Chloride Binding Capacity and Its Effect on the Microstructure of Mortar Made with Marine Sand
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportions and Specimen Preparation
2.3. Test Methods
3. Results and Discussion
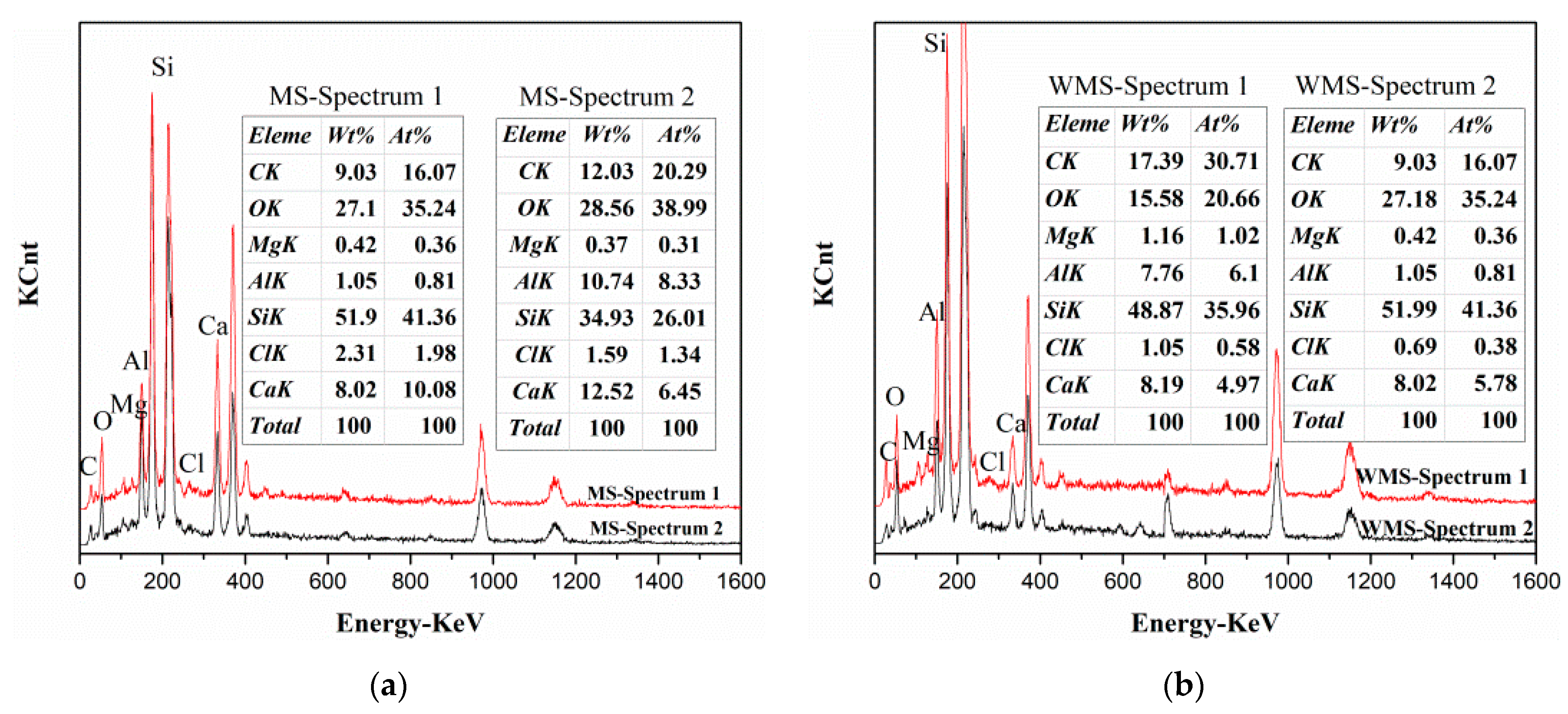
3.1. Diffusion of Chloride Ions from Fine Aggregate to Cement Hydrated Products
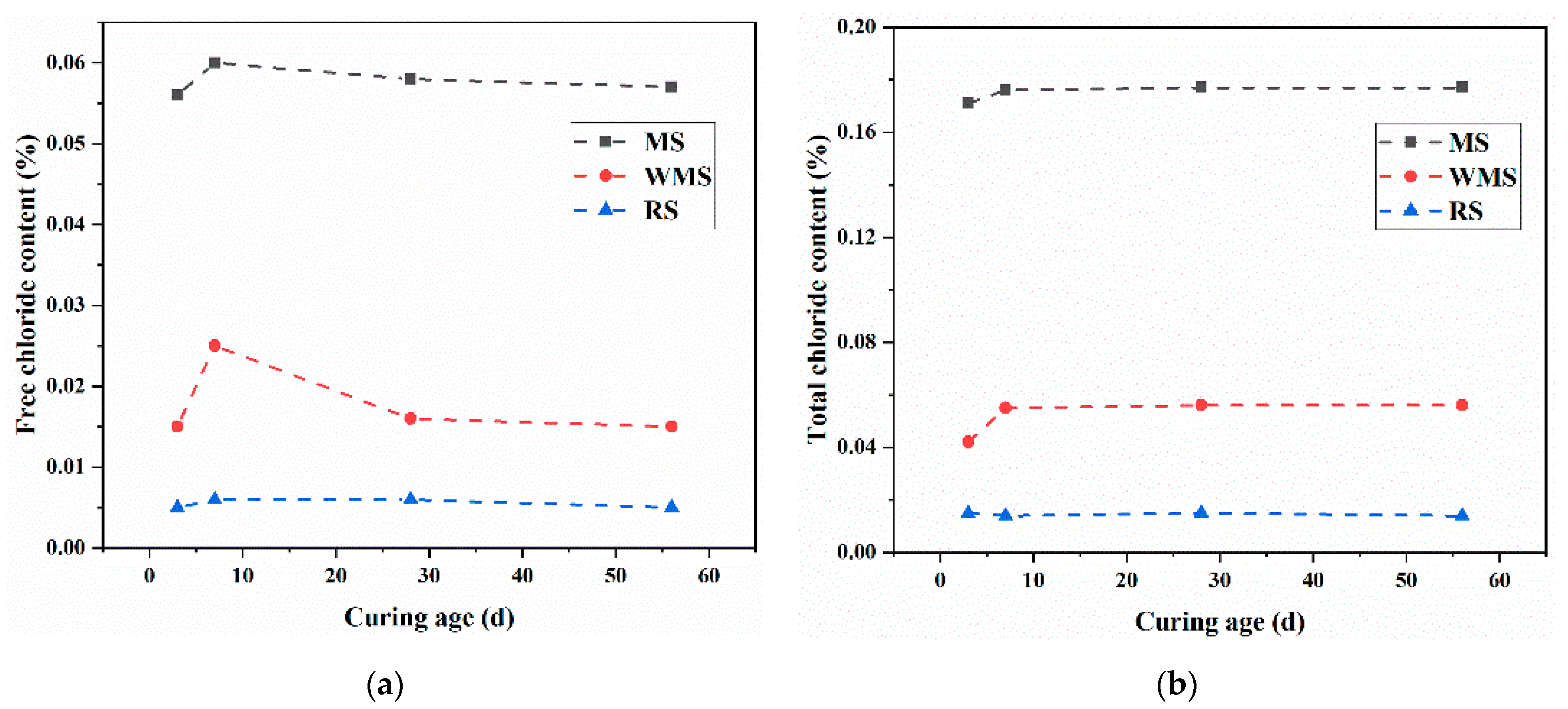
3.2. Free Chloride Content of Mortars at Different Curing Ages
3.3. Total Chloride Content of Mortars at Different Curing Ages
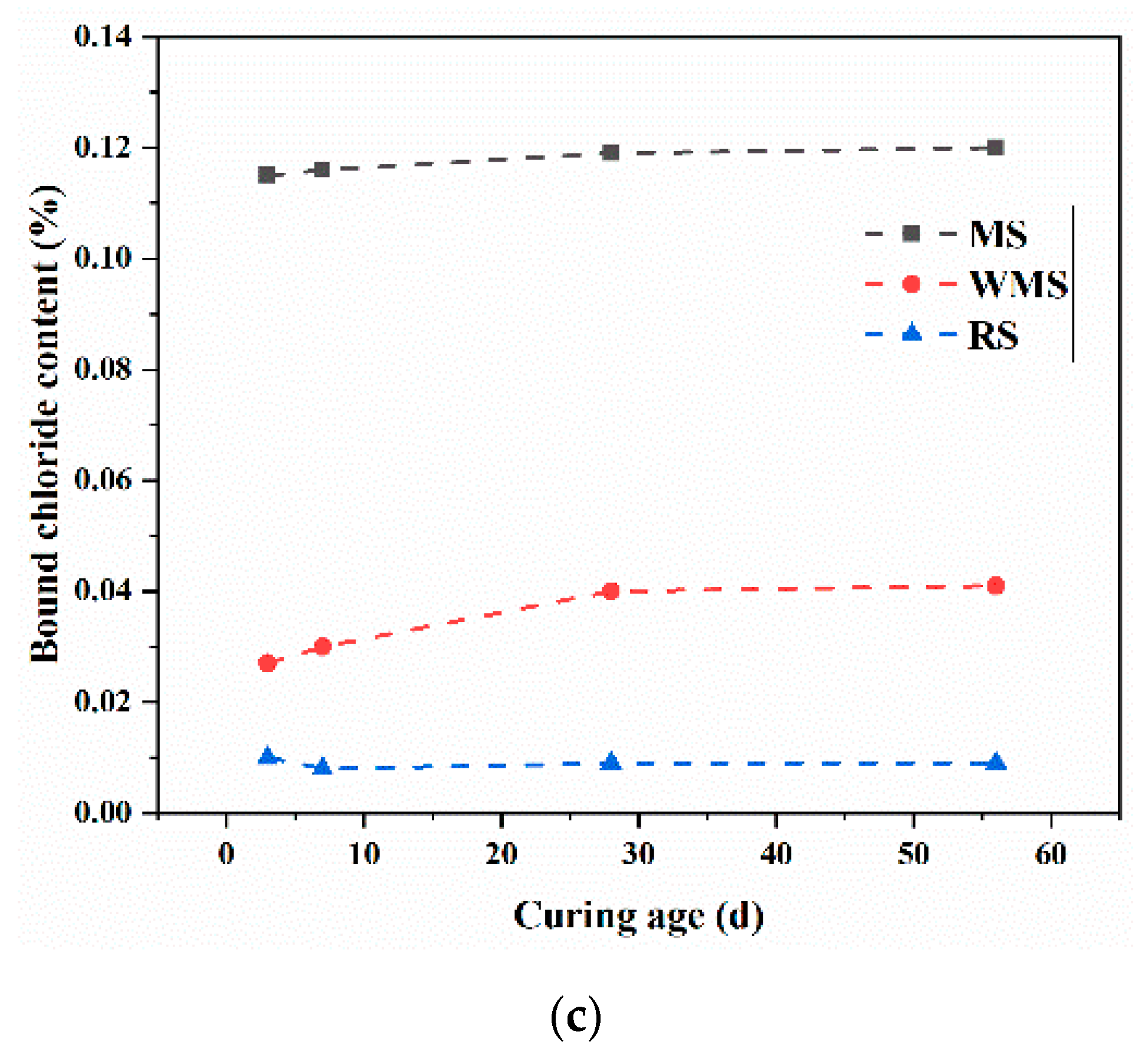
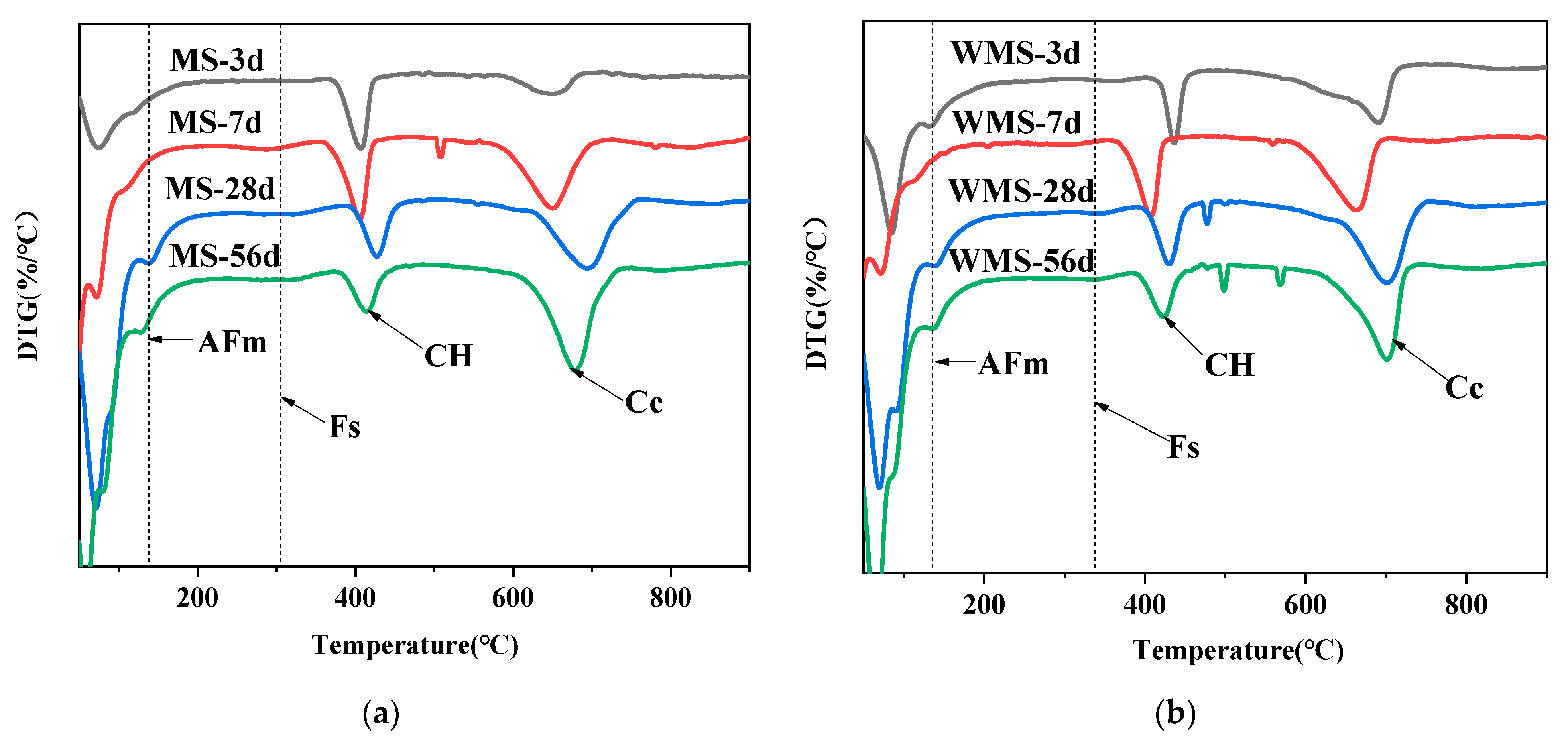
3.4. Bound Chloride Content at Different Curing Ages
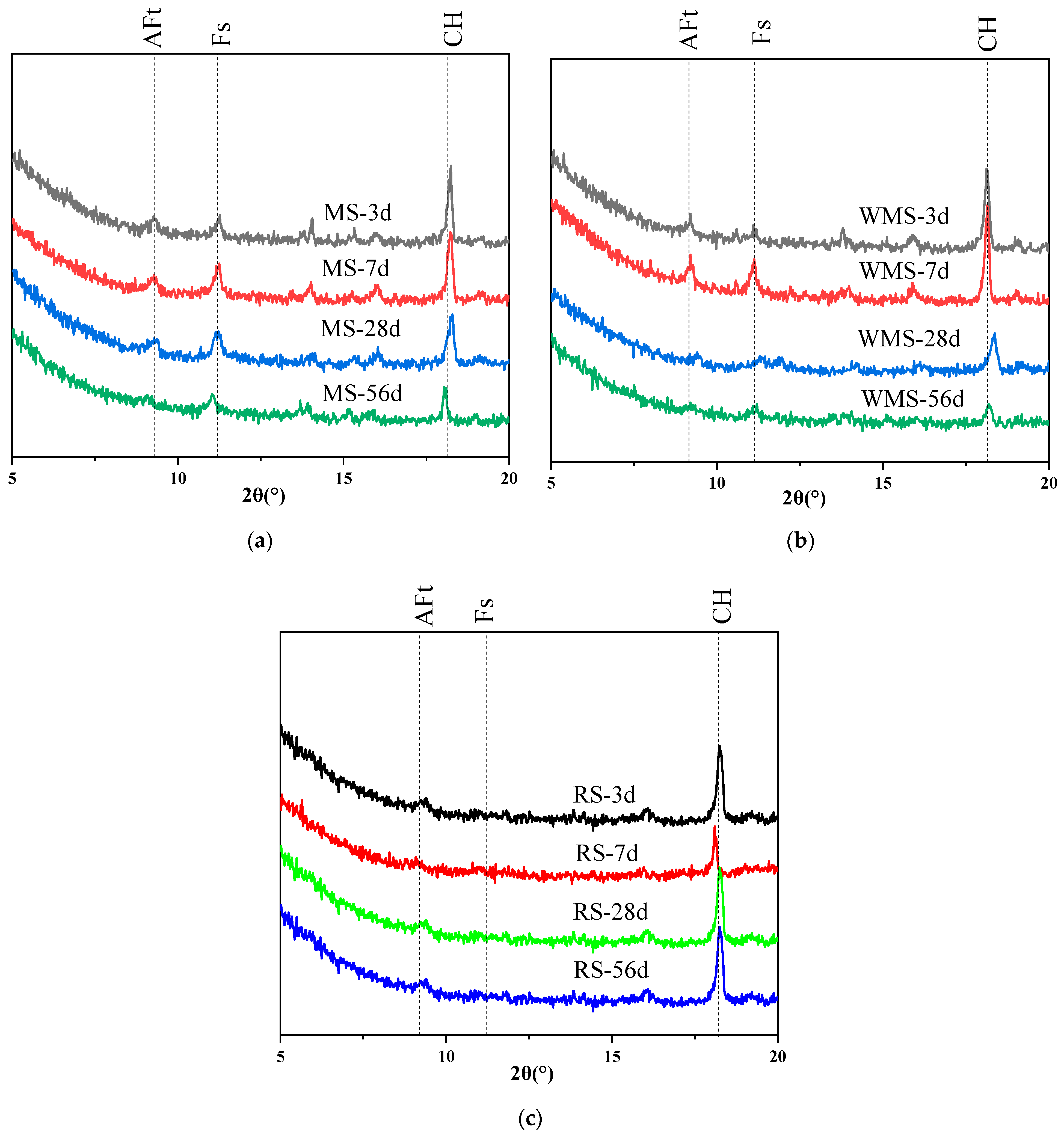
3.5. Chloride Binding Mechanism of Mortars at Different Curing Ages
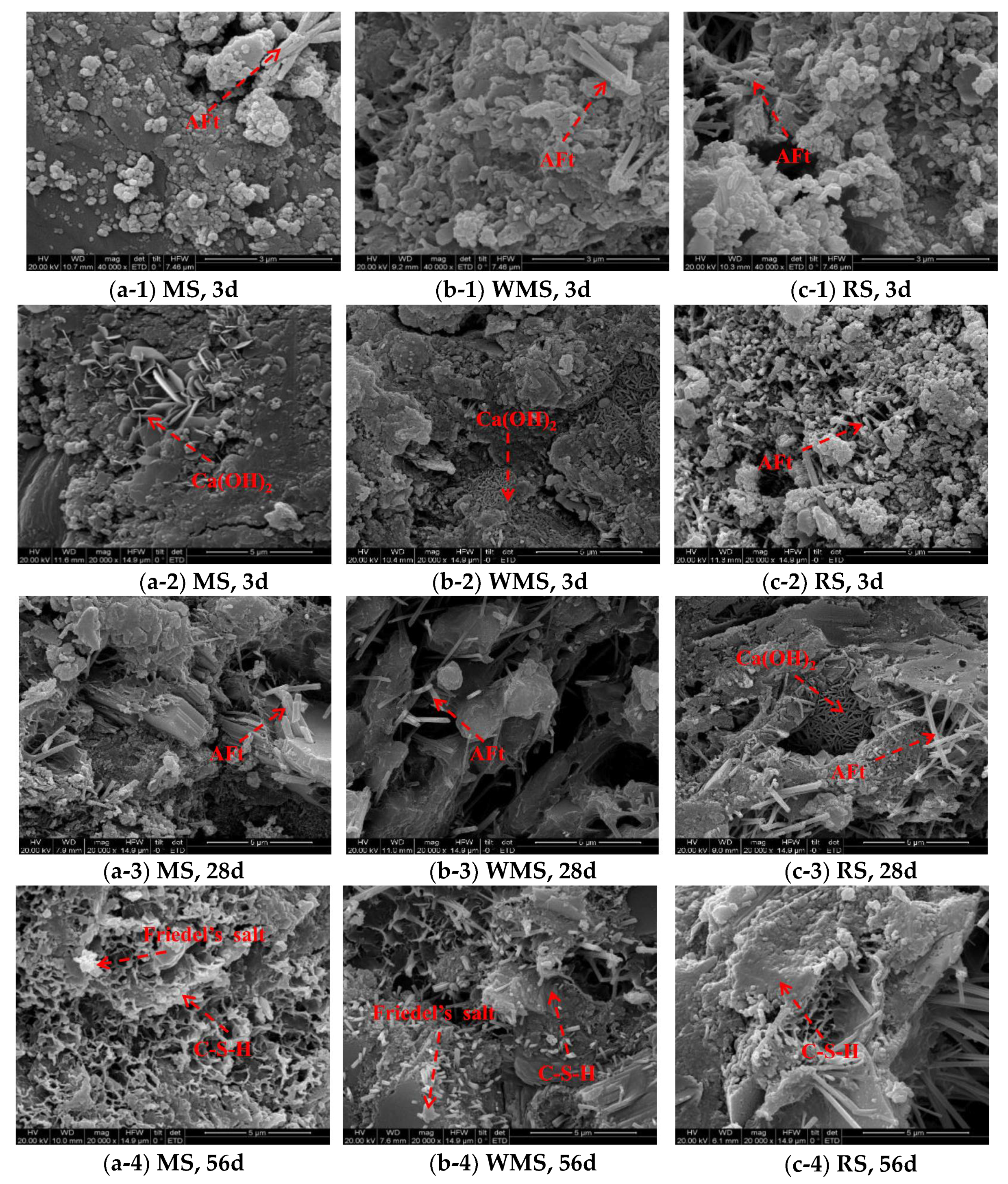
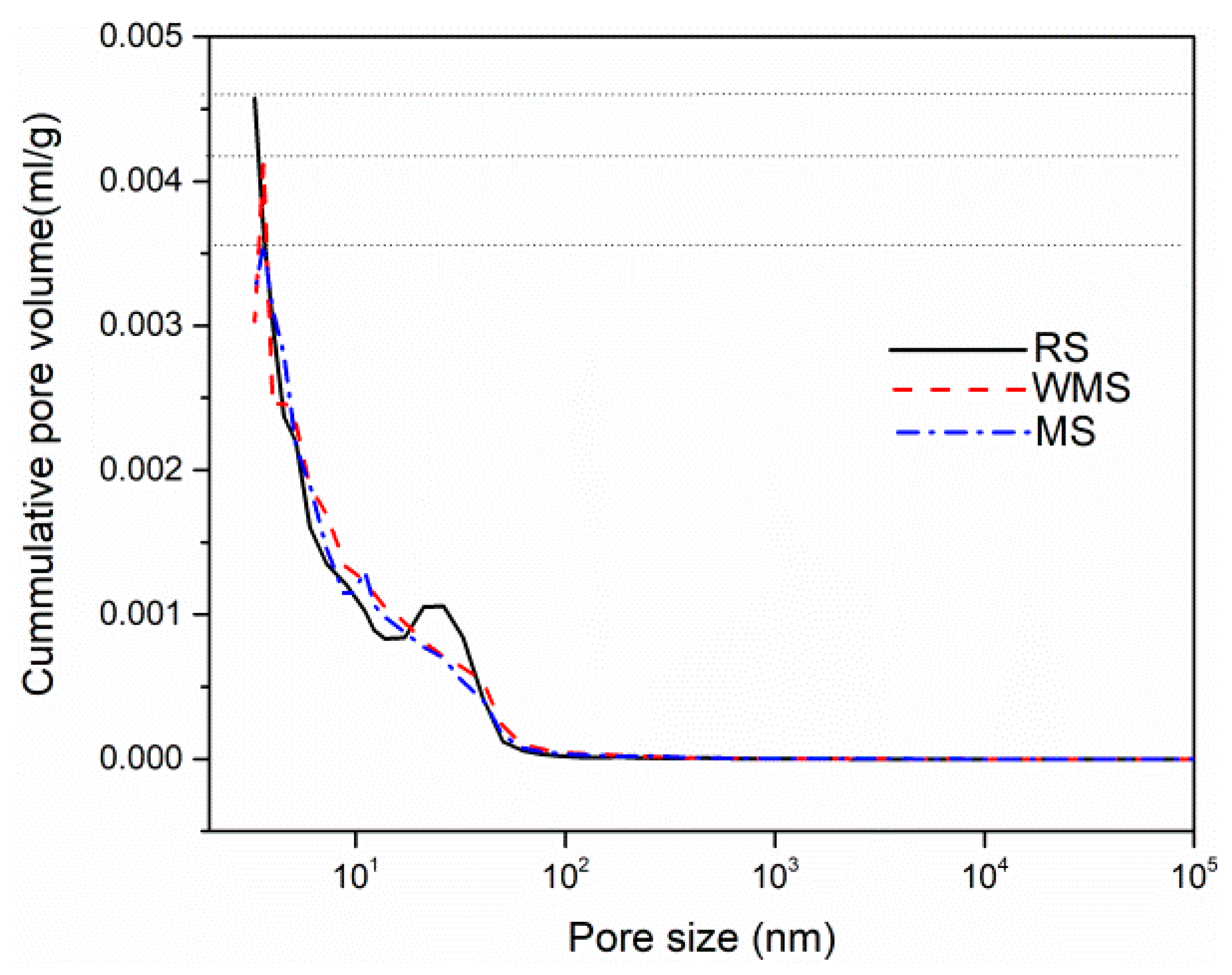
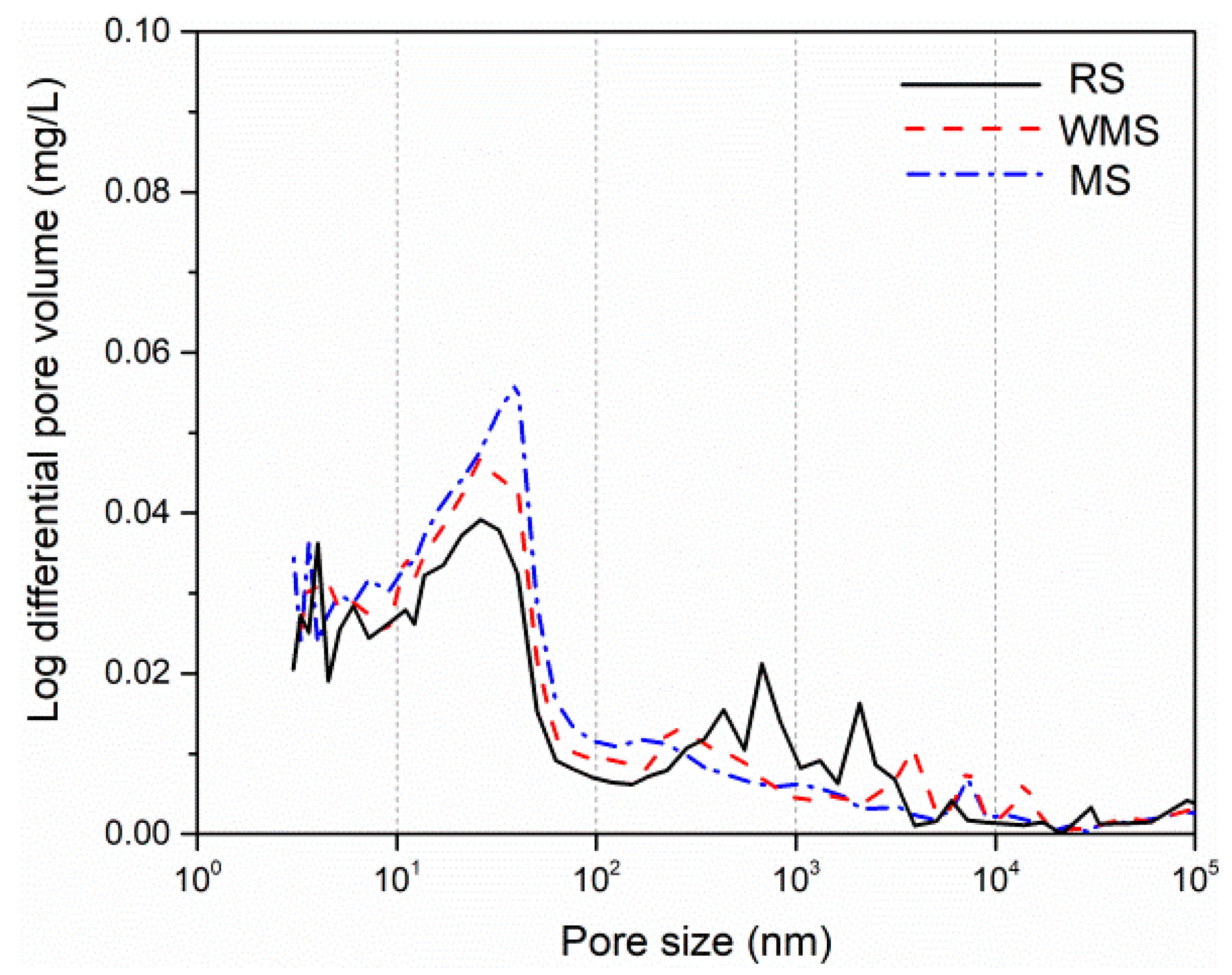
3.6. Effects of Chloride Binding on Micromorphology and Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jikai, Z.; Xu, H.; Lunchao, Z. CT characteristic analysis of sea-sand concrete exposed in simulated marine environment. Constr. Build. Mater. 2020, 268, 121170. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Joo, M.S. Mechanical and durability performance of marine sand and seawater concrete incorporating silicomanganese slag as coarse aggregate. Constr. Build. Mater. 2020, 254, 119195. [Google Scholar] [CrossRef]
- Limeira, J.; Etxeberria, M.; Agullo, L.; Molina, D. Mechanical and durability properties of concrete made with dredged marine sand. Med. Clínica 2011, 141, 4165–4174. [Google Scholar] [CrossRef]
- Chen, C.; Ji, T.; Zhuang, Y.; Lin, X. Workability, mechanical properties and affinity of artificial reef concrete. Constr. Build. Mater. 2015, 98, 227–236. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, G.; Xu, Y. Experimental study on the bond durability between steel-FRP composite bars (SFCBs) and sea sand concrete in ocean environment. Constr. Build. Mater. 2016, 115, 277–284. [Google Scholar] [CrossRef]
- Shang, B.; Li, Y. Application feasibility of HRB400 steel in seawater and marine sand concrete. Mater. Corros. 2018, 69. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.L.; Xian, G.; Wu, G.; Raman, R.K.S.; Al-Saadi, S. Effect of sustained load and seawater and sea sand concrete environment on durability of basalt- and glass-fibre reinforced polymer (B/GFRP) bars. Corros. Sci. 2018, 138, 200. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Y. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Kovler, K.; Roussel, N. Properties of fresh and hardened concrete. Cem. Concr. Res. 2011, 41, 775–792. [Google Scholar] [CrossRef]
- Limeir, J.; Agulló, L.; Etxeberria, M. Dredged marine sand as construction material. Eur. J. Environ. Civ. Eng. 2012, 16, 906–918. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Yu, R.; Huang, Y. Chloride ingress and binding of coral waste filler-coral waste sand marine mortar incorporating metakaolin. Constr. Build. Mater. 2018, 190, 1069–1080. [Google Scholar] [CrossRef]
- Sui, S.; Wilson, W.; Georget, F.; Maraghechi, H.; Scrivener, K.L. Quantification methods for chloride binding in Portland cement and limestone systems. Cem. Concr. Res. 2019, 125, 105864. [Google Scholar] [CrossRef]
- Zibara, H.; Hooton, R.D.; Thomas, M.D.A.; Stanish, K. Influence of the C/S and C/A ratios of hydration products on the chloride ion binding capacity of lime-SF and lime-MK mixtures. Cem. Concr. Res. 2008, 38, 422–426. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Brouwers, H.J.H. Chloride binding related to hydration products: Part I: Ordinary Portland Cement. Cem. Concr. Res. 2012, 42, 282–290. [Google Scholar] [CrossRef]
- Ipavec, A.; Vuk, T.; Gabrovšek, R.; Kaučič, V.E. Chloride binding into hydrated blended cements: The influence of limestone and alkalinity. Cem. Concr. Res. 2013, 48, 74–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, C.; Wang, J.; Gao, Y.; Zhang, J. Sustainability of Reinforced Concrete Beams with/without BF Influenced by Cracking Capacity and Chloride Diffusion. Sustainability 2020, 12, 1054. [Google Scholar] [CrossRef]
- Balonis, M.; Lothenbach, B.; Saout, G.L.; Glasser, F.P. Impact of chloride on the mineralogy of hydrated Portland cement systems. Cem. Concr. Res. 2010, 40, 1009–1022. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; Lothenbach, B.; Weerdt, K.D.; Garzón, S.F.; Enemark-Rasmussen, K.; Skibsted, J. Friedel’s salt profiles from thermogravimetric analysis and thermodynamic modelling of Portland cement-based mortars exposed to sodium chloride solution. Cem. Concr. Compos. 2017, 78, 73–83. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Ying Then, N.W.; Choudhary, A.; Weiss, J. Chloride binding of cement pastes with fly ash exposed to CaCl2 solutions at 5 and 23 °C. Cem. Concr. Compos. 2018, 97, 43–53. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Ben-Yair, M. The effect of chlorides on concrete in hot and arid regions. Cem. Concr. Res. 1974, 4, 405–416. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Li, H.; Mei, J.; Zhang, T.; Chen, P.; Gu, B. Chloride immobilization of cement-based material containing nano-Al2O3. Constr. Build. Mater. 2019, 220, 43–52. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Huang, Y.; Yu, R.; Xiao, X. Modification on the chloride binding capacity of cementitious materials by aluminum compound addition. Constr. Build. Mater. 2019, 222, 15–25. [Google Scholar] [CrossRef]
- Liu, J.; Dong, B.; Xing, F.; Liu, W.; Huo, Y. Simulation experiment and mechanism of the combining of sea sand type chlorine ions cement materials. J. Chin. Ceram. Soc. 2009, 37, 862–866+876. (In Chinese) [Google Scholar] [CrossRef]
- Michel, M.; Georgin, J.F.; Ambroise, J. Improving the mechanical performance of high-grade slag cement by the addition of Portland cement and sulfoaluminate cement. Constr. Build. Mater. 2012, 37, 291–300. [Google Scholar] [CrossRef]
- De Weerdt, K.; Kjellsen, K.O.; Sellevold, E.; Justnes, H. Synergy between fly ash and limestone powder in ternary cements. Cem. Concr. Compos. 2011, 33, 30–38. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, W.; Zhao, T.; Li, Q. Chloride binding in concrete exposed to corrosive solutions. J. Chin. Ceram. Soc. 2009, 37, 1068–1078. (In Chinese) [Google Scholar] [CrossRef]
- Csizmadia, J.; Balázs, G.; Tamás, F.D. Chloride ion binding capacity of aluminoferrites. Cem. Concr. Res. 2001, 31, 577–588. [Google Scholar] [CrossRef]
- Saikia, N.; Kato, S.; Kojima, T. Thermogravimetric investigation on the chloride binding behaviour of MK–lime paste. Thermochim. Acta 2006, 444, 16–25. [Google Scholar] [CrossRef]
- Zajac, M.; Rossberg, A.; Le Saout, G.; Lothenbach, B. Influence of limestone and anhydrite on the hydration of Portland cements. Cem. Concr. Compos. 2014, 46, 99–108. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Liu, W.; Cui, H.; Dong, Z.; Xing, F.; Zhang, H.; Lo, T.Y. Carbonation of concrete made with dredged marine sand and its effect on chloride binding. Constr. Build. Mater. 2016, 120, 1–9. [Google Scholar] [CrossRef]

| Content | SiO2 | CaO | MgO | Fe2O3 | Al2O3 | SO3 | Loss |
|---|---|---|---|---|---|---|---|
| wt% | 19.9 | 63.27 | 1.6 | 2.82 | 4.14 | 4.49 | 0.55 |
| Sand Type | Chloride Content (wt%) | Shell Content (wt%) | Fitness Modulus |
|---|---|---|---|
| RS | 0.005 | 0 | 2.73 |
| MS | 0.236 | 2.4 | 2.75 |
| WMS | 0.058 | 2.2 | 2.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Sun, M.; Tao, T.; Qu, F.; Wang, G.; Zhang, P.; Li, Y.; Duan, J. Chloride Binding Capacity and Its Effect on the Microstructure of Mortar Made with Marine Sand. Sustainability 2021, 13, 4169. https://doi.org/10.3390/su13084169
Sun C, Sun M, Tao T, Qu F, Wang G, Zhang P, Li Y, Duan J. Chloride Binding Capacity and Its Effect on the Microstructure of Mortar Made with Marine Sand. Sustainability. 2021; 13(8):4169. https://doi.org/10.3390/su13084169
Chicago/Turabian StyleSun, Congtao, Ming Sun, Tao Tao, Feng Qu, Gongxun Wang, Peng Zhang, Yantao Li, and Jizhou Duan. 2021. "Chloride Binding Capacity and Its Effect on the Microstructure of Mortar Made with Marine Sand" Sustainability 13, no. 8: 4169. https://doi.org/10.3390/su13084169
APA StyleSun, C., Sun, M., Tao, T., Qu, F., Wang, G., Zhang, P., Li, Y., & Duan, J. (2021). Chloride Binding Capacity and Its Effect on the Microstructure of Mortar Made with Marine Sand. Sustainability, 13(8), 4169. https://doi.org/10.3390/su13084169







