth responses and nutrient absorption characteristics of ardisia pot plants in two growth stages as influenced by nutrient solution strengths. Hortic. Environ. Biotechnol. 2009, 50, 525–531. [Google Scholar]
- Zhang, Q.J.; Yu, X.B.; Qian, J.X.; Xiong, T. Distribution characteristics of plant communities and soil organic matter and main nutrients in the Poyang Lake Nanji Wetland. Acta Ecol. Sin. 2012, 12, 3656–3669. (In Chinese) [Google Scholar] [CrossRef]
- Xu, J.X.; Xu, L.G.; Jiang, J.H.; Wang, X.L.; Chen, Y.L.; Xu, J. Change of vegetation community structure and the rela-tionship between it and soil nutrients in typical beaches in Poyang Lake Area. Wetl. Sci. 2013, 2, 186–191. (In Chinese) [Google Scholar]
- Sun, B.; Hallett, P.D.; Caul, S.; Daniell, T.J.; Hopkins, D.W. Distribution of soil carbon and microbial biomass in arable soils under different tillage regimes. Plant Soil 2011, 338, 17–25. [Google Scholar] [CrossRef]
- Heemsbergen, D.; Berg, M.P.; Loreau, M.; Hal, J.; Faber, J.H.; Verhoef, H. Biodiversity effects on soil processes ex-plained by site-specific functional dissimilarity. Science 2004, 306, 1019–1020. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.; White, D.C.; Peacock, A.; Tilman, D. Plant diversity, soil microbial communities, and ecosys-tem function: Are there any link? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Shine, A. Linkages between plant litter diversity, soil microbial biomass and ecosystem function in temper-ate grasslands. Soil Biol. Biochem. 1999, 31, 317–321. [Google Scholar] [CrossRef]
- Broughton, L.C.; Gross, K.L. Patterns of diversity in plant and soil microbial communities along a productivity gra-dient in a Michigan old field. Oecologia 2000, 125, 420–427. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, J.; Li, R.; Pan, B.; Zhang, L.; Chen, X. Distribution and partitioning of heavy metals in sediments of the Xinjiang River in Poyang Lake Region, China. Environ. Prog. Sustain. Energy 2015, 34, 713–723. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, X.L.; Tang, C.J.; Xu, H.X.; Jiang, X.Y.; Zhang, Y.L. Influence of long-term inundation and nutrient ad-dition on denitrification in sandy wetland sediments from Poyang Lake, a large shallow subtropical lake in China. Environ. Pollut. 2016, 219, 440–449. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Li, X.; Zhang, Q. Factors influencing water level changes in China’s largest freshwater lake, Poyang Lake, in the past 50 years. Water Int. 2014, 39, 983–999. [Google Scholar] [CrossRef]
- Wang, X.L.; Han, J.Y.; Xu, L.G.; Wan, R.G.; Chen, Y.W. Soil characteristics in relation to vegetation communities in the wet-lands of Poyang Lake, China. Wetlands 2014, 34, 829–839. [Google Scholar]
- Tan, Z.; Jiang, J.-H. Spatial–Temporal Dynamics of Wetland Vegetation Related to Water Level Fluctuations in Poyang Lake, China. Water 2016, 8, 397. [Google Scholar] [CrossRef]
- Wu, Z.; Cai, Y.; Liu, X.; Xu, C.P.; Chen, Y.; Zhang, L. Temporal and spatial variability of phytoplankton in Lake Poyang: The largest freshwater lake in China. J. Great Lakes Res. 2013, 39, 476–483. [Google Scholar] [CrossRef]
- Li, B.; Yang, G.S.; Wan, R.G.; Zhang, Y.H.; Dai, X.; Chen, Y.W. Spatiotemporal variability in the water quality of po-yang lake and its associated responses to hydrological conditions. Water 2016, 8, 296–312. [Google Scholar] [CrossRef]
- Shao, M.Q.; Jiang, H.J.; Guo, H.; Zeng, B.B. Abundance, distribution and diversity variations of wintering water birds in Poyang Lake, Jiangxi Province, China. Pak. J. Zool. 2014, 46, 451–462. [Google Scholar]
- Dronova, I.; Beissinger, S.R.; Burnham, J.W.; Gong, P. Landscape-Level Associations of Wintering Waterbird Diversity and Abundance from Remotely Sensed Wetland Characteristics of Poyang Lake. Remote. Sens. 2016, 8, 462. [Google Scholar] [CrossRef]
- Yang, P.; Liu, X.; Xu, B. Spatiotemporal pattern of bird habitats in the Poyang Lake based on Landsat images. Environ. Earth Sci. 2016, 75, 1230. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis: Part 3, Chemical Methods; Bigham, J.M., Ed.; Soil Science Society of America Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis: Part 3; Chemical, Methods, SSSA Book, Series no 5, Bigham, J.M., Eds.; Soil Science Society of America Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Xu, G. Manual of Soil Microbial Analysis; Agricultural Publishing House: Beijing, China, 1986. [Google Scholar]
- Li, Z.; Huang, J.Q.; Li, Y.Y.; Guo, W.; Zhu, J.F. Assessment on soil fertility of Dongting Lake wetland area (China) based on GIS and fuzzy evaluation. J. Central South Univ. 2011, 18, 1465–1472. [Google Scholar] [CrossRef]
- Leivuori, M.; Niemist, Ö.L. Sedimentation of trace metals in the Gulf of Bothnia. Chemosphere 1995, 31, 3839–3856. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Sun, Q.; Xie, Y. Review on the Study of Soil Microorganisms in Wetland Ecosystems. Chin. J. Appl. Environ. Biol. 2013, 19, 547–552. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, X.; Wu, L.; Lu, Y. Community composition of ammonia-oxidizing bacteria and archaea in rice field soil as af-fected by nitrogen fertilization. Syst. Appl. Microbiol. 2009, 32, 27–36. [Google Scholar] [CrossRef]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter sta-bilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Haynes, R. Labile Organic Matter Fractions as Central Components of the Quality of Agricultural Soils: An Overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar] [CrossRef]
- Burger, M.; Jackson, L.E.; Lundquist, E.J.; Louie, D.T.; Miller, R.L.; Rolston, D.E.; Scow, K.M. Microbial responses and nitrous oxide emissions during wetting and drying of organically and conventionally managed soil under to-matoes. Biol. Fertil. Soils 2005, 42, 109–118. [Google Scholar] [CrossRef]
- Cavagnaro, T.R. Soil moisture legacy effects: Impacts on soil nutrients, plants and mycorrhizal responsiveness. Biol. Biochem. 2016, 95, 173–179. [Google Scholar] [CrossRef]
- Ouyang, Q.L.; Gao, G.Q.; Liu, S.S.; Shi, X.L.; Ju, H.Y. Study on Adsorption and Desorption of Suspended Sediments to Phos-phorus in Ganjiang River. Adv. Mater. Res. 2012, 599, 669–672. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.M.; Xin, Z.B.; Wang, J.; Ren, Y.; Li, Q.S. Plant diversity and soil characteristics of different inundation zones in an aquatic-terrestrial ecotone, Li River. Acta Ecol. Sin. 2015, 15, 5121–5130. (In Chinese) [Google Scholar]
- Devi, N.B.; Yadava, P. Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, Northeast India. Appl. Soil Ecol. 2006, 31, 220–227. [Google Scholar] [CrossRef]
- Parr, J.F.; Papendick, R.I. Soil quality, relationship and strategies for sustainable dryland farming system. Ann. Arid Zone 1997, 36, 181–191. [Google Scholar]
- Bai, J.; Gao, H.; Xiao, R.; Wang, J.; Huang, C. A Review of Soil Nitrogen Mineralization as Affected by Water and Salt in Coastal Wetlands: Issues and Methods. CLEAN–Soil Air Water 2012, 40, 1099–1105. [Google Scholar] [CrossRef]
- Huang, L.; Bai, J.; Xiao, R.; Shi, J.; Gao, H. The soil nitrogen dynamics in an inland salt marsh as affected by various experi-mental water levels. Hydrol. Process. 2014, 28, 4708–4717. [Google Scholar] [CrossRef]
- Liu, J.; Fang, S.; Sun, J. Nutrient zoning of Poyang Lake based on aquatic eco-environment indices. Environ. Earth Sci. 2016, 75, 61. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Distribution Characteristics of Phosphorus in the Sediments and Overlying Water of Poyang Lake. PLoS ONE 2015, 10, e0125859. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, L.; Liang, T.; Huang, M. Nitrogen distribution and ammonia release from the overlying water and sedi-ments of Poyang Lake, China. Environ. Earth Sci. 2015, 74, 771–778. [Google Scholar] [CrossRef]
- Edwards, K.A.; Jefferies, R.L. Inter-annual and seasonal dynamics of soil microbial biomass and nutrients in wet and dry low-Arctic sedge meadows. Soil Biol. Biochem. 2013, 57, 83–90. [Google Scholar] [CrossRef]
- Ge, P.; Da, L.; Wang, W.; Xu, X. Seasonal dynamics of dissolved organic carbon, nitrogen and other nutrients in soil of Pinus massoniana stands after pine wilt disease disturbance. J. soil Sci. Plant Nutr. 2014, 14, 75–87. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Bai, J.H.; Liu, Q.; Lu, Q.Q.; Gao, Z.Q.; Wang, J.J. Spatial and seasonal variations of soil carbon and nitro-gen con-tent and stock in a tidal salt marsh with tamarix chinensis, China. Wetlands 2016, 36 (Suppl. 1), S145–S152. [Google Scholar] [CrossRef]

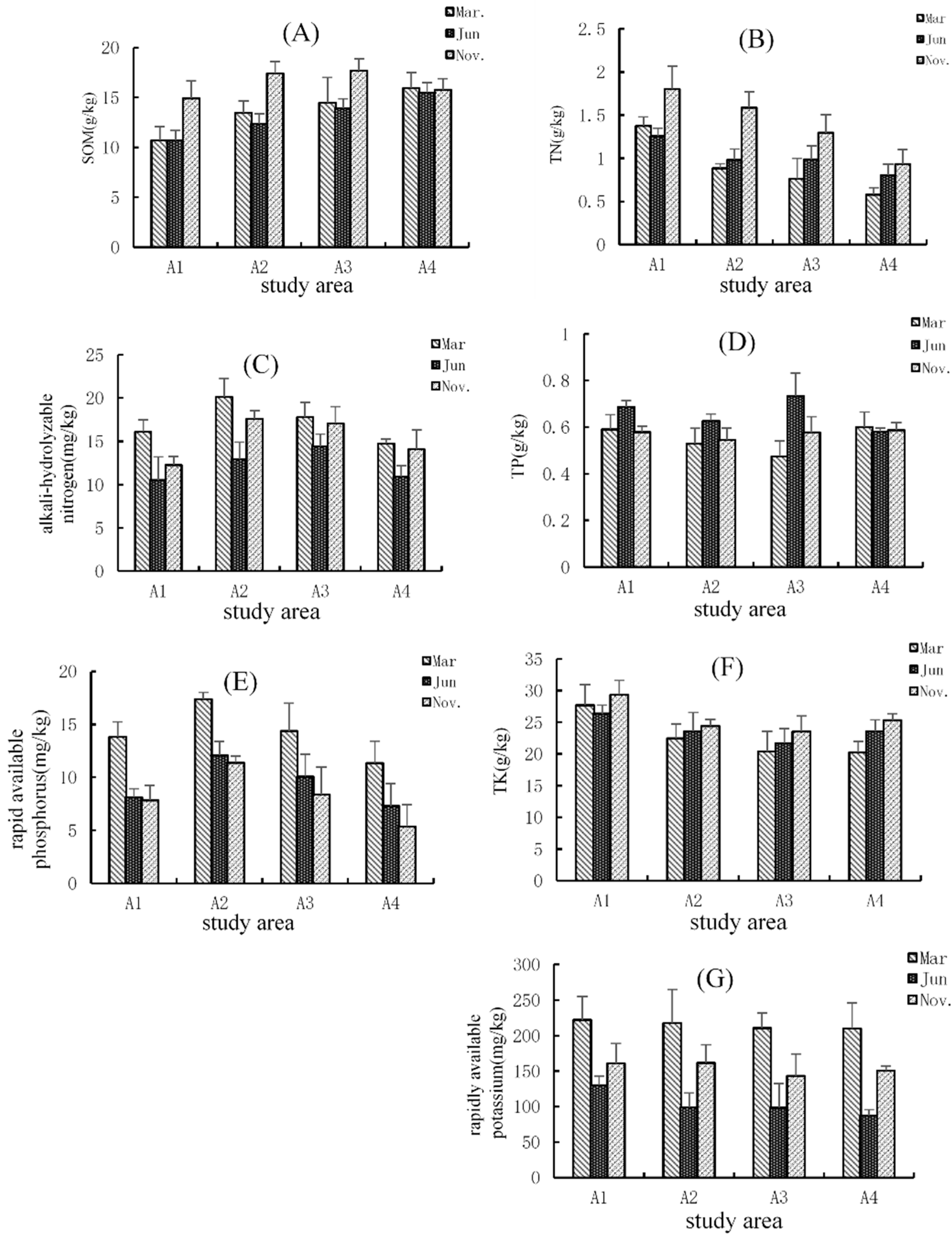
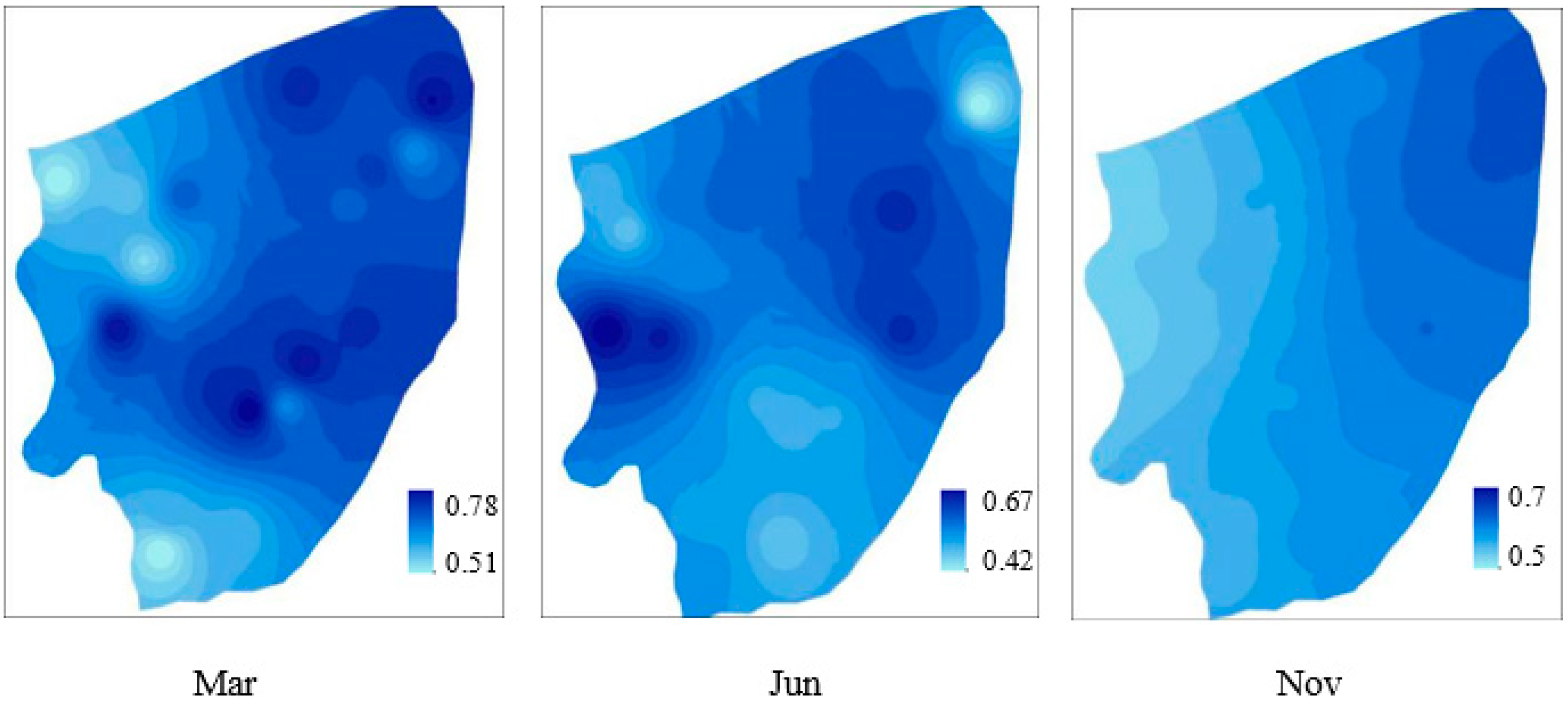
| Study Area | Sampling Sites | Detailed Description |
|---|---|---|
| A1 | S1–S6 | An area located neared the center of Poyang lake with a longer submerged period and rare anthropogenic activities. |
| A2 | S7–S12 | An area located in Ganjiang River downstream in Jiangxi Province, with heavy nutrients, such as nitrogen, phosphorus, and potassium. |
| A3 | S13–S20 | An area has abundant vegetation and few human activities. |
| A4 | S21–S23 | An area located neared the center of Poyang lake with a longer submerged period and rare anthropogenic activities. |
| S-Type | Parabola Type | ||||||
|---|---|---|---|---|---|---|---|
| AK | TP | TK | AN | AP | TN | TOC | |
| Point 1 | 50 | 0.5 | 10 | 10 | 5 | 0.5 | 8.5 |
| Point 2 | 1 | 11.5 | |||||
| Point 3 | 1.5 | 20 | |||||
| Point 4 | 200 | 1.2 | 25 | 90 | 20 | 2 | 25 |
| TN | AN | TP | AP | TK | AK | TOC | ||
|---|---|---|---|---|---|---|---|---|
| Mar | bacteria | 0.06 | −0.21 | −0.04 | −0.45 * | 0.12 | 0.50 * | 0.04 |
| fungi | −0.08 | 0.51 * | −0.46 * | 0.16 | −0.01 | −0.27 | 0.21 | |
| actinomycetes | 0.09 | −0.45 * | 0.40 * | −0.01 | 0.11 | 0.42 | −0.23 | |
| Jun | bacteria | −0.13 | 0.53 ** | 0.48 * | 0.37 | −0.21 | 0.22 | 0.49 * |
| fungi | −0.25 | 0.50 * | 0.17 | 0.02 | −0.12 | 0.43 * | 0.47 * | |
| actinomycetes | 0.00 | 0.51 ** | 0.12 | 0.16 | −0.49 * | −0.46 * | 0.52 ** | |
| Nov | bacteria | −0.45 * | 0.27 | −0.18 | −0.02 | −0.27 | −0.11 | 0.54 ** |
| fungi | −0.14 | 0.55 ** | 0.11 | 0.52 ** | −0.50 * | −0.19 | 0.44 * | |
| actinomycetes | −0.02 | 0.41 * | 0.05 | 0.42 * | −0.47 * | −0.09 | 0.08 |
| Parameters | TN | TP | TK | AN | AP | AK | SOM | |
|---|---|---|---|---|---|---|---|---|
| TN | A | 1 | ||||||
| B | 1 | |||||||
| C | 1 | |||||||
| TP | A | 0.36 | 1 | |||||
| B | 0.153 | 1 | ||||||
| C | 0.108 | 1 | ||||||
| TK | A | 0.627 ** | 0.174 | 1 | ||||
| B | 0.251 | 0.027 | 1 | |||||
| C | 0.438 * | 0.203 | 1 | |||||
| AN | A | 0.023 | −0.33 | 0.025 | 1 | |||
| B | −0.227 | 0.378 | −0.474 * | 1 | ||||
| C | −0.05 | 0.134 | −0.642 ** | 1 | ||||
| AP | A | 0.189 | −0.142 | 0.065 | 0.593 ** | 1 | ||
| B | −0.147 | 0.221 | −0.278 | 0.305 | 1 | |||
| C | 0.247 | 0.123 | −0.269 | 0.402 | 1 | |||
| AK | A | 0.191 | 0.413 | 0.139 | −0.337 | −0.16 | 1 | |
| B | 0.178 | 0.216 | 0.419 * | −0.051 | −0.209 | 1 | ||
| C | 0.315 | 0.161 | 0.292 | −0.153 | 0.048 | 1 | ||
| SOM | A | −0.485 * | −0.251 | −0.453 * | 0.409 | 0.015 | −0.332 | 1 |
| B | −0.312 | 0.112 | −0.386 | 0.523 * | 0.031 | −0.255 | 1 | |
| C | −0.199 | −0.042 | −0.197 | 0.346 | 0.284 | 0.12 | 1 | |
| Sampling Site | TN | Designation Number | TP | Designation Number | Sampling Site | TN | Designation Number | TP | Designation Number |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.29 | 2.34 | 0.66 | 1.10 | 13 | 0.87 | 1.58 | 0.74 | 1.23 |
| 2 | 1.32 | 2.39 | 0.74 | 1.23 | 14 | 0.95 | 1.73 | 0.86 | 1.43 |
| 3 | 1.06 | 1.93 | 0.70 | 1.17 | 15 | 1.01 | 1.83 | 0.88 | 1.47 |
| 4 | 1.34 | 2.44 | 0.68 | 1.13 | 16 | 0.95 | 1.73 | 0.78 | 1.30 |
| 5 | 1.32 | 2.39 | 0.68 | 1.13 | 17 | 1.09 | 1.99 | 0.68 | 1.13 |
| 6 | 1.20 | 2.19 | 0.66 | 1.10 | 18 | 0.98 | 1.78 | 0.72 | 1.20 |
| 7 | 1.23 | 2.24 | 0.62 | 1.03 | 19 | 1.32 | 2.39 | 0.62 | 1.03 |
| 8 | 0.92 | 1.68 | 0.68 | 1.13 | 20 | 0.73 | 1.32 | 0.58 | 0.97 |
| 9 | 0.95 | 1.73 | 0.58 | 0.97 | 21 | 0.76 | 1.37 | 0.58 | 0.97 |
| 10 | 0.84 | 1.53 | 0.64 | 1.07 | 22 | 0.98 | 1.78 | 0.56 | 0.93 |
| 11 | 1.04 | 1.88 | 0.62 | 1.03 | 23 | 0.67 | 1.22 | 0.60 | 1.00 |
| 12 | 0.90 | 1.63 | 0.62 | 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wei, J.; Li, Y.; Duan, M.; Nwankwegu, A.S.; Norgbey, E. The Influence of Seasonal Water Level Fluctuations on the Soil Nutrients in a Typical Wetland Reserve in Poyang Lake, China. Sustainability 2021, 13, 3846. https://doi.org/10.3390/su13073846
Zhang S, Wei J, Li Y, Duan M, Nwankwegu AS, Norgbey E. The Influence of Seasonal Water Level Fluctuations on the Soil Nutrients in a Typical Wetland Reserve in Poyang Lake, China. Sustainability. 2021; 13(7):3846. https://doi.org/10.3390/su13073846
Chicago/Turabian StyleZhang, Shuangshuang, Jin Wei, Yiping Li, Maoqing Duan, Amechi S. Nwankwegu, and Eyram Norgbey. 2021. "The Influence of Seasonal Water Level Fluctuations on the Soil Nutrients in a Typical Wetland Reserve in Poyang Lake, China" Sustainability 13, no. 7: 3846. https://doi.org/10.3390/su13073846
APA StyleZhang, S., Wei, J., Li, Y., Duan, M., Nwankwegu, A. S., & Norgbey, E. (2021). The Influence of Seasonal Water Level Fluctuations on the Soil Nutrients in a Typical Wetland Reserve in Poyang Lake, China. Sustainability, 13(7), 3846. https://doi.org/10.3390/su13073846








