Energy Treatment of Solid Municipal Waste in Combination with Biomass by Decentralized Method with the Respect to the Negative Effects on the Environment
Abstract
:1. Introduction
2. Materials and Methods
- Energy properties of municipal solid waste (biofuels and non-hazardous municipal textile waste).
- The migration of heavy metals (HM).
- The formation and migration of polycyclic aromatic hydrocarbons (PAH).
2.1. Energy Properties
- Elemental composition of fuel (C, H, N, O, S)
- Calorific values or combustion heat
- Humidity
- Ash and biomass content
- Emission factor
- Density
- Analysis of the elemental composition of the fuel was carried out according to EN 15,407 “Solid recovered fuels. Methods for determining the carbon (C), hydrogen (H) and nitrogen (N) content” [14].
- Analysis of the calorific value and combustion heat of the fuel was performed according to the standard EN 15,400 “Solid recovered fuels. Determination of fuel calorific value” [15].
- Fuel moisture content analysis was performed based on EN 15,414 “Solid recovered fuels. Determination of the moisture content using the oven drying method” [16].
- The ash content analysis of the fuel was performed according to standard EN 15,403 “Solid recovered fuels. Determination of ash content” [17].
- Analysis of the biomass content determination of the fuel was performed based on standard EN 15,440: “Solid recovered fuels. Methods for determining the biomass content” [18].
2.2. The Migration of Heavy Metals
- Antimony, arsenic, lead, chromium, cobalt, copper, manganese, nickel, and vanadium: Maximum content in a flue gas 0.5 mg/m3;
- Cadmium and thallium: Maximum content in a flue gas 0.05 mg/m3;
- Mercury: Maximum content in a flue gas 0.05 mg/m3.
- Focusing with polycapillary optics on a beam with a diameter of 25 µm and interaction depth of 10–1000 µm;
- A wide-angle camera and two coaxial digital microscopes, 10× and 100×, to search the test site in the sample;
- Mosaic scanning when measuring large objects;
- An X-ray tube with a Rh target, excitation maximum of 50 kV/600 µA, and 5 filters;
- An SDD (Silicon Drift Detector) with a resolution of 145 eV and active area of 30 mm2.
2.3. Polycyclic Aromatic Hydrocarbons
3. Results
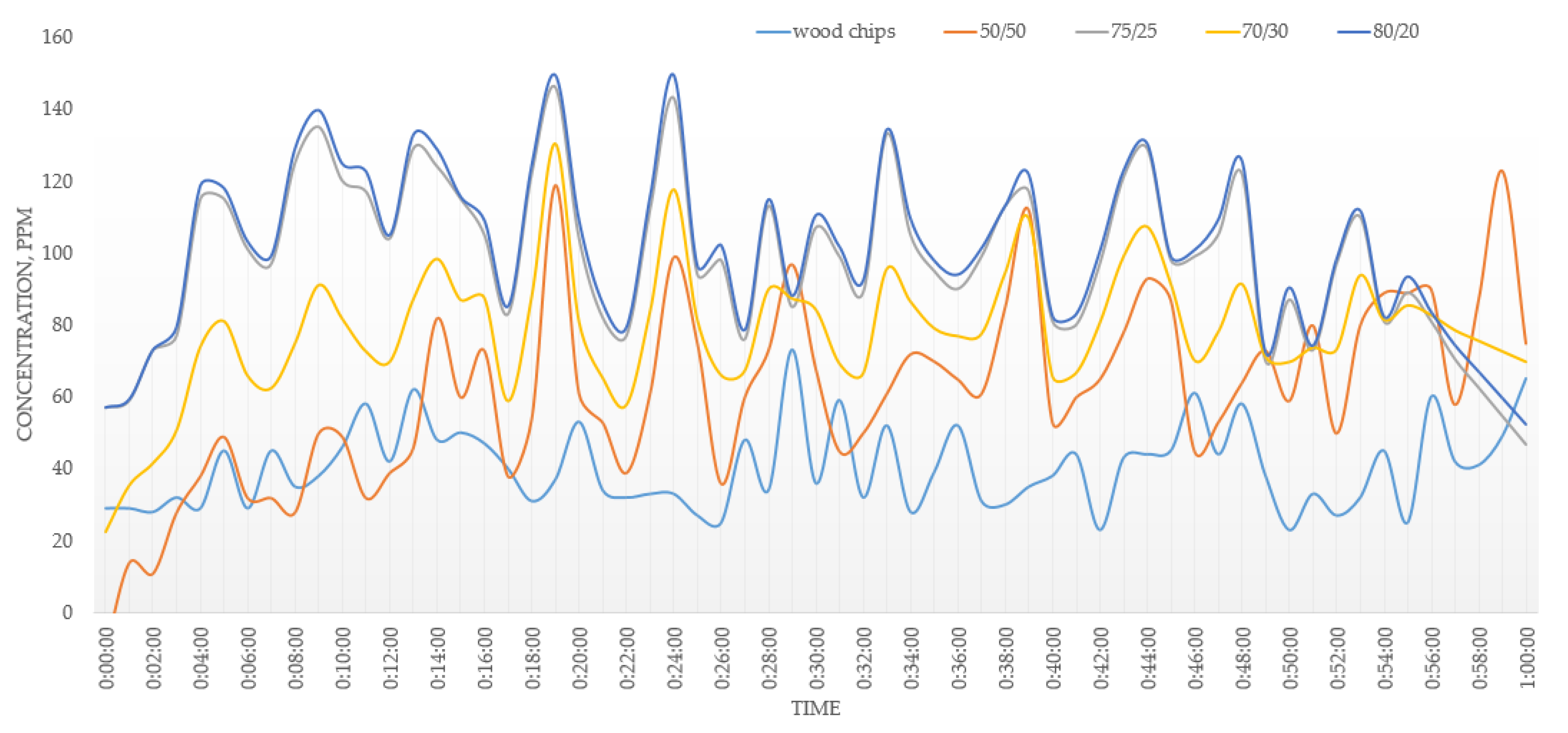
3.1. Energy Properties
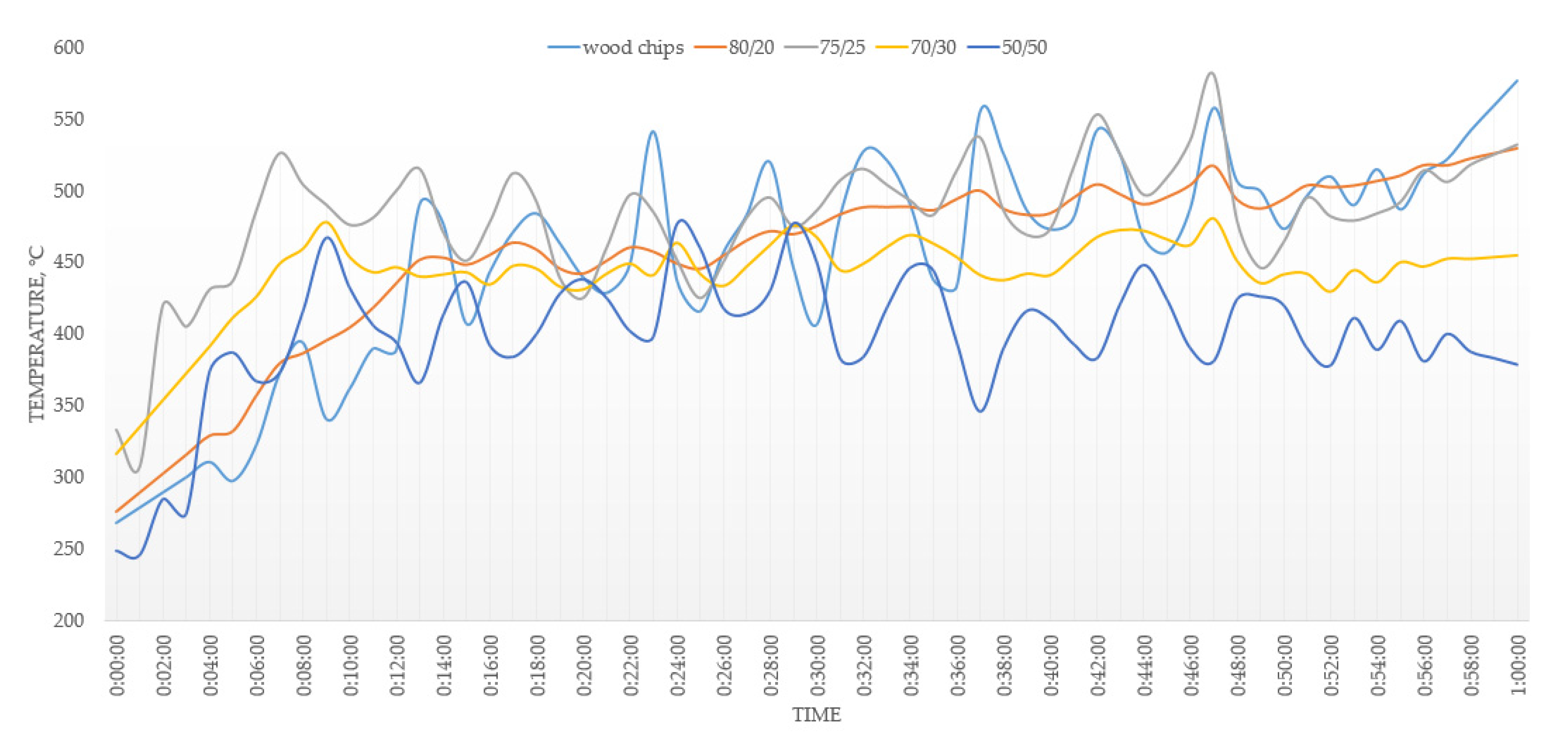
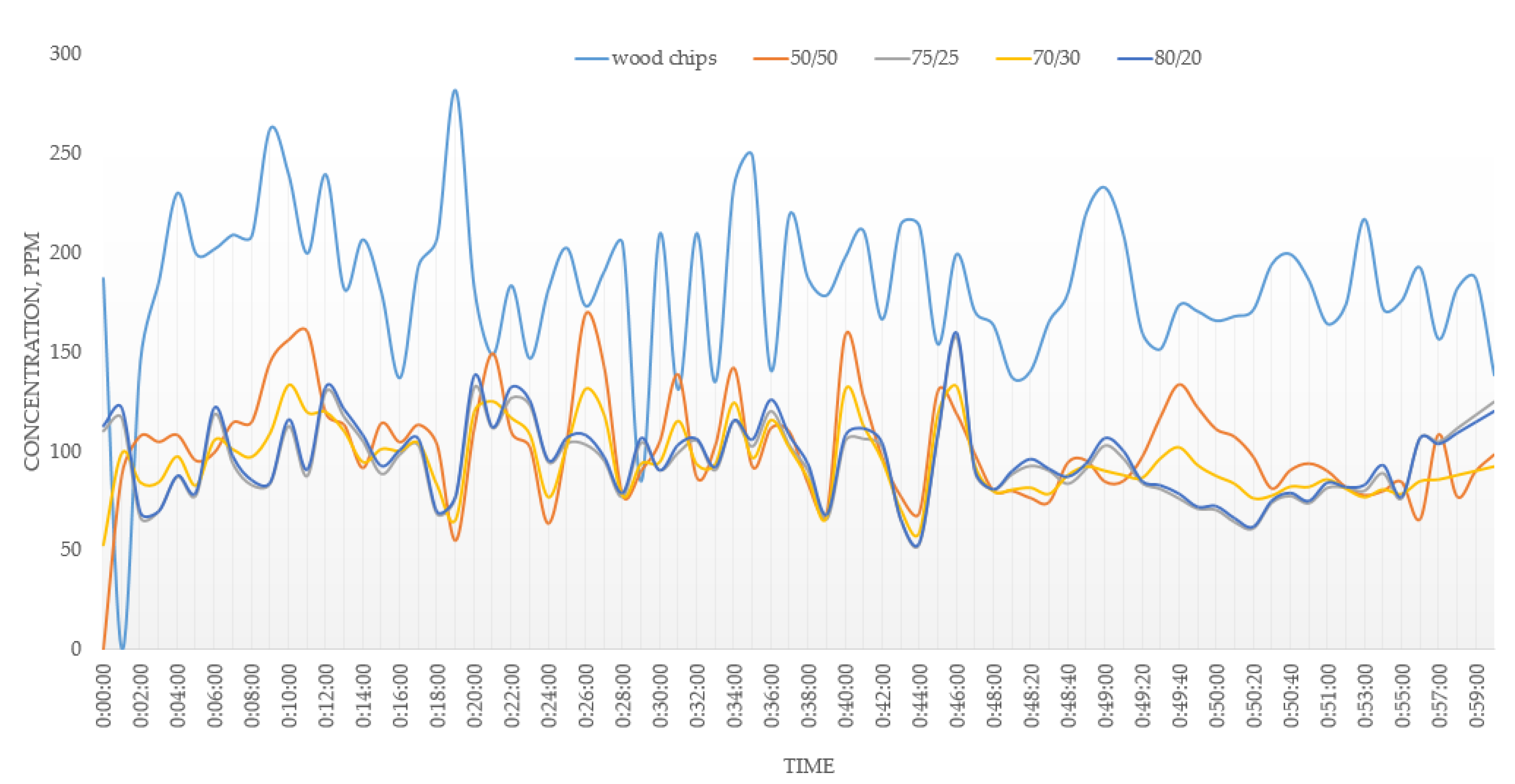
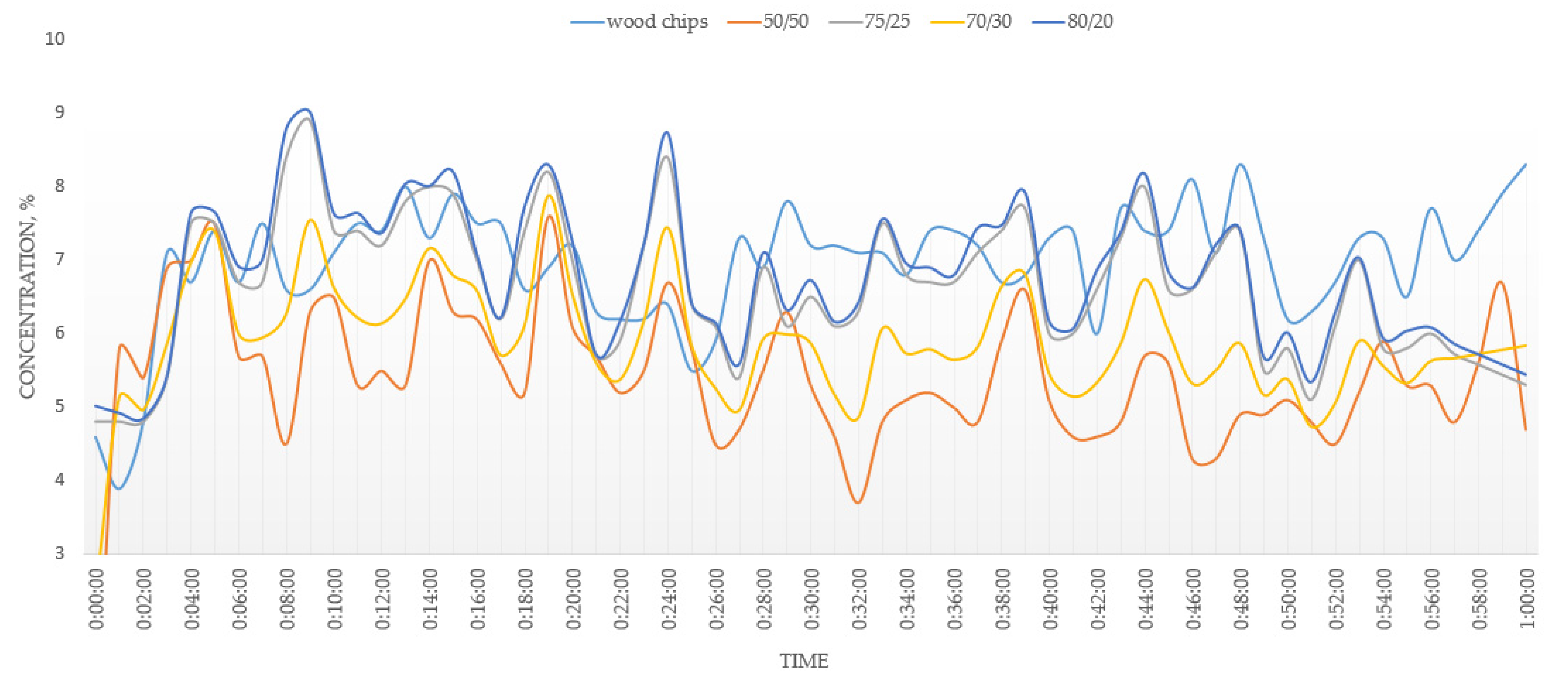
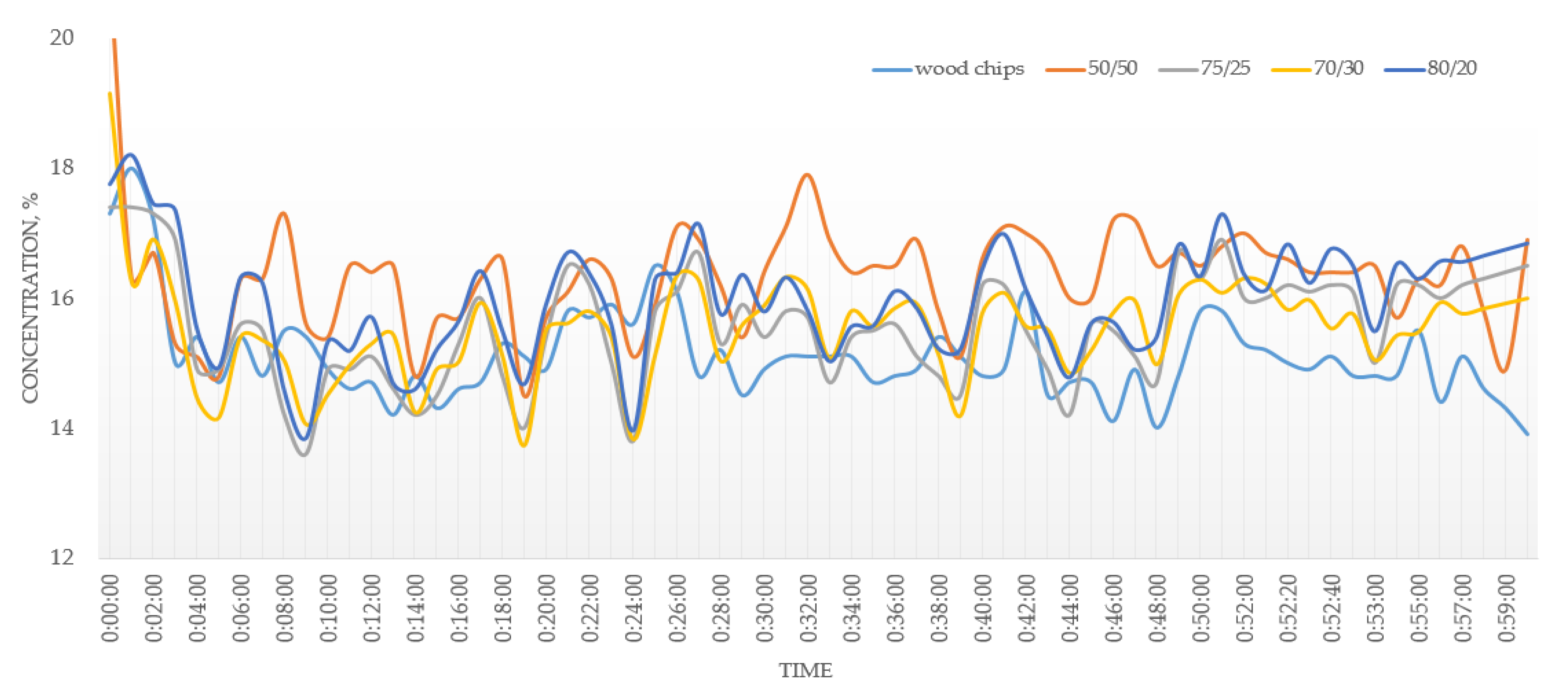
3.2. Combustion
3.3. The Migration of Heavy Metals
3.4. Polycyclic Aromatic Hydrocarbons
4. Discussion
5. Conclusions
- An addition of 20–30% of MSW to the fuel mixture showed an improvement in the operating parameters of the combustion process. The addition of 50% of MSW will lead to undesirable effects during the combustion process due to the different physics of the combustion of MSW and wood chips. With concentrations of MSW in the fuel of more than 30%, the emission limits cannot be met.
- During the combustion of the fuel mixtures, approximately 55% of the HM from the biomass and MSW passed into the ash. Analysis confirmed that the co-combustion of dirty fuels and biomass reduces the risk of releasing minerals and HM from the fuel into the natural environment. The HM concentration in the flue gases was in the range of 0.4 mg/m3, which is 20% lower than emission limits.
- Analysis of PAHs in MSW and fuel mixtures containing MSW showed a risk of increasing PAH concentrations in flue gases. Although the concentration of PAHs in the flue gas was within the appropriate limits, it is necessary to thoroughly control the chimney system of the combustion equipment in order to eliminate the increase in PAH content in the outdoor environment, especially in residential areas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Parliament. Council of the European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Union 2009. Available online: http://data.europa.eu/eli/dir/2009/28/oj (accessed on 24 January 2021).
- Taskin, A.; Demir, N. Life cycle environmental and energy impact assessment of sustainable urban municipal solid waste collection and transportation strategies. Sustain. Cities Soc. 2020, 61, 102339. [Google Scholar] [CrossRef]
- Calma, J. The COVID-19 pandemic is generating tons of medical waste. Verge 2020. Available online: https://www.theverge.com/2020/3/26/21194647/the-covid-19-pandemic-is-generating-tons-of-medical-waste (accessed on 12 February 2021).
- Dharmaraj, S.; Ashokkumar, V.; Hariharan, S.; Manibharathi, A.; Show, P.L.; Chong, C.T.; Ngamcharussrivichai, C. The COVID-19 pandemic face mask waste: A blooming threat to the marine environment. Chemosphere 2021, 272, 129601. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, B.N.; Anantharama, V. Repercussions of COVID-19 pandemic on municipal solid waste management: Challenges and opportunities. Sci. Total Environ. 2020, 743, 140693. [Google Scholar] [CrossRef] [PubMed]
- World Health Organiyation. Water, Sanitation, Hygiene, and Waste Management for SARS-CoV-2, the Virus that Causes COVID-19. Available online: https://www.who.int/publications/i/item/wter-sanitation-hygiene-and-waste-management-for-the-covid-19-virus-interim-guidance (accessed on 3 March 2021).
- European Parliament; Council of the European Union. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Off. J. Eur. Union 2008. Available online: http://data.europa.eu/eli/dir/2008/98/oj (accessed on 2 February 2021).
- European Commission. Guidance on Municipal Waste Data Collection. Unit E2–Environmental Statistics and Accounts; Sustainable Development; Eurostat: Luxembourg, 2017. [Google Scholar]
- Environmental Protection Agency. Advancing Sustainable Materials Management. 2019. Available online: https://www.epa.gov/sites/production/files/2019-11/documents/2017_facts_and_figures_fact_sheet_final.pdf (accessed on 3 March 2021).
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Ministry of the Environment of the Slovak Republic. Decree of the Ministry of the Environment of the Slovak Republic No. 365/2015 Coll. 2015. Available online: https://www.slov-lex.sk/pravne-predpisy/SK/ZZ/2015/365/20160101 (accessed on 1 March 2021).
- Ministry of the Environment of the Slovak Republic. Decree of the Ministry of the Environment of the Slovak Republic No 410/2012 Implementing Certain Provisions of the Clean Air Act, As Amended; Ministry of the Environment of the Slovak Republic: Bratislava, Slovakia, 2012. [Google Scholar]
- Slovak Technical Standard. STN EN ISO 18135. Solid Biofuels-Sampling (ISO 18135:2017); Slovak Technical Standard: Bratislava, Slovakia, 2017. [Google Scholar]
- European Committee for Standardization. EN 15407:2011. Solid Recovered Fuels. Methods for the Determination of Carbon (C), Hydrogen (H) and Nitrogen (N) Content; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- European Committee for Standardization. EN 15400:2011. Solid Recovered Fuels. Determination of Fuel Calorific Value; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- European Committee for Standardization. EN 15414-3:2011. Solid Recovered Fuels. Determination of Moisture Content Using the Oven Dry Method. Moisture in General Analysis Sample; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- European Committee for Standardization. EN 15403:2011. Solid Recovered Fuels. Determination of Ash Content; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- European Committee for Standardization. EN 15440:2011. Solid Recovered Fuels. Methods for the Determination of Biomass Content; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- Slovak Technical Standard. STN EN 15309. Characterization of Waste and Soil-Determination of Elemental Composition by X-Ray Fluorescence; Slovak Technical Standard: Bratislava, Slovakia, 2011. [Google Scholar]
- European Parliament; Council of the European Union. Decree of Ministry of the Environment of the Slovak Republic no. 382/2018 Coll. about landfill and storage of waste mercury. Off. J. Eur. Union 2019. [Google Scholar]
- Slovak Technical Standard. STN EN 15527. Characterization of Waste. Determination of Polycyclic Aromatic Hydrocarbons (PAH) in Waste Using Gas Chromatography Mass Spectrometry (GC/MS); Slovak Technical Standard: Bratislava, Slovakia, 2009. [Google Scholar]
- Pastircakova, K. Determination of trace metal concentrations in ashes from various biomass materials. Energy Educ. Sci. Technol. 2004, 13, 97–104. [Google Scholar]
- Löser., C.; Ulbricht, H.; Hoffmann, P.; Seidel, H. Composting of Wood Containing Polycyclic Aromatic Hydrocarbons (PAHs). Compost Sci. Util. 2013, 7, 16–32. [Google Scholar] [CrossRef]
- Glushkov, D.; Paushkina, K.; Shabardin, D.; Strizhak, P.; Gutareva, N. Municipal solid waste recycling by burning it as part of composite fuel with energy generation. J. Environ. Manag. 2019, 231, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Wang, Y.; Wang, J.; Zhang, Y.; Pan, W.-P. Arsenic release and transformation in co-combustion of biomass and coal: Effect of mineral elements and volatile matter in biomass. Bioresour. Technol. 2020, 297, 122388. [Google Scholar] [CrossRef] [PubMed]
- New York State Energy Research and Development Authority. Elemental Analysis of Wood Fuels, Northeast States for Coordinated Air Use Management (NESCAUM); New York State Energy Research and Development Authority: Boston, MA, USA, 2013.
- Kliucininkas, L.; Krugly, E.; Stasiulaitiene, I.; Radziuniene, I.; Prasauskas, T.; Jonusas, A.; Kauneliene, V.; Martuzevicius, D. Indooreoutdoor levels of size segregated particulate matter and mono/polycyclic aromatic hydrocarbons among urban areas using solid fuels for heating. Atmos. Environ. 2014, 97, 83–93. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B.; Wheeler, A.J.; Héroux, M.È.; Wallace, L.A. Source apportionment of indoor and outdoor volatile organic compounds at homes in Edmonton, Canada. Build. Environ. 2015, 90, 114–124. [Google Scholar] [CrossRef]
- Stasiulaitiene, I.; Krugly, E.; Prasauskas, T.; Ciuzas, D.; Kliucininkas, L.; Kauneliene, V.; Martuzevicius, D. Infiltration of outdoor combustion-generated pollutants to indoors due to various ventilation regimes: A case of a single-family energy efficient building. Build. Environ. 2019, 157, 235–241. [Google Scholar] [CrossRef]


| Year of Manufacture | Fuel | Minimum Power (kW) | Nominal Power (kW) | Heat Output in Fuel at Rated Heat Output (kW) | Boiler Class * |
|---|---|---|---|---|---|
| 2011 | Wood chips, pellets | 24.9 | 83.0 | 90.1 | 3 |
| Parameters | Measuring Range |
|---|---|
| O2 | 0–21 Vol.% O2 |
| NO | 0–3000 ppm NO |
| 0–6160 mg/m3 NO | |
| NO2 | 0–500 ppm NO2 |
| 0–1030 mg/m3 NO2 | |
| NOx (NO+ NO2) | 0–3500 ppm NOx |
| 0–7190 mg/m3 NOx | |
| SO2 | 0–5000 ppm SO2 |
| 0–14,650 mg/m3 SO2 | |
| CO2 | 0–25 Vol.% CO2 |
| CO | 0–10,000 ppm CO |
| 0–12,560 mg/m3 CO | |
| Absolute pressure | 40–1200 hPa |
| Flue gas moisture | 2–31% H2O |
| 15–70 °C td | |
| Temperature (flue gas temperature) | –40 to +1200 °C |
| Flow speed | 5–40 m/s |
| 0–50% hPa |
| Element | Wood Chips | MSW | 50/50 | 70/30 | 75/25 | 80/20 |
|---|---|---|---|---|---|---|
| C (%) | 53.55 | 64.27 | 53.82 | 55.31 | 56.74 | 54.21 |
| H (%) | 6.4 | 9.0 | 7.05 | 7.17 | 7.23 | 6.9 |
| N (%) | 0.28 | 0.49 | 0.48 | 0.46 | 0.42 | - |
| O (%) | 39.64 | 26.214 | 38.54 | 36.87 | 35.37 | 38.42 |
| S (%) | 0.13 | 0.026 | 0.11 | 0.19 | 0.24 | - |
| Element | Wood Chips | MSW | 50/50 | 70/30 | 75/25 | 80/20 |
|---|---|---|---|---|---|---|
| Calorific value, MJ/kg | 22.53 | 27.09 | 15.82 | 22.02 | 20.67 | 19.32 |
| Humidity, % | 27.46 | 6.59 | 9.27 | 6.96 | 6.63 | 6.30 |
| Ash content, % | 0.83 | 7.47 | 4.54 | 4.02 | 3.65 | 3.28 |
| Biomass content, % | 93.28 | 21.82 | 72.33 | 84.25 | 83.16 | 82.07 |
| Volatiles, % | 80.87 | 86.6 | 79.2 | 82.63 | 78.3 | 73.97 |
| Emission factor tCO2/TJ | 88.66 | 86.99 | 124.7 | 91.34 | 100.6 | 82.64 |
| Density, kg/m3 | 222.1 | 80.0 | 104.8 | 107.8 | 103.8 | 99.8 |
| Element | Wood Chips | MSW | 50/50 | 70/30 | 75/25 | 80/20 |
|---|---|---|---|---|---|---|
| C (%) | 5.31 | - | 7.53 | 4.25 | 3.91 | 4.91 |
| H (%) | 0.47 | - | 0.87 | 0.42 | 0.3 | 0.45 |
| N (%) | 0.37 | - | 0.38 | 0.36 | 0.36 | 0.37 |
| O (%) | - | - | - | - | - | - |
| S (%) | 0.08 | - | 0.05 | 0.07 | 0.09 | 0.07 |
| Element | Wood Chips | MSW | 50/50 | 70/30 | 75/25 | 80/20 |
|---|---|---|---|---|---|---|
| Cd + Tl | 0.1 | 7.92 | 3.98 | 2.44 | 2.09 | 1.65 |
| Hg | 0.6904 | 0.265 | 0.512 | 0.6836 | 0.7135 | 0.5947 |
| Sb + As + Pb + Cr + Co + Cu + Mn + Ni +V | 41.9856 | 111.03 | 48.697 | 67.63 | 64.53 | 61.48 |
| Zn | 3.49 | 135 | 114.4 | 42.943 | 36.3675 | 29.792 |
| Element | Wood Chips | MSW | 50/50 | 70/30 | 75/25 | 80/20 |
|---|---|---|---|---|---|---|
| Cd + Tl | 0.00415 | - | 2.437 | 1.801302 | 1.301 | 1.34608 |
| Hg | 0.00083 | - | - | 0.0005 | 0.001 | 0.001034 |
| Sb + As + Pb + Cr + Co + Cu + Mn + Ni +V | 52.79846 | - | 20.87539 | 57.868725 | 110.18024 | 111.896596 |
| Zn | 1.24417 | - | 110.856 | 21.23812 | 41.318 | 43.32475 |
| Element | Wood Chips | MSW | 50/50 | 75/25 | ||||
|---|---|---|---|---|---|---|---|---|
| Ash, mg/kg | Carryover, mg/m3 | Ash, mg/kg | Carryover, mg/m3 | Ash, mg/kg | Carryover, mg/m3 | Ash, mg/kg | Carryover, mg/m3 | |
| acenaften | - | - | - | - | - | - | - | 0.075 |
| acenaftylen | - | - | - | - | - | - | - | - |
| benzo(a)antracen | - | - | - | - | - | - | 0.008213 | 0.0098 |
| benzo(a)pyren | - | - | - | - | - | - | 0.002701 | - |
| benzo(b)fluoranten | - | - | - | - | - | - | 0.006899 | 0.0501 |
| benzo(ghi)perylen | - | - | - | - | - | - | 0.002081 | - |
| benzo(k)fluoranten | - | - | - | - | - | - | 0.002665 | - |
| fenantren | 0.000564 | - | - | 0.064 | 0.00876 | 0.0652 | 0.022375 | 0.1142 |
| fluoranten | - | - | - | - | - | - | 0.023944 | 0.1075 |
| fluoren | - | - | - | - | - | - | 0.002373 | 0.0676 |
| chryzen | - | - | - | - | - | - | 0.009162 | 0.0938 |
| indeno (123cd)pyren | - | - | - | - | - | - | 0.0023 | - |
| naftalen | 0.000432 | - | - | - | 0.03423 | 0.0517 | 0.017081 | 0.1221 |
| pyren | - | - | - | - | 0.00390 | 0.0460 | 0.017958 | 0.0804 |
| antracen | - | - | - | - | - | - | 0.003066 | 0.0479 |
| dibenzo(ah)antracen | - | - | - | - | - | - | - | - |
| Sum PAU | 0.000996 | - | - | 0.064 | 0.04689 | 0.1631 | 0.120818 | 0.7688 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimar, M.; Kulikova, O.; Kulikov, A.; Fedak, M. Energy Treatment of Solid Municipal Waste in Combination with Biomass by Decentralized Method with the Respect to the Negative Effects on the Environment. Sustainability 2021, 13, 4405. https://doi.org/10.3390/su13084405
Rimar M, Kulikova O, Kulikov A, Fedak M. Energy Treatment of Solid Municipal Waste in Combination with Biomass by Decentralized Method with the Respect to the Negative Effects on the Environment. Sustainability. 2021; 13(8):4405. https://doi.org/10.3390/su13084405
Chicago/Turabian StyleRimar, Miroslav, Olha Kulikova, Andrii Kulikov, and Marcel Fedak. 2021. "Energy Treatment of Solid Municipal Waste in Combination with Biomass by Decentralized Method with the Respect to the Negative Effects on the Environment" Sustainability 13, no. 8: 4405. https://doi.org/10.3390/su13084405






