Local Drivers Associated to Temporal Spectral Response of Chlorophyll-a in Mangrove Leaves
Abstract
:1. Introduction
2. Materials and Methods
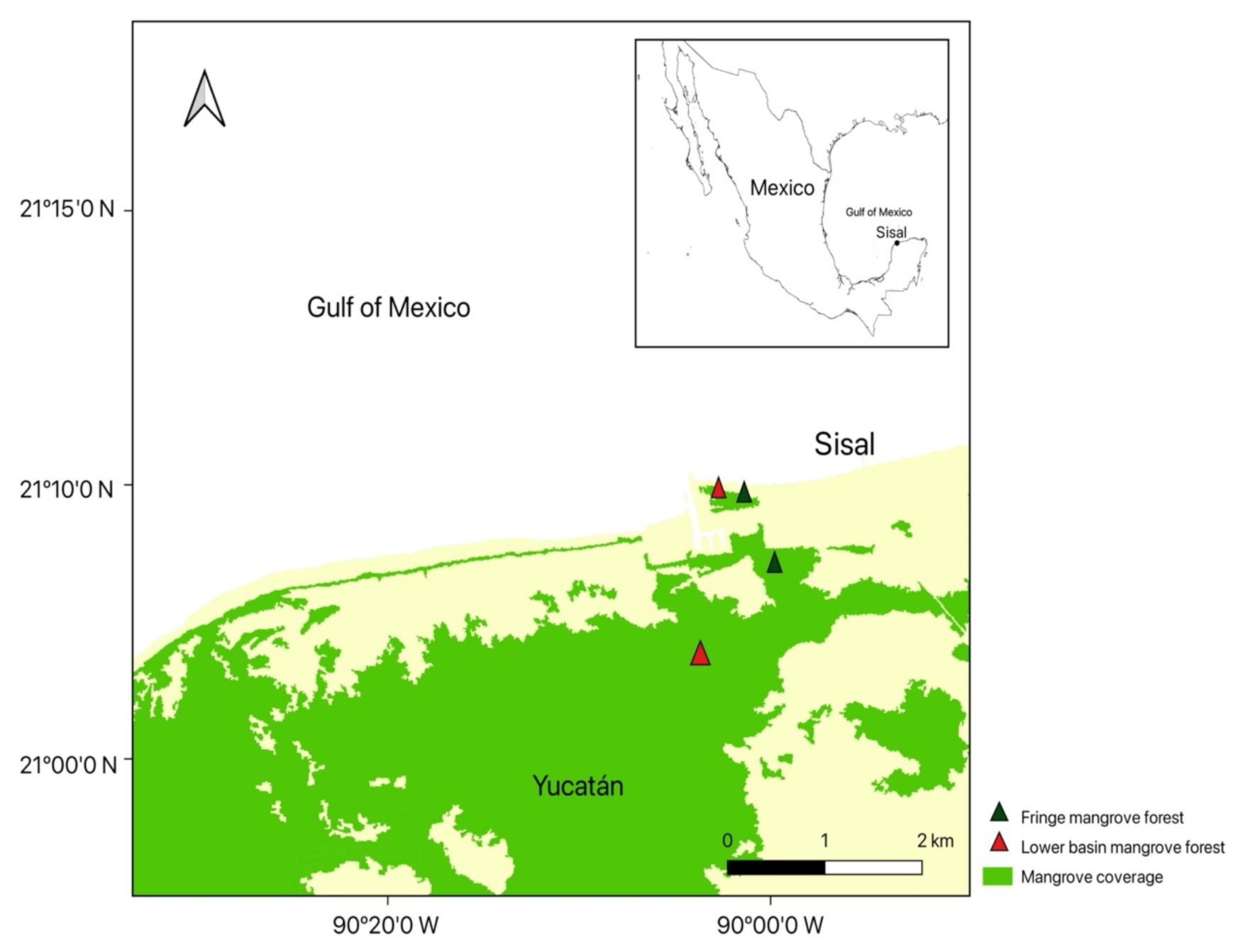
2.1. Study Area
Data collection
2.2. Data Analysis
3. Results
3.1. Fringe Mangrove Forest
3.2. Lower Basin Mangrove Forest
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, M. Valuing ecosystem services of Sundarbans Mangrove forest: Approach of choice experiment. Glob. Ecol. Conserv. 2020, 24, e01273. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.; Bouillon, S.; Rodney, S.; Björk, M.; Duarte, C.; Lovelock, C.; Schlesinger, W.; Silliman, B. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Donato, D.; Kauffman, J.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangrove among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Alongi, D. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, J.; Smoak, J.; Smith, T.; Sanders, C. Temporal variability of carbón and nutrient burial, sediment accretion and mass accumulation over the past century in a carbonate platform mangrove forest of the Florida Everglades. J. Geophys. Res. Biogeosci. 2014, 17. [Google Scholar] [CrossRef] [Green Version]
- Alongi, D.; Sasekumar, A.; Chong, V.; Pfitzner, J.; Trott, L.; Tirendi, F.; Dixon, P.; Brunskill, G. Sediment accumulation and organic material flux in a managed mangrove ecosystem: Estimates of land-ocean-atmosphere exchange in peninsular Malaysia. Mar. Geol. 2004, 208, 383–402. [Google Scholar] [CrossRef]
- Chen, S.; Chen, B.; Chen, G.; Ji, J.; Yu, W.; Liao, J.; Chen, G. Higher soil organic carbon sequestration potencial at a rehabilitated mangrove comprised of Aegiceras corniculatum compared to Kandelia obovata. Sci. Total Environ. 2021, 752, 142279. [Google Scholar] [CrossRef]
- Senger, D.; Saavedra, D.; Engel, S.; Schnurawa, M.; Moosdorf, N.; Gillis, L. Impacts of wetland dieback on carbon dynamics: A comparison between intact and degraded mangroves. Sci. Total Environ. 2021, 753, 141817. [Google Scholar] [CrossRef]
- Thom, B. Coastal landforms and geomorphic processes. In The Mangrove Ecosystem: Research Methods; Snedake, S., Snedaker, J., Eds.; UNESCO: París, France, 1984; pp. 3–17. [Google Scholar]
- Duke, N.; Ball, M.; Ellison, J. Factors influencing biodiversity and distributional gradients in mangrove. Glob. Ecol. Biogeogr. 1998, 7, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Twilley, R.; Gottfriedb, R.; Rivera-Monroy, V.; Zhanga, W.; Montaño-Armijosc, M.; Boderod, A. An approach and preliminary model of integrating ecological and economic constraints of environmental quality in the Guayas river estuary, Ecuador. Environ. Sci. Policy 1998, 4, 271–288. [Google Scholar] [CrossRef]
- Balke, T.; Fries, D. Geomorphic knowledge for mangrove restoration: A pan-tropical categorization. Earth Surf. Process. Landf. 2016, 2, 231–239. [Google Scholar] [CrossRef]
- Alongi, D.; Pfitzner, J.; Trott, L.; Tirendi, F.; Dixon, P.; Klumpp, D. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuar. Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Adame, M.; Teutli, C.; Santini, N.; Caamal, J.; Zaldívar-Jiménez, A.; Hernández, R.; Herrera-Silveira, J. Root biomass and production of mangroves surrounding a karstic oligotrophic coastal lagoon. Wetlands 2014, 34, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Moroyoqui-Rojo, L.; Flores-Verdugo, F.; Escobedo-Urias, D.; Herrera-Moreno, M. Nutrient dynamics in a closed system with mangrove seedlings and poecilid fishes. Bull. Mar. Sci. 2007, 3, 929–930. [Google Scholar]
- Croft, H.; Chen, J.; Froelich, N.; Chen, B.; Staebler, R. Seasonal controls of canopy chlorophyll content on forest carbon uptake: Implications for GPP modeling. J. Geophys. Res. Biosgeosci. 2015, 120, 1576–1586. [Google Scholar] [CrossRef] [Green Version]
- Comparini, D.; Masi, E.; Pandolfi, C.; Sabbatini, L.; Dolfi, M.; Morosi, S.; Mancuso, S. Stem electrical properties associated with water stress conditions in olive tree. Agric. Water Manag. 2020, 234, 12. [Google Scholar] [CrossRef]
- Flores-de-Santiago, F.; Kovacs, J.; Flores-Verdugo, F. Seasonal changes in leaf chlorophyll a content and morphology in a sub-tropical mangrove forest of the Mexican Pacific. Mar. Ecol. Prog. Ser. 2012, 444, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Flores-de-Santiago, F.; Kovacs, J.; Wang, J.; Flores-Verdugo, F.; Zhang, C.; González-Farías, F. Examining the influence of seasonality, condition, and species composition on mangrove leaf pigment contents and laboratory based spectroscopy data. Remote Sens. 2016, 8, 226. [Google Scholar] [CrossRef] [Green Version]
- Flores-Verdugo, F.; Zebadua-Penagos, F.; Flores-de-Santiago, F. Assessing the influence of artifically constructed channels in the growth of afforested Black mangrove (Avicennia germinans) within arid coastal region. J. Environ. Manag. 2015, 160, 113–120. [Google Scholar] [CrossRef]
- Gilman, E.; Ellison, J.; Duke, N.; Field, C. Threats to mangrove from climate change and adaptation options: a review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Ball, M. Salinity tolerance in the mangroves Aegiceras corniculatum and Avicennia marina. I. Water use in relation to growth, carbon partitioning and salt balance. Aust. J. Plant Physiol. 1988, 15, 447–464. [Google Scholar] [CrossRef]
- Biriukova, K.; Celesti, M.; Evdokimov, A.; Pacheco-Labrador, J.; Julitta, T.; Migliavacca, M.; Giardino, C.; Miglietta, F.; Colombo, R.; Panigada, C.; et al. Effects of varying solar view geometry and canopy structure on solar induced chlorophyll fluorescence and PRI. Int. J. Appl. Earth Obs. 2020, 89, 17. [Google Scholar] [CrossRef]
- Richardson, A.; Dulgan, S.; Berlyn, G. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Flores-de-Santiago, F.; Kovacs, J.; Flores-Verdugo, F. The influence of seasonality in estimating mangrove leaf chlorophyll-a content from hyperspectral data. Wetl. Ecol. Manag. 2013, 21, 193–207. [Google Scholar] [CrossRef]
- Wollschlaeger, J.; Roettgers, R.; Petersen, W.; Wiltshire, K. Performance of absorption coefficient measurements for the in situ determination of chlorophyll-a and total suspended matter. J. Exp. Mar. Biol. Ecol. 2014, 453, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Duoliang, L. Development of in situ sensors for chlorophyll concentration measurement. J. Sens. 2015, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Hussain, A. Chemo-metric analysis of carotenoids, chloropylls, and antioxidant activity of Trifolium hybridum. Heliyon 2020, 6, e03195. [Google Scholar] [CrossRef] [Green Version]
- Kalmatskaya, O.; Trubitsin, B.; Suslichenko, I.; Karavaev, V.; Tikhonov, A. Electron transport in Tradescantia leaves acclimated to high and low light: Thermoluminescence, PAM-fluorometry, and EPR studies. Photosynth. Res. 2020, 146, 123–141. [Google Scholar] [CrossRef]
- Zeng, J.; Ping, W.; Sanaeifar, A.; Xu, X.; Luo, W.; Sha, J.; Huang, Z.; Huang, Y.; Liu, X.; Zhang, B.; et al. Quantitative visualization of photsynthetic pigments in tea leaves based on Raman spectroscopy and calibration model transfer. Plant Mehods. 2021, 17, 13. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent advances in carbón and nitrogen metabolism in C3 plants. Int. J. Mol. Sci. 2021, 1, 1–39. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, Y.; Dechant, B.; Lee, H.; Kim, H.; Kornfeld, A.; Berry, J. Solar-induced chlorophyll fluorescence is non-linearly related to canopy photosynthesis in a temperate evergreen needleleaf forest during the fall transition. Remote Sens. Environ. 2021, 258, 112362. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.; Liu, Y.; Flores-Verdugo, F.; Flores-de-Santiago, F. Separating mangrove species and conditions using laboratory hyperspectral data: A case study of a degraded mangrove forest of the Mexican Pacific. Remote Sens. 2014, 6, 11673–11688. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.; Kaufman, Y.; Merzlyak, M. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Pastor-Guzman, J.; Atkinson, P.; Dash, J.; Rioja-Nieto, R. Spatiotemporal variation in mangrove chlorophyll concentration using Landsat 8. Remote Sens. 2015, 7, 14530–14558. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, Y.; Kovacs, J.; Flores-Verdugo, F.; Flores-de-Santiago, F.; Chen, K. Spectral response to varying levels of leaf pigments collected from a degraded mangrove forest. J. Appl. Remote Sens. 2012, 6, 063501. [Google Scholar] [CrossRef] [Green Version]
- Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad. Available online: https://www.gob.mx/conabio/prensa/el-sistema-de-monitoreo-de-los-manglares-de-mexico-presenta-nueva-cartografia-de-la-distribucion-de-manglares-en-2020-262804 (accessed on 8 April 2021).
- Zamora-Luria, J.C.; Perera-Burgos, J.A.; González-Calderón, A.; Marín, L.E.; Leal-Bautista, R.M. Control of fracture networks on a coastal karstic aquifer: A case study from northeastern Yucatán Peninsula (Mexico). Hydrogeol. J. 2020, 28, 2765–2777. [Google Scholar] [CrossRef]
- CONAFOR. Procedimientos de Muestreo; Inventario Nacional Forestal y de Suelos: Zapopan, Mexico, 2015; p. 261. [Google Scholar]
- Boone, J.; Donato, D.; Adame, M. Protocolo para la Medición, Monitoreo y Reporte de la Estructura, Biomasa y Reservas de Carbono de los Manglares, Documento de Trabajo 117; CIFOR: Bogor, Indonesia, 2013. [Google Scholar]
- Agraz-Hernández, C.; Noriega-Trejo, R.; López-Portillo, J.; Flores-Verdugo, F.; Jiménez-Zacarías, J. Guía de Campo. Identificación de los Manglares en México; EPOMEX, INECOL-CONAFOR: Campeche, México, 2006. [Google Scholar]
- McCullagh, P.; Nelder, J. Generalized Linear Models. Chapman. Hall. 1989, 2, 511. [Google Scholar]
- Zuur, A.; Ieno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef] [Green Version]
- Akaike, H. A new look at the Statistical Identificación Model. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Pastor-Guzman, J.; Dash, J.; Atkinson, P. Remote sensing of mangrove forest phenology and its environmental drivers. Remote Sens. Environ. 2018, 205, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Batllori-Sampedro, E.; González-Piedra, J.; Díaz-Sosa, J.; Febles-Patrón, J. Caracterización hidrológica de la región costera noroccidental del estado de Yucatán, México. Investig. Geográficas 2006, 59, 74–92. [Google Scholar] [CrossRef]
- Aranda-Cicerol, N.; Herrera-Silveira, J.; Comín, F. Nutrient water quality in a tropical coastal zone with groundwater discharge, northwest Yucatán, Mexico. Estuar. Coast. Shelf Sci. 2006, 68, 445–454. [Google Scholar] [CrossRef]
- Medina-Calderon, J.; Mancera-Pineda, J.; Castañeda-Moya, E.; Rivera-Monroy, V. Hydroperiod and salinity interactions control mangrove root dynamics in a karstic oceanic island in the Caribbean Sea (San Andres, Colombia). Front. Mar. Sci. 2021, 7, 598132. [Google Scholar] [CrossRef]
- Hernández, C.; Zaragoza, C.; Iriarte-Vivar, S.; Flores-Verdugo, F.; Casasola, P. Forest structure, productivity and species phenology of mangroves in the La Mancha lagoon in the Atlantic coast of Mexico. Wetl. Ecol. Manag. 2011, 19, 273–293. [Google Scholar] [CrossRef]
- Herrera-Silveira, J. Lagunas costeras de Yucatán (SE, México): Investigación, diagnóstico y manejo. Ecotrópicos 2006, 2, 94–108. [Google Scholar]
- Unger, I.; Kennedy, A.; Muzika, R. Flooding effects on soil microbial communities. Appl. Soil. Ecol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Thatoi, H.; Behera, B.; Mishra, R.; Dutta, S. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann. Microbiol. 2013, 63, 1–19. [Google Scholar] [CrossRef]
- Moorthy, P.; Kathiresan, K. Photosynthetic efficiency in Rhizophoracean mangrove with reference to compartmentalization of photosynthetic pigments. Rev. Biol. Trop. 1999, 47, 21–25. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, M.; Villeda-Chávez, E.; Vázquez-Lule, A.; Bejarano, M.; Cruz-López, M.; Olguín, M.; Villela-Gaytán, S.; Flores, R. Métodos Para la Caracterización de los Manglares Mexicanos: Un Enfoque Espacial Multiescala; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Tlalpan, Mexico, 2018. [Google Scholar]
- Gutierrez-Mendoza, J.; Herrera-Silveira, J. Almacenes de Carbono en Manglares de Tipo Chaparro en un Escenario Cárstico; Programa Mexicano del Carbono, Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional, Unidad Mérida, Centro de Investigación y Asistencia en Tecnologia y Diseño del Estado de Jalisco: Jalisco, Mexico, 2014. [Google Scholar]
- Zaldívar-Jiménez, A.; Herrera-Silveira, J.; Teutli Hernández, C.; Comín, F.; Andrade, J.; Coronado-Molina, C.; Pérez-Ceballos, R. Conceptual framework for mangrove restoration in the Yucatán Peninsula. Ecol. Restor. 2010, 28, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez- Rodríguez, J.; Mancera-Pineda, J.; Rodríguez, J. Validación y aplicación de un modelo de restauración de manglar basado en individuos para tres especies en la Ciénaga Grande de Santa Marta. Caldasia 2016, 38, 285–299. [Google Scholar] [CrossRef]
- Devaney, J.; Marone, D.; McElwain, J. Impact of soil salinity on mangroves restoration in a semiarid region: Study from the Saloum Delta, Senegal. Restor. Ecol. 2021, 29, e13186. [Google Scholar] [CrossRef]


| Species | Data | Min | Max | Mean | ±SD | ±SE |
|---|---|---|---|---|---|---|
| R. mangle | H (m) | 2.70 | 3.30 | 3.02 | 0.02 | 0.01 |
| DBH (cm) | 2.60 | 4.20 | 3.44 | 0.36 | 0.07 | |
| BA (cm2) | 5.31 | 13.85 | 9.46 | 1.93 | 0.39 | |
| Chl-a (μg per cm−2) | 24.83 | 44.81 | 34.08 | 2.66 | 0.54 | |
| A. germinans | H (m) | 2.50 | 3.20 | 2.81 | 0.04 | 0.01 |
| DBH (cm) | 3.10 | 4.70 | 3.82 | 0.21 | 0.04 | |
| BA (cm2) | 7.55 | 17.35 | 11.57 | 1.27 | 0.26 | |
| Chl-a (μg per cm−2) | 4.86 | 28.60 | 14.63 | 4.91 | 1.00 |
| Species | Data | Min | Max | Mean | ±SD | ±SE |
|---|---|---|---|---|---|---|
| R. mangle | H (m) | 2.40 | 3.40 | 2.93 | 0.07 | 0.01 |
| DBH (cm) | 1.90 | 6.00 | 3.80 | 0.18 | 0.04 | |
| BA (cm2) | 2.84 | 28.27 | 12.72 | 0.72 | 0.15 | |
| Chl-a (μg cm−2) | 9.55 | 36.27 | 24.35 | 3.56 | 0.73 | |
| A. germinans | H (m) | 2.50 | 3.30 | 2.89 | 0.24 | 0.05 |
| DBH (cm) | 1.90 | 3.30 | 2.57 | 0.21 | 0.04 | |
| BA (cm2) | 2.84 | 8.55 | 5.32 | 1.62 | 0.33 | |
| Chl-a (μg cm−2) | 17.48 | 36.27 | 27.22 | 3.69 | 0.75 |
| Data | Deviance | Residuals Deviance | D2 | F | Pr (>F) |
|---|---|---|---|---|---|
| Ti | 0.68 | 47.24 | 1.41 | 13.89 | 2 × 10−04 ** |
| Si | 0.32 | 46.92 | 0.67 | 6.57 | 1.09 × 10−02 * |
| Oi | 0.11 | 46.80 | 0.23 | 2.33 | 0.12 |
| fL | 0.25 | 46.55 | 0.53 | 5.27 | 2.26 × 10−02 * |
| Hrel | 0.04 | 46.50 | 0.09 | 0.92 | 0.33 |
| Tamb | 0.09 | 46.41 | 0.18 | 1.85 | 0.17 |
| DBH | 5.34 | 41.07 | 11.15 | 109.19 | 2.2 × 10−16 ** |
| H | 2.49 | 38.57 | 5.20 | 50.98 | 1.2 × 10−11 ** |
| MF | 0.52 | 38.05 | 1.09 | 10.68 | 1.2 × 10−03 * |
| Spp | 14.62 | 23.42 | 30.51 | 298.83 | 2.2 × 10−16 ** |
| Time | 2.54 | 20.87 | 5.31 | 2.26 | 1.2 × 10−03 * |
| MF:Spp | 6.29 | 14.58 | 13.12 | 128.53 | 2.2 × 10−16 ** |
| Spp:Time | 3.18 | 11.40 | 6.64 | 2.82 | 4.07 × 10−05 ** |
| Data | Deviance | Residuals Deviance | D2 | F | Pr (>F) |
|---|---|---|---|---|---|
| Ti | 0.68 | 47.24 | 1.41 | 13.97 | 2.0 × 10−04 ** |
| Si | 0.32 | 46.92 | 0.67 | 6.61 | 1.07 × 10−02 * |
| fL | 0.15 | 46.77 | 0.31 | 3.09 | 7.96 × 10−02 |
| DBH | 5.46 | 41.30 | 11.40 | 112.27 | 2.2 × 10−16 ** |
| MF | 3.6 × 10−03 | 41.30 | 0.01 | 0.07 | 0.78 |
| Spp | 17.19 | 24.11 | 35.87 | 353.16 | 2.2 × 10−16 ** |
| Time | 3.18 | 20.92 | 6.64 | 2.84 | 3.58 × 10−05 ** |
| MF:Spp | 6.29 | 14.62 | 13.14 | 129.41 | 2.2 × 10−16 ** |
| Spp:Time | 3.13 | 11.49 | 6.53 | 2.79 | 4.77 × 10−05 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos-Basto, B.; Herrera-Silveira, J.; Bataller, É.; Rioja-Nieto, R. Local Drivers Associated to Temporal Spectral Response of Chlorophyll-a in Mangrove Leaves. Sustainability 2021, 13, 4636. https://doi.org/10.3390/su13094636
Castellanos-Basto B, Herrera-Silveira J, Bataller É, Rioja-Nieto R. Local Drivers Associated to Temporal Spectral Response of Chlorophyll-a in Mangrove Leaves. Sustainability. 2021; 13(9):4636. https://doi.org/10.3390/su13094636
Chicago/Turabian StyleCastellanos-Basto, Blanca, Jorge Herrera-Silveira, Érick Bataller, and Rodolfo Rioja-Nieto. 2021. "Local Drivers Associated to Temporal Spectral Response of Chlorophyll-a in Mangrove Leaves" Sustainability 13, no. 9: 4636. https://doi.org/10.3390/su13094636






