Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid Extraction Conditions
2.3. Analyses
2.4. Statistics
3. Results and Discussion
3.1. Optimum Conditions for Lipid Extraction
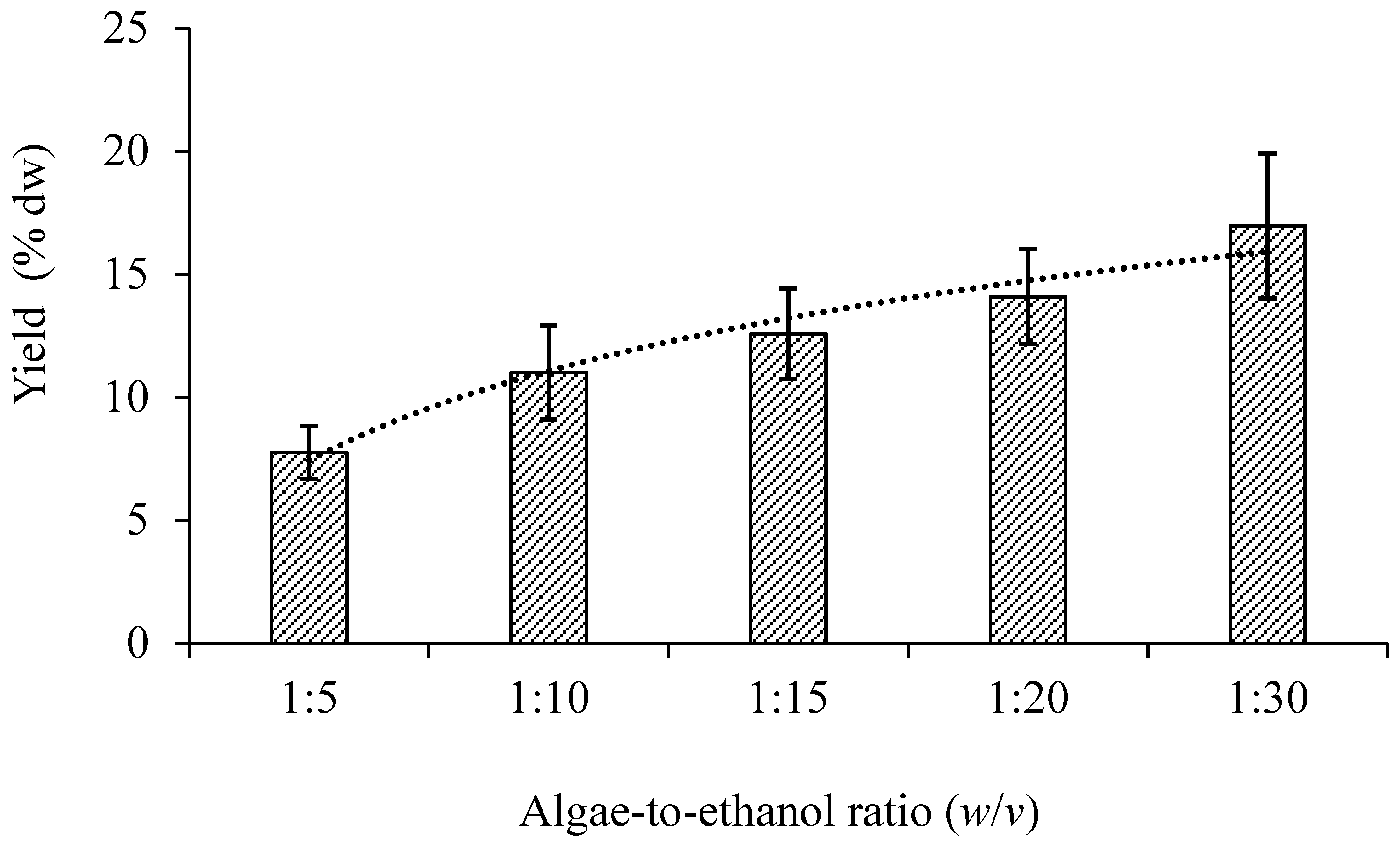
3.1.1. Sample-to-Solvent Ratio
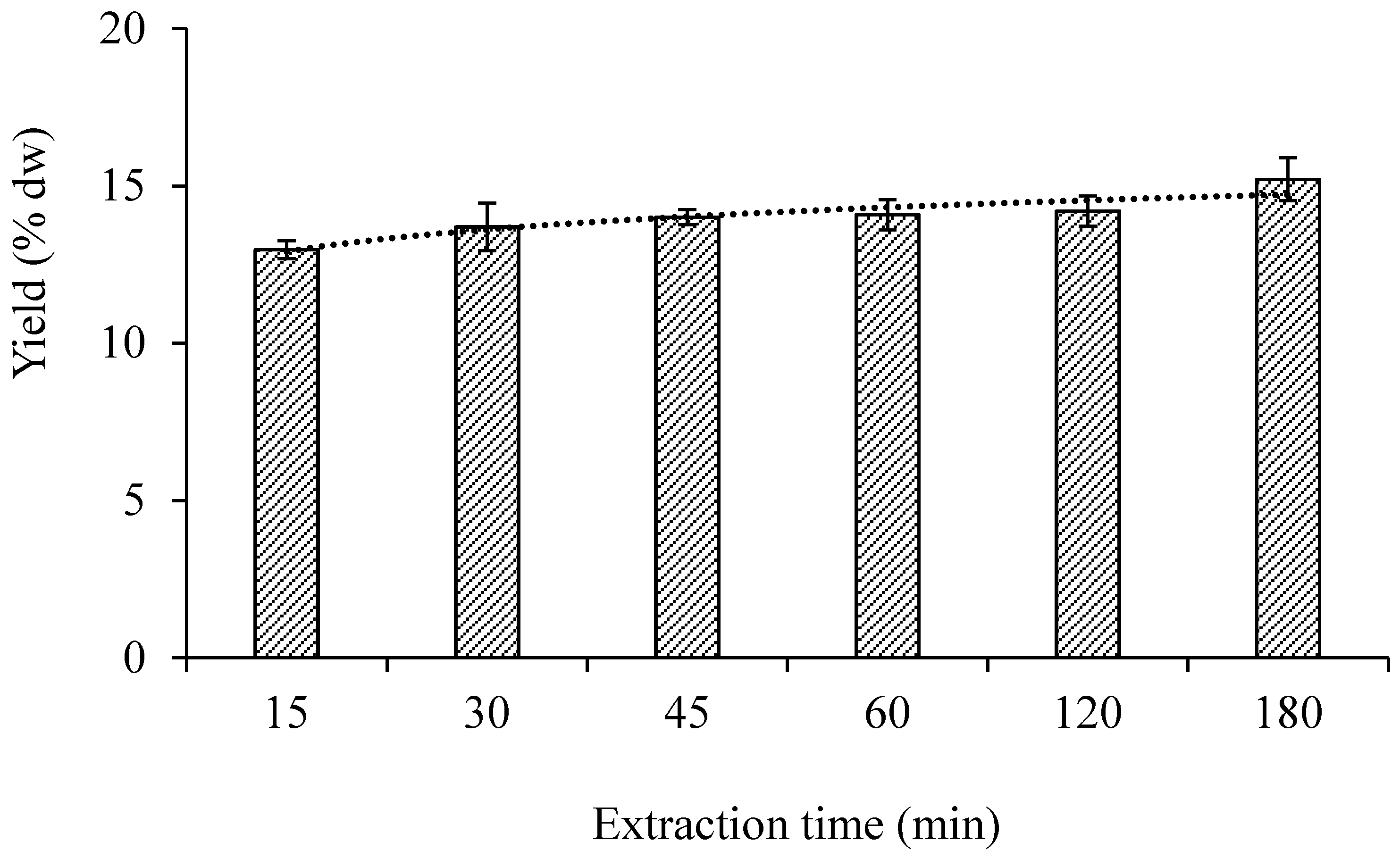
3.1.2. Extraction Time
3.1.3. Repetition Number of Extractions
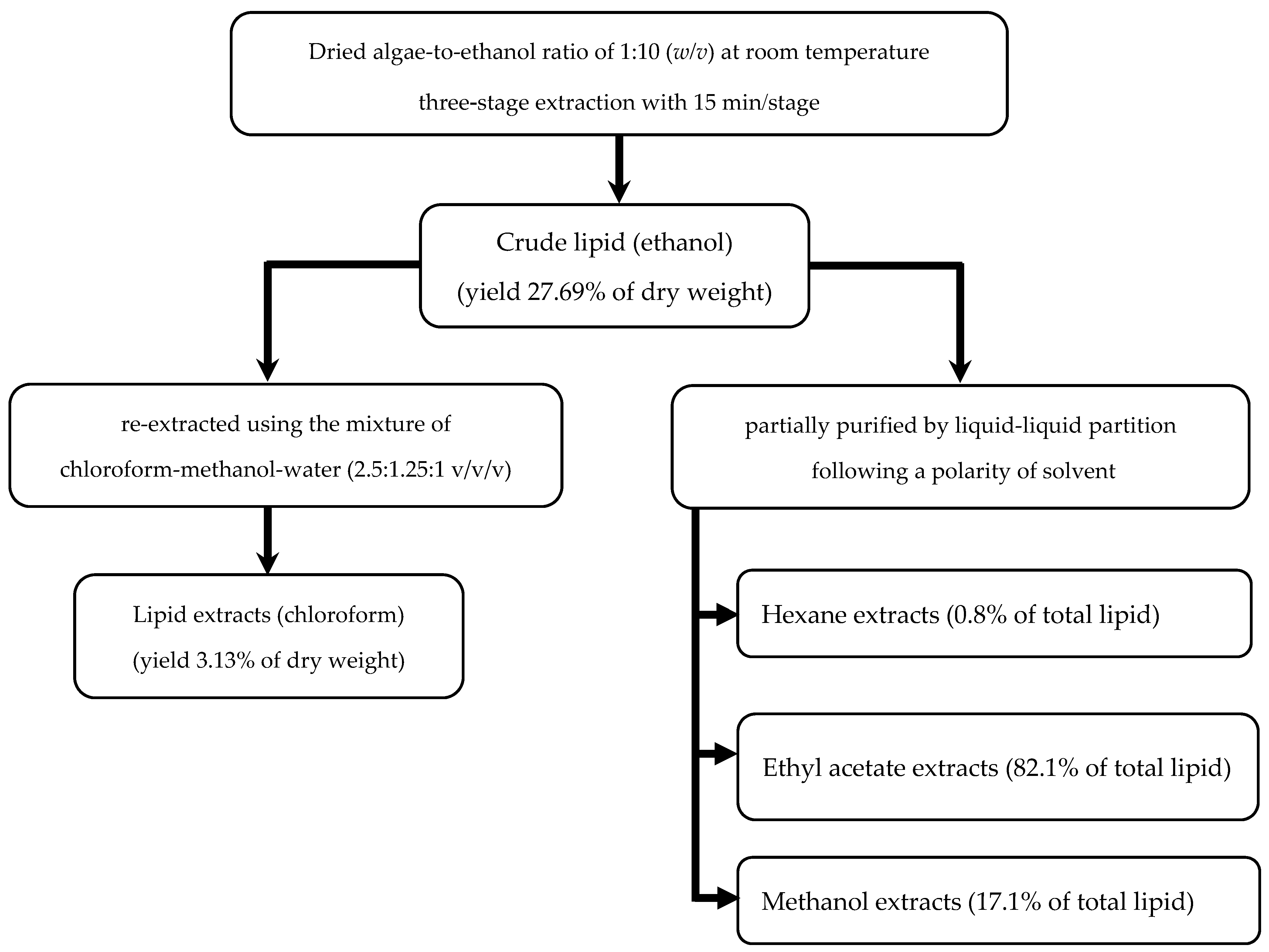
3.2. Compositions of Lipid Extracts
3.3. Antioxidant Properties and Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ratana-arporn, P.; Chirapart, A. Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulate. Nat. Sci. 2006, 40, 75–83. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, M.N.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ueng, J.P.; Tsai, G.J. Proximate composition, total phenolic content, and antioxidant activity of seagrape (Caulerpa lentillifera). J. Food Sci. 2011, 76, C950–C958. [Google Scholar] [CrossRef]
- Sarini, A.W.; Nor’Aishah, H.; Mohd Zaini, N. Determination of antioxidant activity for seven types of macroalgae. In Proceedings of the 5th International Conference on Food Engineering and Biotechnology, IPCBEE, Penang, Malaysia, 12–14 March 2014; Volume 65, pp. 51–55. [Google Scholar]
- Sharma, B.R.; Rhyu, D.Y. Anti-diabetic effects of Caulerpa lentillifera: Stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes. Asian Pac. J. Trop. Biomed. 2014, 4, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.R.; Kim, H.J.; Rhyu, D.Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 2015, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sabirin, F.; Kazi, J.A.; Ibrahim, I.; Rashit, M.M.A. Screening of seaweeds potential against oral infections. J. Appl. Sci. Res. 2015, 11, 1–6. [Google Scholar]
- West, E.J.; West, R.J. Growth and survival of the invasive alga, Caulerpa taxifolia, in different salinities and temperatures: Implications for coastal lake management. Hydrobiologia 2007, 577, 87–94. [Google Scholar] [CrossRef]
- Ukabi, S.; Dubinsky, Z.; Steinberger, Y.; Israel, A. Temperature and irradiance effects on growth and photosynthesis of Caulerpa (Chlorophyta) species from the eastern Mediterranean. Aquat. Bot. 2013, 104, 106–110. [Google Scholar] [CrossRef]
- Gao, X.; Choi, H.G.; Park, S.K.; Sun, Z.M.; Nam, K.W. Assessment of optimal growth conditions for cultivation of the edible Caulerpa okamurae (Caulerpales, Chlorophyta) from Korea. J Appl. Phycol. 2019, 31, 1855–1862. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Srinorasing, T.; Chirasuwan, N.; Tamtin, M.; Bunnag, B. The potential of polysaccharide extracts from Caulerpa lentillifera waste. Int. J. Biol. Macromol. 2020, 161, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiancui, L.; Rongli, N.; Xiao, F.; Lijun, H.; Lixin, Z. Macroalgae as a source of alpha-glucosidase inhibitors. Chin. J. Oceanol. Limnol. 2005, 23, 354–356. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.M.; Nguyen, T.N.; Nguyen, X.H.; Huynh, N.N.T.; Min, S.B. Screening of α-glucosidase inhibitory activity of Vietnamese medicinal plants: Isolation of active principles from Oroxylum indicum. Nat. Prod. Sci. 2012, 18, 47–51. [Google Scholar]
- Telagari, M.; Hullatti, K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantumcaudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [PubMed] [Green Version]
- Yen, F.S.; Wei, J.C.C.; Lin, M.C.; Hsu, C.C.; Hwu, C.M. Long-term outcomes of adding alpha-glucosidase inhibitors in insulin-treated patients with type 2 diabetes. BMC Endocr. Disord. 2021, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Singh, B.; Arora, R.; Arora, S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complement. Altern. Med. 2019, 19, 74. [Google Scholar] [CrossRef] [Green Version]
- Sato, N.; Murata, N. Membrane lipid. Meth. Enzymol. 1988, 167, 251–259. [Google Scholar]
- Lapage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction of purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Othman, R.; Amin, N.A.; Sani, M.S.A.; Fadzillah, N.A.; Jamaludin, M.A. Carotenoid and chlorophyll profiles in five species of Malaysian seaweed as potential halal active pharmaceutical ingredient (API). Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 1610–1616. [Google Scholar]
- Wu, X.J.; Hansen, C. Antioxidant capacity, phenolic content, and polysaccharide content of Lentinus edodes grown in whey permeate-based submerged culture. J. Food Sci. 2008, 73, M1–M8. [Google Scholar] [CrossRef] [PubMed]
- Pallab, K.; Tapan, K.B.; Tapas, K.P.; Ramen, K. Estimation of total flavonoids content (TFC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivum linn. J. Drug Deliv. Ther. 2013, 3, 33–37. [Google Scholar]
- Li, X.; Zhou, A.; Han, Y. Anti-oxidation and anti-microorganism activities of purification polysaccharide from Lygodium japonicum in vitro. Carbohydr. Polym. 2006, 66, 34–42. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.A.; Zaidul, I.S.M.; Ghafoor, K.; Sahena, F.; Hakim, M.A.; Rafii, M.Y.; Abir, H.M.; Bostanudin, M.F.; Perumal, V.; Khatib, A. In vitro antioxidant and, α-glucosidase inhibitory activities and comprehensive metabolite profiling of methanol extract and its fractions from Clinacanthus nutans. BMC Complement. Altern. Med. 2017, 17, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, B.; Zhang, Q.; Qu, Y.; Xu, H.; Li, G. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemose. J. Appl. Phycol. 2012, 24, 1527–1536. [Google Scholar] [CrossRef]
- Chirasuwan, N.; Chaiklahan, R.; Kittakoop, P.; Chanasattru, W.; Ruengjitchatchawalya, M.; Tanticharoen, M.; Bunnag, B. Anti HSV–1 activity of sulphoquinovosyl diacylglycerol isolated from Spirulina platensis. Sci. Asia 2009, 35, 137–141. [Google Scholar] [CrossRef]
- Blažina, M.; Iveša, L.; Najdek, M. Caulerpa racemosa: Adaptive varieties studied by fatty acid composition (Northern Adriatic Sea, Vrsar, Croatia). Eur. J. Phycol. 2009, 44, 183–189. [Google Scholar] [CrossRef]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Osuna-Ruiz, I.; López-Saiz, C.M.; Burgos-Hernández, A.; Velázquez, C.; Nieves-Soto, M.; Hurtado-Oliva, M.A. Antioxidant, antimutagenic and antiproliferative activities in selected seaweed species from Sinaloa, Mexico. Pharm. Biol. 2016, 54, 2196–2210. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, H.J.; Kim, M.S.; Park, C.M.; Rhyu, D.Y. Caulerpa okmurae extract inhibits adipogenesis in 3T3–L1 adipocytes and prevents high–fat diet–induced obesity in C57BL/6 mice. Nutr. Res. 2017, 47, 44–52. [Google Scholar] [CrossRef]
- Rahman, S.M.; Neaz, S.; Alam, M.M.; Nur, J. Hypolipidemic activity of ethanolic extract of Caulerpa recemosa. BIRDEM Med. J. 2019, 9, 197–201. [Google Scholar] [CrossRef]

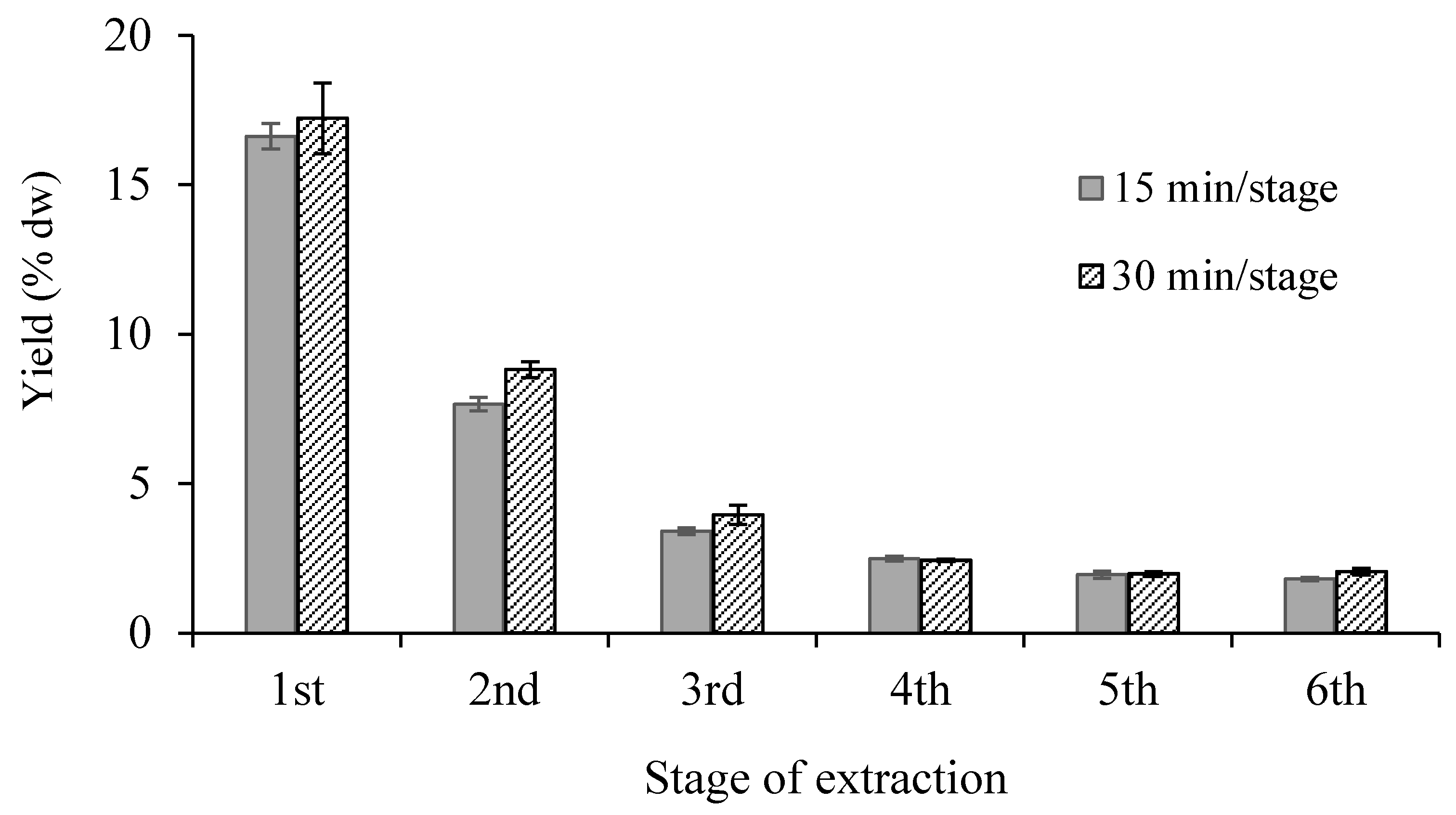
| Number of Stage | Total Time of Extraction (min) | Total Volume of Ethanol (mL) * | Total Yield (% dw) | |||
|---|---|---|---|---|---|---|
| 15 | 30 | 15 | 30 | 15 | 30 | |
| 1 | 15 | 30 | 150 | 150 | 16.62 | 17.23 |
| 2 | 30 | 60 | 300 | 300 | 24.28 | 26.04 |
| 3 | 45 | 90 | 450 | 450 | 27.69 | 30.00 |
| 4 | 60 | 600 | 30.18 | |||
| Compositions | Value |
|---|---|
| Chlorophyll a (µg/mg of extract) | 1.77 ± 0.25 |
| Chlorophyll b (µg/mg of extract) | 0.91 ± 0.09 |
| Carotenoids (µg/mg of extract) | 0.70 ± 0.09 |
| Total fatty acid (TFA; % weight of lipid extract) | 58.60 ± 4.95 |
| Fatty acid compositions (% mol.) | |
| Myristic acid (14:0) | 4.89 ± 0.36 |
| Palmitic acid (16:0) | 74.48 ± 3.24 |
| Palmitoleic acid (16:1n7) | 6.82 ± 0.38 |
| Stearic acid (18:0) | 2.50 ± 0.28 |
| Vaccenic acid (18:1n7) | 3.03 ± 0.28 |
| Oleic acid (18:1n9) | 2.92 ± 0.39 |
| Linoleic acid (18:2) | 2.69 ± 0.38 |
| Linoleic acid (18:3) | 0.93 ± 0.13 |
| Arachidonic acid (20:4) | 0.95 ± 0.09 |
| Eicosapentaenoic acid (20:5) | 1.01 ± 0.34 |
| Saturated fatty acids (SFA) | 82.56 ± 2.37 |
| Monounsaturated fatty acids (MFA) | 12.68 ± 0.44 |
| Polyunsaturated fatty acids (PFA) | 3.09 ± 0.69 |
| Characteristic | Crude Lipid | Purified Lipid |
|---|---|---|
| Total phenolic (mg GAE/g sample) | 2.07 ± 0.34 | 17.46 ± 2.24 |
| Total flavonoids (mg QE/g sample) | 5.40 ± 0.76 | 55.48 ± 7.78 |
| IC50 ABTS (mg/mL) | 1.67 ± 0.09 | 0.21 ± 0.06 |
| IC50 DPPH (mg/mL) | 3.55 ± 0.53 | 0.43 ± 0.11 |
| IC50 α-glucosidase (mg/mL) | 8.97 ± 0.19 | Not determined |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinorasing, T.; Chirasuwan, N.; Bunnag, B.; Chaiklahan, R. Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy. Sustainability 2021, 13, 4491. https://doi.org/10.3390/su13084491
Srinorasing T, Chirasuwan N, Bunnag B, Chaiklahan R. Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy. Sustainability. 2021; 13(8):4491. https://doi.org/10.3390/su13084491
Chicago/Turabian StyleSrinorasing, Thanyarat, Nattayaporn Chirasuwan, Boosya Bunnag, and Ratana Chaiklahan. 2021. "Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy" Sustainability 13, no. 8: 4491. https://doi.org/10.3390/su13084491





