Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of the Essential Oil
2.2. Gas Chromatography-Mass Spectrometry Analysis
2.3. Antioxidant Activity
2.4. Antibacterial Activity
2.4.1. Preparation of the Strains
2.4.2. Disc Diffusion Method
2.4.3. Determination of MIC and MBC with the Broth Dilution Method
2.4.4. Determination of the Percentage of Sublethal-Injured Salmonella
2.4.5. In vitro Effect of TV-EO on the Growth of Salmonella
2.5. Predictive Model
2.6. In situ Antimicrobial Activity of TV-EO in Poultry Minced Meat
2.7. Test on Poultry Minced Meat: Sensory Analysis
2.8. Statistical Analysis
3. Results
3.1. Essential Oil Content and GC-MC Analysis
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
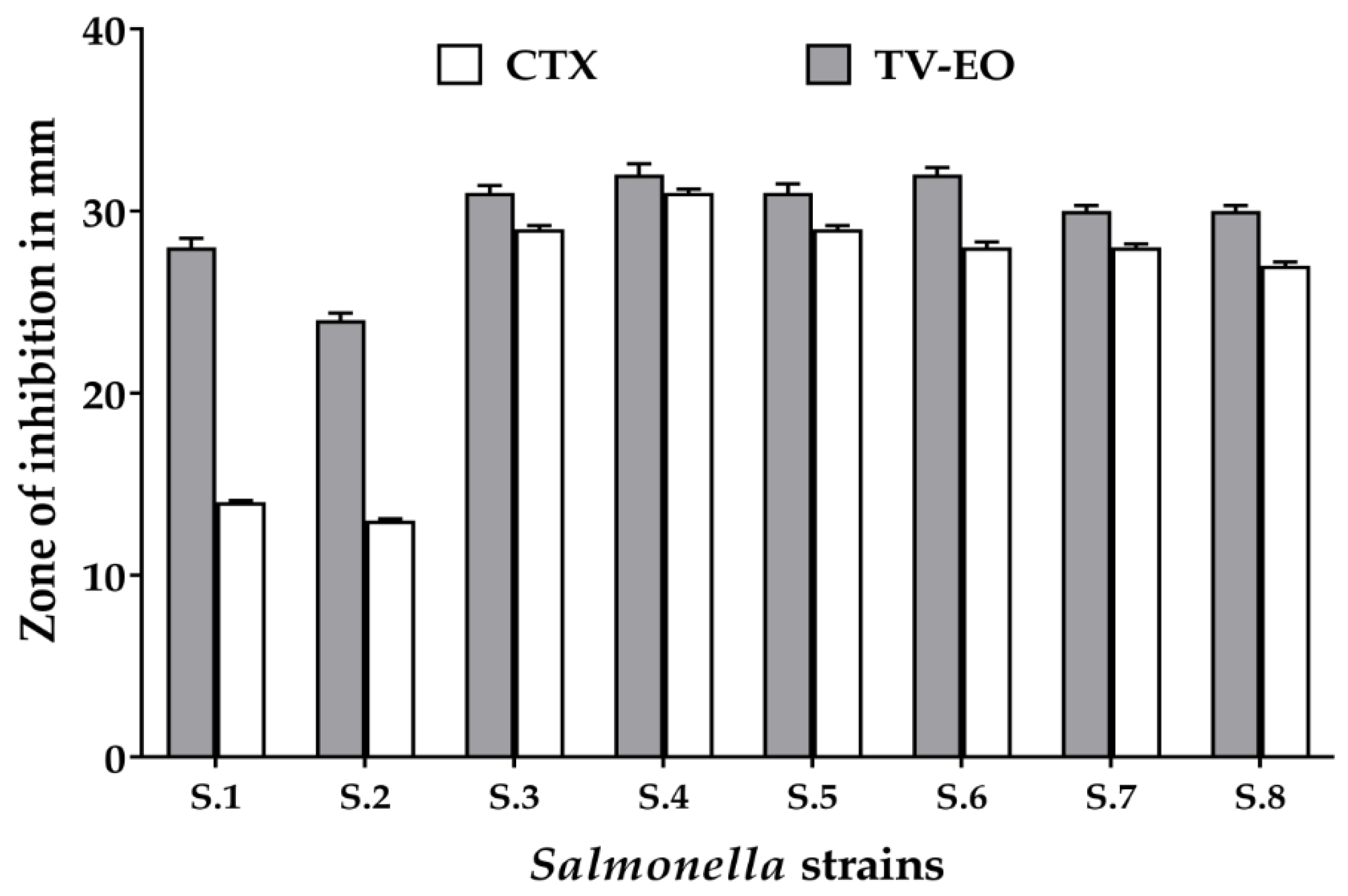
3.3.1. Disc Diffusion Method, MIC, MBC, and Percentage of Sublethal-Injured Cells
3.3.2. In Vitro Effect of TV-EO on the Growth of Salmonella
3.4. Predictive Model
3.5. Antimicrobial Activity in Poultry Minced Meat
3.6. Sensorial Analysis
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassman, K.G.; Grassini, P. A global perspective on sustainable intensification research. Nat. Sustain. 2020, 3, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Del Nobile, M.A.; Lucera, A.; Costa, C.; Conte, A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef] [Green Version]
- Trabelsi, N.; Nalbone, L.; Marotta, S.M.; Taamali, A.; Abaza, L.; Giarratana, F. Effectiveness of five flavored Tunisian olive oils on Anisakis larvae type 1: Application of cinnamon and rosemary oil in industrial anchovy marinating process. J. Sci. Food Agric. 2019, 99, 4808–4815. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, F.; Muscolino, D.; Ragonese, C.; Beninati, C.; Sciarrone, D.; Ziino, G.; Mondello, L.; Giuffrida, A.; Panebianco, A. Antimicrobial activity of combined thyme and rosemary essential oils against Listeria monocytogens in Italian mortadella packaged in modified atmosphere: Thyme & Rosemary EOs vs L. monocytogenes. J. Essent. Oil Res. 2016, 28, 467–474. [Google Scholar] [CrossRef]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E. Production of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications, 3rd ed.; Husnu Can Baser, K., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 83–90. [Google Scholar]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Elsharkawy, E.R.; Ed-Dra, A.; Alghanem, S.; Abdallah, E.M. Comparative studies of chemical compostion, antimicrobial and antioxidant activity of essential oil of some species from genus Artemisia. J. Nat. Remedies 2018, 18, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Giarratana, F.; Muscolino, D.; Panebianco, F.; Patania, A.; Benianti, C.; Ziino, G.; Giuffrida, A. Activity of R (+) limonene against Anisakis larvae. Ital. J. Food Saf. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Ed-Dra, A.; Filali, F.R.; Lo Presti, V.; Zekkori, B.; Nalbone, L.; Bouymajane, A.; Trabelsi, N.; Lamberta, F.; Bentayeb, A.; Giuffrida, A.; et al. Chemical composition, antioxidant capacity and antibacterial action of five Moroccan essential oils against Listeria monocytogenes and different serotypes of Salmonella enterica. Microb. Pathog. 2020, 149, 104510. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, F.; Muscolino, D.; Beninati, C.; Giuffrida, A.; Panebianco, A. Activity of Thymus vulgaris essential oil against Anisakis larvae. Exp. Parasitol. 2014, 142, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Ozogul, Y.; Boğa, E.K.; Akyol, I.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Köşker, A.R. Antimicrobial activity of thyme essential oil nanoemulsions on spoilage bacteria of fish and food-borne pathogens. Food Biosci. 2020, 36, 100635. [Google Scholar] [CrossRef]
- Ziani, K.; Chang, Y.; McLandsborough, L.; McClements, D.J. Influence of surfactant charge on antimicrobial efficacy of surfactant-stabilized thyme oil nanoemulsions. J. Agric. Food Chem. 2011, 59, 6247–6255. [Google Scholar] [CrossRef]
- Moazeni, M.; Davari, A.; Shabanzadeh, S.; Akhtari, J.; Saeedi, M.; Mortyeza-Semnani, K.; Abastabar, M.; Nabili, M.; Moghadam, F.H.; Roohi, B.; et al. In vitro Antifungal Activity of Thymus vulgaris Essential Oil nanoemulsion. J. Herb. Med. 2021, 100452. [Google Scholar] [CrossRef]
- Thanissery, R.; Smith, D.P. Marinade with thyme and orange oils reduces Salmonella Enteritidis and Campylobacter coli on inoculated broiler breast fillets and whole wings. Poult. Sci. 2014, 93, 1258–1262. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Attia, R.A.; Mahmoud, A.E.; Farrag, H.M.M.; Makboul, R.; Mohamed, M.E.; Ibraheim, Z. Effect of myrrh and thyme on Trichinella spiralisenteral and parenteral phases with inducible nitric oxide expression in mice. Mem. Inst. Oswaldo Cruz 2015, 110, 1035–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Huo, X.; Zhou, X.; Zhao, D.; He, W.; Liu, S.; Liu, H.; Feng, T.; Wang, C. Acaricidal activity and synergistic effect of thyme oil constituents against carmine spider mite (Tetranychus Cinnabarinus (Boisduval)). Molecules 2017, 22, 1873. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Food Safety. 2017. Available online: http://www.who.int/en/news-room/fact-sheets/detail/food-safety (accessed on 16 June 2018).
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on monitoring and assessment of the public health risk of “Salmonella Typhimurium-like” strains. EFSA J. 2010, 8, 1826. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel). Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596. [Google Scholar] [CrossRef]
- Doyle, M.P.; Diez-Gonzalez, F.; Hill, C. Food Microbiology: Fundamentals and Frontiers, 5th ed.; John Wiley & Sons: Washington, DC, USA, 2020; pp. 225–262. [Google Scholar]
- Ed-Dra, A.; Karraouan, B.; El Allaoui, A.; Khayatti, M.; El Ossmani, H.; Rhazi Filali, F.; ElMdaghri, N.; Bouchrif, B. Antimicrobial resistance and genetic diversity of Salmonella Infantis isolated from foods and human samples in Morocco. J. Glob. Antimicrob. Resist. 2018, 14, 297–301. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Filali, F.R.; Karraouan, B.; El Allaoui, A.; Aboulkacem, A.; Bouchrif, B. Prevalence, molecular and antimicrobial resistance of Salmonella isolated from sausages in Meknes, Morocco. Microb. Pathog. 2017, 105, 340–345. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.; Ed-Dra, A.; Li, X.; Peng, X.; Xia, L.; Guo, Q.; Yao, G.; Yue, M. Prevalence and genomic investigation of Salmonella isolates recovered from animal food-chain in Xinjiang, China. Food Res. Int. 2021, 110198. [Google Scholar] [CrossRef]
- Murgia, M.; Bouchrif, B.; Timinouni, M.; Al-Qahtani, A.; Al-Ahdal, M.N.; Cappuccinelli, P.; Rubino, S.; Paglietti, B. Antibiotic resistance determinants and genetic analysis of Salmonella enterica isolated from food in Morocco. Int. J. Food Microbiol. 2015, 215, 31–39. [Google Scholar] [CrossRef]
- Mejlholm, O.; Dalgaard, P. Antimicrobial effect of essential oils on the seafood spoilage micro-organism Photobacterium phosphoreum in liquid media and fish products. Lett. Appl. Microbiol. 2002, 34, 27–31. [Google Scholar] [CrossRef]
- Giarratana, F.; Nalbone, L.; Ziino, G.; Giuffrida, A.; Panebianco, F. Characterization of the temperature fluctuation effect on shelf life of an octopus semi-preserved product. Ital. J. Food Saf. 2020, 9. [Google Scholar] [CrossRef] [Green Version]
- Fennane, M.; Ibn Tattou, M.; Mathez, J.; Ouyahya, A.; EL Oualidi, J. Flore Pratique du Maroc. Manuel de Détermination des Plantes Vasculaires, 1.; Série Botanique, N., Ed.; Série Bota; Travaux de l’Institut Scientifique: Rabat, Morocco, 1999. [Google Scholar]
- Ed-Dra, A.; Filali, F.R.; Khayi, S.; Oulghazi, S.; Bouchrif, B.; El Allaoui, A.; Ouhmidou, B.; Moumni, M. Antimicrobial resistance, virulence genes, and genetic diversity of Salmonella enterica isolated from sausages. Eur. J. Microbiol. Immunol. 2019, 9, 56–61. [Google Scholar] [CrossRef]
- Mazzarrino, G.; Paparella, A.; Chaves-López, C.; Faberi, A.; Sergi, M.; Sigismondi, C.; Compagnode, D.; Serio, A. Salmonella enterica and Listeria monocytogenes inactivation dynamics after treatment with selected essential oils. Food Control 2015, 50, 794–803. [Google Scholar] [CrossRef]
- Silva-Angulo, A.B.; Zanini, S.F.; Rosenthal, A.; Rodrigo, D.; Klein, G.; Martínez, A. Comparative study of the effects of citral on the growth and injury of Listeria innocua and Listeria monocytogenes cells. PLoS ONE 2015, 10, e0114026. [Google Scholar] [CrossRef] [Green Version]
- Giuffrida, A.; Giarratana, F.; Valenti, D.; Muscolino, D.; Parisi, R.; Parco, A.; Marotta, S.; Ziino, G.; Panebianco, A. A new approach to predict the fish fillet shelf-life in presence of natural preservative agents. Ital. J. Food Saf. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Gumudavelli, V.; Subbiah, J.; Thippareddi, H.; Velugoti, P.R.; Froning, G. Dynamic predictive model for growth of Salmonella enteritidis in egg yolk. J. Food Sci. 2007, 72, 254–262. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 6579-1. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization. ISO 6658. Sensory Analysis—Methodology—General Guidance; International Organization for Standardization; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization. ISO 8586. Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- International Organization for Standardization. ISO 8589. Sensory Analysis—General Guidance for the Design of Test Rooms; International Organization for Standardization; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Smaoui, S.; Hsouna, A.B.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Najah, S.; Bouaziz, M.; Mellouli, L. Bio-preservative effect of the essential oil of the endemic Mentha piperita used alone and in combination with BacTN635 in stored minced beef meat. Meat Sci. 2016, 117, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Albarracín, W.; Sánchez, I.C.; Grau, R.; Barat, J.M. Salt in food processing; usage and reduction: A review. Int. J. Food Sci. 2011, 46, 1329–1336. [Google Scholar] [CrossRef]
- Fadil, M.; Fikri-Benbrahim, K.; Rachiq, S.; Ihssane, B.; Lebrazi, S.; Chraibi, M.; Haloui, T.; Farah, A. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella Typhimurium: Optimization of antibacterial activity by mixture design methodology. Eur. J. Pharm. Biopharm. 2018, 126, 211–220. [Google Scholar] [CrossRef]
- Nezhadali, A.; Nabavi, M.; Rajabian, M.; Akbarpour, M.; Pourali, P.; Amini, F. Chemical variation of leaf essential oil at different stages of plant growth and in vitro antibacterial activity of Thymus vulgaris Lamiaceae, from Iran. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Atti-Santos, A.C.; Pansera, M.R.; Paroul, N.; Atti-Serafini, L.; Moyna, P. Seasonal variation of essential oil yield and composition of Thymus vulgaris L. (Lamiaceae) from South Brazil. J. Essent. Oil Res. 2004, 16, 294–295. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Aludatt, M.H.; Al-Tawaha, A.R.; Ereifej, K.; Almajwal, A.; Odat, N.; Al Khateeb, W. Seasonal variation in essential oil yield and composition from Thymus vulgaris L. during different growth stages in the south of Jordan. Nat. Prod. Res. 2012, 26, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.; McFeeters, R.; Setzer, W. Essential oil characterization of Thymus vulgaris from various geographical locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Imelouane, B.; Amhamdi, H.; Wathelet, J.P.; Ankit, M.; Khedid, K.; El Bachiri, A. Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from eastern Morocco. Int. J. Agric. Biol. 2009, 11, 205–208. [Google Scholar]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Khaneghah, A.M.; Gavahian, M.; Gomez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Kodal Coşkun, B.; Çalikoǧlu, E.; Karagöz Emiroǧlu, Z.; Candoǧan, K. Antioxidant active packaging with soy edible films and oregano or thyme essential oils for oxidative stability of ground beef patties. J. Food Qual. 2014, 37, 203–212. [Google Scholar] [CrossRef]
- Aljabeili, H.S.; Barakat, H.; Abdel-Rahman, H.A. Chemical Composition, Antibacterial and Antioxidant Activities of Thyme Essential Oil (Thymus vulgaris). Food Nutr. Sci. 2018, 09, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Guimarães, A.C.; Fronza, M.; Endringer, D.C.; Scherer, R. Seasonal variation affects the composition and antibacterial and antioxidant activities of Thymus vulgaris. Ind. Crops Prod. 2017, 95, 543–548. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef] [Green Version]
- Paulus, D.; Luchesi, L.A.; Busso, C.; Frata, M.T.; Oliveira, P.J.B. de Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils of Four Species of the Lamiaceae Family. Eur. J. Med. Plants 2020, 129–140. [Google Scholar] [CrossRef]
- Aeschbach, R.; Löliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef] [Green Version]
- Guo-Xiang, L.; Zai-Qun, L. Unusual antioxidant behavior of α- and λ-terpinene in protecting methyl linoleate, DNA, and erythrocyte. J. Agric. Food Chem. 2009, 57, 3943–3948. [Google Scholar] [CrossRef]
- Milde, J.; Elstner, E.F.; Graßmann, J. Synergistic inhibition of low-density lipoprotein oxidation by rutin, γ-terpinene, and ascorbic acid. Phytomedicine 2004, 11, 105–113. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Sato, K.; Krist, S.; Buchbauer, G. Antimicrobial effect of vapours of geraniol, (R)-(-)-linalool, terpineol, γ-terpinene and 1,8-cineole on airbone microbes using an airwasher. Flavour Fragr. J. 2007, 22, 435–437. [Google Scholar] [CrossRef]
- Bartkiene, E.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; Zavistanaviciute, P.; Starkute, V.; Zokaityte, E.; Lele, V.; Dauksiene, A.; Grashorn, M.; et al. Study of the antibiotic residues in poultry meat in some of the EU countries and selection of the best compositions of lactic acid bacteria and essential oils against Salmonella enterica. Poult. Sci. 2020, 99, 4065–4076. [Google Scholar] [CrossRef] [PubMed]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Marotta, S.M.; Giarratana, F.; Parco, A.; Neri, D.; Ziino, G.; Giuffrida, A.; Panebianco, A. Evaluation of the antibacterial activity of bergamot essential oils on different Listeria monocytogenes strains. Ital. J. Food Saf. 2016, 5. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. essential oils: Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Joković, N.; Jokanović, M.; Ivić, M.; Šojić, B.; Škaljac, S.; Stojanović, P.; Mihajilov-Krstev, T. Inhibition of Salmonella Enteritidis growth and storage stability in chicken meat treated with basil and rosemary essential oils alone or in combination. Food Control 2018, 90, 332–343. [Google Scholar] [CrossRef]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Jia, S.; Zhang, L.; Luo, Y. The effect of essential oils on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Int. J. Food Microbiol. 2018, 266, 52–59. [Google Scholar] [CrossRef]
- Boutoial, K.; García, V.; Rovira, S.; Ferrandini, E.; Abdelkhalek, O.; López, M.B. Effect of feeding goats with distilled and non-distilled thyme leaves (Thymus zygis subp. gracilis) on milk and cheese properties. J. Dairy Res. 2013, 80, 448. [Google Scholar] [CrossRef]
- Boskovic, M.; Djordjevic, J.; Ivanovic, J.; Janjic, J.; Zdravkovic, N.; Glisic, M.; Glamoclija, N.; Baltic, B.; Djordjevic, V.; Baltic, M. Inhibition of Salmonella by thyme essential oil and its effect on microbiological and sensory properties of minced pork meat packaged under vacuum and modified atmosphere. Int. J. Food Microbiol. 2017, 258, 58–67. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Van der Meeren, P.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef] [Green Version]




| Strains | ID Strains | Information | Serotype | Origin |
|---|---|---|---|---|
| Salmonella enterica | S1 | MG869132 * | Typhimurium | turkey sausages |
| S2 | KX355308 * | Typhimurium | turkey sausages | |
| S3 | KX355300 * | Kentucky | beef sausages | |
| S4 | KX355309 * | Corvallis | turkey sausages | |
| S5 | KX355310 * | Kentucky | turkey sausages | |
| S6 | KX355311 * | Saintpul | turkey sausages | |
| S7 | MG869130 * | Kentucky | turkey sausages | |
| S8 | KX355302 * | Kentucky | beef sausages |
| N° | Component | RIobs | RIlit | Formula | Method of Identification | Concentration (Peak Area %) |
|---|---|---|---|---|---|---|
| 1 | α-Pinene | 941 | 933 | C10H16 | RI, MSL | 0.659 |
| 2 | Camphene | 952 | 953 | C10H16 | RI, MSL | 0.318 |
| 3 | β-Pinene | 982 | 978 | C10H16 | RI, MSL | 0.060 |
| 4 | 3-Carene | 1012 | 1009 | C10H16 | RI, MSL | 2.180 |
| 5 | β-Phellandrene | 1032 | 1023 | C10H16 | RI, MSL | 0.080 |
| 6 | O-Cymene | 1024 | 1024 | C10H14 | RI, MSL | 0.073 |
| 7 | p-Cymene | 1025 | 1025 | C10H14 | RI, MSL | 15.660 |
| 8 | Limonene | 1029 | 1030 | C10H16 | RI, MSL | 0.528 |
| 9 | γ-Terpinene | 1058 | 1058 | C10H16 | RI, MSL | 13.308 |
| 10 | cis-Sabinene hydrate | 1066 | 1069 | C10H18 | RI, MSL | 0.489 |
| 11 | Borneol | 1169 | 1173 | C10H18O | RI, MSL | 0.721 |
| 12 | α-Terpineol | 1193 | 1195 | C10H18O | RI, MSL | 0.657 |
| 13 | Thymol | 1302 | 1293 | C10H14O | RI, MSL | 38.677 |
| 14 | Carvacrol | 1310 | 1300 | C10H14O | RI, MSL | 14.889 |
| 15 | β-Caryophyllene | 1426 | 1424 | C15H24 | RI, MSL | 2.728 |
| 16 | Caryophyllene oxide | 1575 | 1587 | C15H24O | RI, MSL | 0.538 |
| Total identified (%) | 91.565 | |||||
| Monoterpene hydrocarbons | 33.355 | |||||
| Sesquiterpene hydrocarbons | 2.728 | |||||
| Oxygenated monoterpenes | 54.944 | |||||
| Oxygenated sesquiterpenes | 0.538 | |||||
| Ascorbic Acid | Quercetin | BHT | TV-EO | |
|---|---|---|---|---|
| IC50 (mg/mL) | 0.031 ± 0.001 A | 0.012 ± 0.002 B | - | 0.29 ± 0.04 C |
| EC50 (mg/mL) | 0.095 ± 0.002 A | 0.019 ± 0.003 B | - | 0.74 ± 0.08 C |
| RC50 (mg/mL) | - | 0.052 ± 0.007 A | 0.063 ± 0.004 A | 0.59 ± 0.06 C |
| % Sublethal-Injured Cell | ID Strain | MIC | MBC | r | ||
|---|---|---|---|---|---|---|
| Control | TV-EO | |||||
| Salmonella | 3.72 ± 0.04 | 7.99 ± 0.08 | S1 | 0.5% | 1% | 2 |
| S2 | 0.5% | 1% | 2 | |||
| S3 | 0.5% | 1% | 2 | |||
| S4 | 0.5% | 1% | 2 | |||
| S5 | 0.5% | 1% | 2 | |||
| S6 | 0.5% | 1% | 2 | |||
| S7 | 0.5% | 1% | 2 | |||
| S8 | 0.5% | 1% | 2 | |||
| 20 °C | 10 °C | |||||||
|---|---|---|---|---|---|---|---|---|
| % TV-EO | 0% | 0.5% | 0.4% | 0.25% | 0% | 0.5% | 0.4% | 0.25% |
| ξ values | 1 | 0.4 | 0.55 | 0.7 | 1 | −2.5 | 0.85 | 0.95 |
| Q0 | 0.5 | 0.03 | 0.03 | 0.5 | 0.7 | 0.7 | 0.7 | 0.7 |
| Nmax | 8.6 | 8.6 | 8.6 | 8.6 | 10.6 | 10.6 | 10.6 | 10.6 |
| 20 °C | 10 °C | |||
|---|---|---|---|---|
| ξ Included | ξ Not Included | ξ Included | ξ Not Included | |
| 0.50% TV-EO | 0.4089 | 1.4310 | 0.0683 | 6.5311 |
| 0.40% TV-EO | 0.1615 | 1.1290 | 0.3421 | 0.6519 |
| 0.25% TV-EO | 0.2385 | 0.4418 | 0.6474 | 0.5521 |
| Bacteria | Time (Days) | Concentrations of TV-EO | ||||
|---|---|---|---|---|---|---|
| 0% (Control) | 0.125% | 0.25% | 0.5% | 1% | ||
| Salmonella | 0 | 3.150 ± 0.0065 A | 3.150 ± 0.0065 A | 3.151 ± 0.0065 A | 3.151 ± 0.0065 A | 3.151 ± 0.0065 A |
| 1 | 3.015 ± 0.100 A | 3.067 ± 0.0368 A | 2.933 ± 0.0429 A | 2.951 ± 0.0685 A | 0.92 ± 0.1651 C | |
| 3 | 3.075± 0.0362 A | 3.037± 0.0169 A | 2.841 ± 0.0883 A | 2.806± 0.0192 A | 0.000 ± 0.000 B | |
| 6 | 2.963± 0.0267 A | 2.923± 0.0439 A | 2.648 ± 0.0963 A | 2.612 ± 0.0450 A | 0.000 ± 0.000 B | |
| 9 | - | - | - | 0.500 ± 0.1707 B | 0.000 ± 0.000 B | |
| 13 | - | - | - | 0.000 ± 0.000 B | 0.000 ± 0.000 B | |
| Enterobacteriaceae | 0 | 4.276 ± 0.0032 D | 4.276 ± 0.0032 D | 4.276 ± 0.0032 D | 4.276 ± 0.0032 D | 4.276 ± 0.0032 D |
| 1 | 4.430 ± 0.0455 D | 4.576 ± 0.0154 D | 4.475 ± 0.0625 D | 3.782 ± 0.0708 D | 2.539 ± 0.3373 G | |
| 3 | 5.346± 0.1386 E | 4.979± 0.0354 D | 4.878 ± 0.0649 D | 4.396 ± 0.0123 D | 2.522 ± 0.1283 G | |
| 6 | 6.555± 0.1079 F | 6.374± 0.0977 F | 5.972 ± 0.1298 EF | 5.481 ± 0.0708 E | 2.50 ± 0.067 G | |
| 9 | - | - | - | 6.212 ± 0.0433 F | 2.00 ± 0.2814 G | |
| 13 | - | - | - | 6.539 ± 0.2003 F | 1.739 ± 0.445 G | |
| Parameters | Time (Days) | Concentrations of TV-EO | ||||
|---|---|---|---|---|---|---|
| 0% (Control) | 0.125% | 0.25% | 0.5% | 1% | ||
| Color | 0 | 10 ± 0.000 L | 10 ± 0.000 L | 10 ± 0.000 L | 10 ± 0.000 L | 10 ± 0.000 L |
| 1 | 8.25 ± 0.353 IJ | 9 ± 0.707 K | 9 ± 0.707 K | 9 ± 0.000 K | 9 ± 0.000 K | |
| 3 | 6.25 ± 0.353 DE | 7.5 ± 0.707 GH | 8 ± 000 HI | 8.75 ± 0.353 JK | 8.75 ± 0.353 JK | |
| 6 | 4.25 ± 0.353 A | 5.25 ± 0.353 BC | 5 ± 0.000 B | 8 ± 0.00 HI | 8.25 ± 0.353 IJ | |
| 9 | - | - | - | 6.75 ± 0.353 EF | 7.25 ± 0.353 FG | |
| 13 | - | - | - | 5.5 ± 0.00 BC | 5.75 ± 0.353 CD | |
| Flavor | 0 | 10 ± 0.000 J | 10 ± 0.000 J | 10 ± 0.000 J | 10 ± 0.000 J | 10 ± 0.000 J |
| 1 | 8 ± 0.00 F | 8.25 ± 0.353 FG | 8.5 ± 0.353 GH | 8.75 ± 0.000 HI | 9.75 ± 0.353 J | |
| 3 | 6.75 ± 0.353 D | 6.75 ± 0.353 D | 6.75 ± 0.353 D | 8 ± 0.000 F | 9 ± 0.000 I | |
| 6 | 4.25 ± 0.353 A | 5 ± 0.00 B | 5.25 ± 0.353 B | 7.25 ± 0.353 E | 8.25 ± 0.353 FG | |
| 9 | - | - | - | 6.75 ± 0.353 D | 7.25 ± 0.353 E | |
| 13 | - | - | - | 5.25 ± 0.353 B | 6 ± 0.000 C | |
| Taste | 0 | 10 ± 0.000 M | 10 ± 0.000 M | 10 ± 0.000 M | 10 ± 0.000 M | 10 ± 0.000 M |
| 1 | 8.017 ± 0.206 V | 8.326 ± 0.553 V | 9.052 ± 0.051 W | 8.706 ± 0.124 W | 9.543 ± 0.395 M | |
| 3 | 6.331 ± 0.332 E | 7.077 ± 0.329 Y | 7.128 ± 0.176 Y | 8.121 ± 0.164 V | 8.723 ± 0.254 W | |
| 6 | 4.664 ± 0.487 N | 5.864 ± 0.116 O | 5.3 ± 0.511 O | 7.293 ± 0.156 Y | 8.016 ± 0.134 V | |
| 9 | - | - | - | 6.864 ± 0.320 E | 6.96 ± 0.053 EY | |
| 13 | - | - | - | 5.178 ± 0.307 O | 5.657 ± 0.493 O | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ed-Dra, A.; Nalbone, L.; Filali, F.R.; Trabelsi, N.; El Majdoub, Y.O.; Bouchrif, B.; Giarratana, F.; Giuffrida, A. Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica. Sustainability 2021, 13, 4594. https://doi.org/10.3390/su13084594
Ed-Dra A, Nalbone L, Filali FR, Trabelsi N, El Majdoub YO, Bouchrif B, Giarratana F, Giuffrida A. Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica. Sustainability. 2021; 13(8):4594. https://doi.org/10.3390/su13084594
Chicago/Turabian StyleEd-Dra, Abdelaziz, Luca Nalbone, Fouzia Rhazi Filali, Najla Trabelsi, Yassine Oulad El Majdoub, Brahim Bouchrif, Filippo Giarratana, and Alessandro Giuffrida. 2021. "Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica" Sustainability 13, no. 8: 4594. https://doi.org/10.3390/su13084594
APA StyleEd-Dra, A., Nalbone, L., Filali, F. R., Trabelsi, N., El Majdoub, Y. O., Bouchrif, B., Giarratana, F., & Giuffrida, A. (2021). Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica. Sustainability, 13(8), 4594. https://doi.org/10.3390/su13084594









