The Physico-Chemical and Mineralogical Characterization of Mg-Rich Synthetic Gypsum Produced in a Rare Earth Refining Plant
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
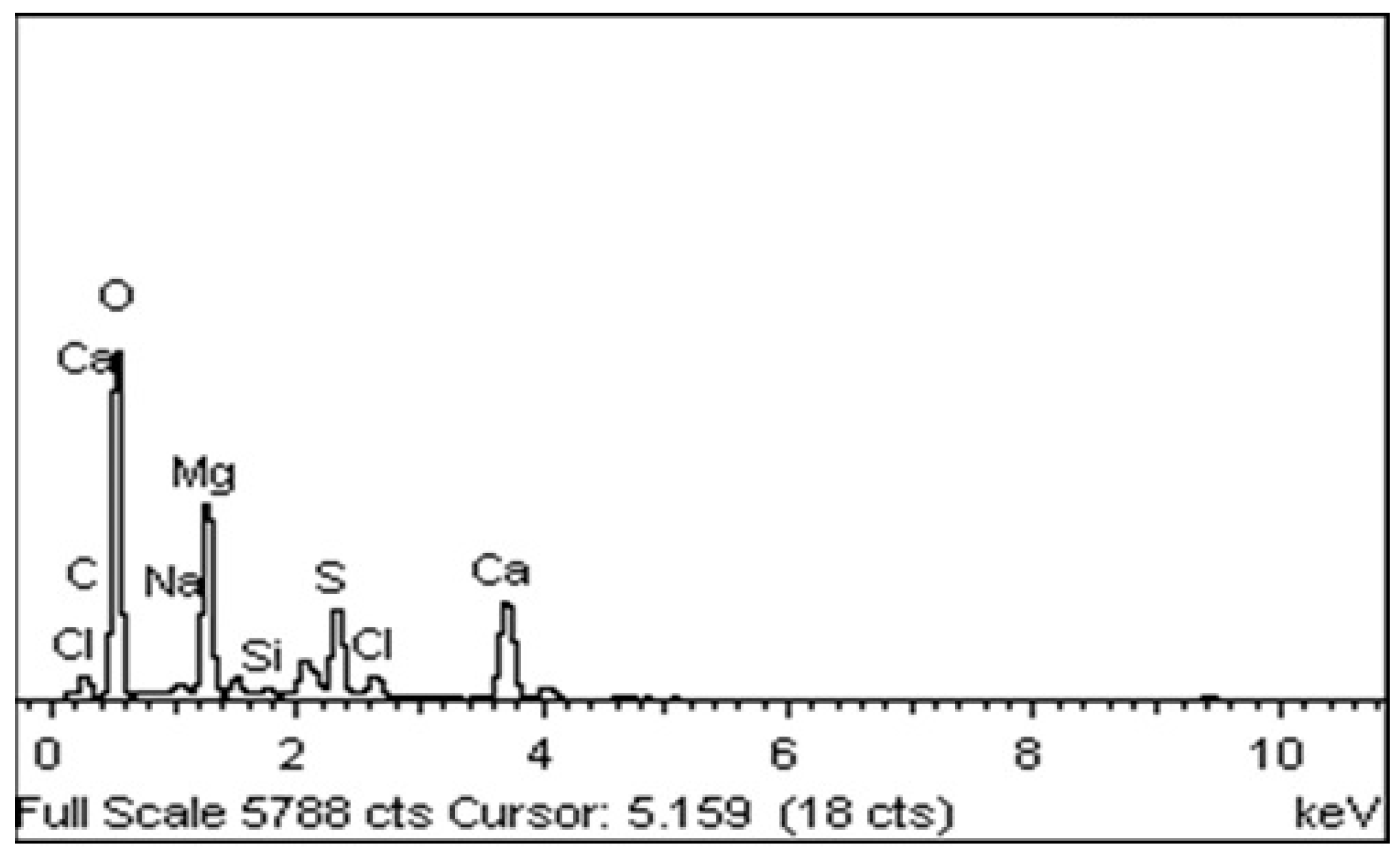
3.1. Physico-Chemical Characterization
3.2. X-ray Diffraction
3.3. FTIR Spectroscopy
3.4. Morphology of MRSG
3.5. Thermal Analysis
3.6. Environmental Impact Assessment of MRSG Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, C.H. Biochemistry of the Lanthanides; Springer Science and Business Media: Berlin, Germany, 2013. [Google Scholar]
- Henderson, P. Rare earth element geochemistry. Developments in Geochemistry; Elsevier: Amsterdam, The Netherlands, 1984; Volume 2. [Google Scholar]
- Wen, B.; Liu, Y.; Hu, X.Y.; Shan, X.Q. Effect of earthworms (Eisenia fetida) on the fractionation and bioavailability of rare earth elements in nine Chinese soils. Chemosphere 2006, 63, 1179–1186. [Google Scholar] [CrossRef]
- Tyler, G. Rare earth elements in soil and plant systems-A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Aguar, P. Report on Trans-Atlantic Workshop on Rare Earth Elements and Other Critical Materials for a Clean Energy Future; European Union: Brussels, Belgium, 2011. [Google Scholar]
- Brioschi, L.; Steinmann, M.; Lucot, E.; Pierret, M.C.; Stille, P.; Prunier, J.; Badot, P.M. Transfer of rare earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE. Plant Soil 2013, 366, 143–163. [Google Scholar] [CrossRef]
- Lynas. Available online: https://www.lynascorp.com/sustainability/residue-tailings-management (accessed on 12 June 2020).
- Goonan, T.G. Rare Earth Elements: End Use and Recyclability; US Department of the Interior, US Geological Survey: Reston, VA, USA, 2011.
- Ayanda, A.F.; Shamshuddin, J.; Fauziah, C.I.; Radziah, O. Utilization of magnesium-rich synthetic gypsum as magnesium fertilizer for oil palm grown on acidic soil. PLoS ONE 2020, 16, 15. [Google Scholar] [CrossRef]
- Sahibin, A.R.; Shamshuddin, J.; Fauziah, C.I.; Radziah, O.; Razi, I.W.; Enio, M.S. Impact of Mg rich synthetic gypsum application on the environment and palm oil quality. Sci. Total Environ. 2019, 652, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Illera, V.; Vizcayno, C.; García-González, M.T. Evaluation of industrial by-products as soil acidity amendments: Chemical and mineralogical implications. Eur. J. Soil Sci. 2003, 54, 411–422. [Google Scholar] [CrossRef]
- Anawar, H.M.; Akter, F.; Solaiman, Z.M.; Strezov, V. Biochar: An emerging panacea for remediation of soil contaminants from mining, industry and sewage wastes. Pedosphere 2015, 25, 654–665. [Google Scholar] [CrossRef]
- Lee, X.J.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Ng, H.K. Biochar potential evaluation of palm oil wastes through slow pyrolysis: Thermochemical characterization and pyrolytic kinetic studies. Bioresour. Technol. 2017, 236, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Kuan, S.H.; Saw, L.H.; Ghorbani, Y. A review of rare earths processing in Malaysia. In Proceedings of the Inemeritus Prof. Dr. Faizah Binti Mohd Sharoum Chairman UMT International Annual Symposium on Sustainability Science and Management-UMTAS, Kuala Terengganu, Malaysia, 9–11 July 2016; Volume 42, p. 105. [Google Scholar]
- US EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, in: Selected Analytical Methods for Environmental Remediation and Recovery (SAM). 2018. Available online: https://www.epa.gov/homeland-security-research/epa-method-3050b-acid-digestion-sediments-sludges-and-soils (accessed on 5 February 2020).
- Boumans, P.W.J.M. Inductively Coupled Plasma Emission Spectroscopy, Part 1: Methodology, Instrumentation and Performance; Wiley-Interscience: Hoboken, NJ, USA, 1987. [Google Scholar]
- Navarro, C.; Díaz, M.; Villa-García, M.A. Physico-chemical characterization of steel slag. Study of its behavior under simulated environmental conditions. Environ. Sci. Technol. 2010, 44, 5383–5388. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.H.; Lo, S.L. Chemical and spectroscopic analysis of organic matter transformations during composting of pig manure. Environ. Pollut. 1999, 104, 189–196. [Google Scholar] [CrossRef]
- Fauziah, C.I. Solid Waste Management: An Inaugural Lecture; Universiti Putra Malaysia Press: Serdang, Malaysia, 2019. [Google Scholar]
- Li, J.Y.; Liu, Z.D.; Zhao, W.Z.; Masud, M.M.; Xu, R.K. Alkaline slag is more effective than phosphogypsum in the amelioration of subsoil acidity in an Ultisol profile. Soil Till. Res. 2015, 149, 21–32. [Google Scholar] [CrossRef]
- Valle, L.A.; Rodrigues, S.L.; Ramos, S.J.; Pereira, H.S.; Amaral, D.C.; Siqueira, J.O.; Guilherme, L.R. Beneficial use of a by-product from the phosphate fertilizer industry in tropical soils: Effects on soil properties and maize and soybean growth. J. Clean. Prod. 2016, 112, 113–120. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Ismail, H. Reactions of ground magnesium limestone and gypsum in soils with variable-charge minerals. Soil Sci. Soc. Am. J. 1995, 59, 6–12. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Daud, W.N.; Ismail, R.; Ishak, C.F.; Panhwar, Q.A. Ultisols & Oxisols: Enchancing Their Productivity for Oil Palm, Rubber and Cocoa Cultivation; Universiti Putra Malaysia Press: Serdang, Malaysia, 2015. [Google Scholar]
- Daud, W.N. Rubber Plantation: Soil Management & Nutritional Requirement; Universiti Putra Malaysia Press: Serdang, Malaysia, 2013. [Google Scholar]
- Alloway, B.J. Sources of heavy metals and metalloids in soils. In Heavy Metals in Soils; Springer: Dordrecht, Germany, 2013; pp. 11–50. [Google Scholar]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Sari, N.A.; Fauziah, C.I.; Rosenani, A.B. Characterization of oil palm empty fruit bunch and rice husk biochars and their potential to adsorb arsenic and cadmium. Am. J. Agric. Biol. Sci. 2014, 9, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Samsuri, A.W.; Sadegh-Zadeh, F.; She-Bardan, B.J. Adsorption of As(III) and As(V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Fauziah, C.I.; Nur Hanani, M.; Zauyah, S.; Samsuri, A.W.; Rosazlin, A. Co-application of red gypsum and sewage sludge on acidic tropical soils. Comm. Soil Sci. Plan. 2011, 42, 2561–2571. [Google Scholar] [CrossRef]
- Nur Hanani, M.; Fauziah, C.I.; Samsuri, A.W.; Zauyah, S. Utilization of drinking-water treatment residue to immobilize copper and zinc in sewage-sludge-amended soils. Land Contam. Reclamat. 2008, 16, 319–332. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Lou, W.; Guan, B.; Wu, Z. Dehydration behavior of FGD gypsum by simultaneous TG and DSC analysis. J. Therm. Anal. Calorim. 2011, 104, 661–669. [Google Scholar] [CrossRef]
- Caillahua, M.C.; Moura, F.J. Technical feasibility for use of FGD gypsum as an additive setting time retarder for Portland cement. J. Mater. Res. Technol. 2018, 7, 190–197. [Google Scholar] [CrossRef]
- Li, J.; Verweij, R.A.; van Gestel, C.A. Lanthanum toxicity to five different species of soil invertebrates in relation to availability in soil. Chemosphere 2018, 193, 412–420. [Google Scholar] [CrossRef]
- Larsson, M.A.; Baken, S.; Gustafsson, J.P.; Hadialhejazi, G.; Smolders, E. Vanadium bioavailability and toxicity to soil microorganisms and plants. Environ. Toxicol. Chem. 2013, 32, 2266–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.N.; Tabatabai, M.A. Effects of trace elements on nitrification in soils. J. Environ. Qual. 1978, 7, 293. [Google Scholar] [CrossRef]
- Gupta, D.K.; Deb, U.; Walther, C.; Chatterjee, S. Strontium in the ecosystem: Transfer in plants via root system. In Behaviour of Strontium in Plants and the Environment; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Li, J.Y.; Wang, N.; Xu, R.K.; Tiwari, D. Potential of industrial byproducts in ameliorating acidity and aluminum toxicity of soils under tea plantation. Pedosphere 2010, 20, 645–654. [Google Scholar] [CrossRef]
- Queralt, I.; Juliá, R.; Plana, F.; Bischoff, J.L. A hydrous Ca-bearing magnesium carbonate from playa lake sediments, Salines Lake, Spain. Am. Mineral. 1997, 82, 812–819. [Google Scholar] [CrossRef]
- Mayo, D.W.; Foil, A.M.; Hannah, R.W. Course Notes on the Interpretation of Infrared and Raman Spectra; Wiley-Interscience: New York, NY, USA, 2004; pp. 316–318. [Google Scholar]
- Van der Marel, H.W.; Beutelspacher, H. Atlas of Infrared Spectroscopy of Clay Minerals and Their Admixtures; Elsevier: Amsterdam, The Netherlands, 1976; pp. 224–229. [Google Scholar]
- Namduri, H.; Nasrazadani, S. Quantative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Hirsch, J.; Lowry, S.R.; Dowd, M. X-ray fluorescence and FT-IR identification of strontium and carbonate in domestic and imported gypsum drywall. Spectroscopy 2010, 25, 30. [Google Scholar]
- Chernysh, Y.; Balintova, M.; Plyatsuk, L.; Holub, M.; Demcak, S. The influence of phosphogypsum addition on phosphorus release in biochemical treatment of sewage sludge. Int. J. Environ. Res. Public Health 2018, 15, 1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazi, W.K.; Hugi, E.; Wullschleger, L.; Frank, T.H. Gypsum board in fire—modeling and experimental validation. J. Fire Sci. 2007, 25, 267–282. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Waligora, J.; Bulteel, D.; Degrugilliers, P.; Damidot, D.; Potdevin, J.L.; Measson, M. Chemical and mineralogical characterizations of LD converter steel slags: A multi-analytical techniques approach. Mater. Charact. 2010, 61, 39–48. [Google Scholar] [CrossRef]
- Masaguer, V.; Oulego, P.; Collado, S.; Villa-García, M.A.; Díaz, M. Characterization of sinter flue dust to enhance alternative recycling and environmental impact at disposal. J. Waste Manag. 2018, 79, 251–259. [Google Scholar] [CrossRef]
- Setién, J.; Hernández, D.; González, J.J. Characterization of ladle furnace basic slag for use as a construction material. Constr. Build. Mater. 2009, 23, 1788–1794. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [Green Version]
- Turan, V.; Khan, S.A.; Iqbal, M.; Ramzani, P.M.; Fatima, M. Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotoxicol. Environ. Saf. 2018, 161, 409–519. [Google Scholar] [CrossRef] [PubMed]
- Zarcinas, B.A.; Ishak, C.F.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia. Environ. Geochem. Health 2004, 26, 343–357. [Google Scholar] [CrossRef]
- US EPA. Guidance for Developing Ecological Soil Screening Levels. 2007. Available online: https://www.epa.gov/sites/production/files/201509/documents/ecossl_guidance_chapters.pdf (accessed on 5 February 2018).





| Properties | Magnesium Rich Synthetic Gypsum |
|---|---|
| pH H2O | 9.2 |
| Electrical Conductivity (EC) (dS m−1) | 7.1 |
| Moisture (%) | 26.7 |
| C (%) | 1.12 |
| N (%) | 0.05 |
| S (%) | 0.41 |
| Ca (mg kg−1) | 17,243 |
| P (mg kg−1) | 234 |
| Mg (mg kg−1) | 14,396 |
| K (mg kg−1) | 49 |
| Na (mg kg−1) | 288 |
| Fe (mg kg−1) | 1368 |
| Mn (mg kg−1) | 1175 |
| Cu (mg kg−1) | 127 |
| Al (mg kg−1) | 350 |
| Cd (mg kg−1) | 234 |
| Zn (mg kg−1) | 38 |
| Loss of ignition (LO1) | 19.39 |
| LODs | 0.05 μg/g |
| Chemical Composition | Amount in % |
|---|---|
| CaO | 59.63 |
| SO3 | 29.58 |
| CeO2 | 3.12 |
| Cl | 2.05 |
| La2O3 | 2.04 |
| Fe2O | 1.62 |
| MnO | 1.02 |
| V2O5 | 0.37 |
| ZnO | 0.21 |
| SrO | 0.21 |
| Y2O3 | 0.07 |
| ZrO2 | 0.04 |
| CuO | 0.04 |
| No. | Wave No. (cm−1) | Vibration | Functional Groups |
|---|---|---|---|
| 1 | 3695 | O-H stretch | Bonded and nonbonded hydroxyl group and water |
| 2 | 3487 | O-H stretch | Bonded and nonbonded hydroxyl group and water |
| 3 | 3392 | O-H stretch | Bonded and nonbonded hydroxyl group and water |
| 4 | 1681 | Stretching | Water molecules |
| 5 | 1618 | Stretching | Water molecules |
| 6 | 1440 | C-H deformation, CO2 stretch; C-O stretch | Alkenes carboxylates and carbonates |
| 7 | 1099 | Si-O stretch; OH vibration | Silica and silicates; aluminum oxyhydroxide and iron oxyhydroxides |
| 8 | 665 | SO42− | Sulfates |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayanda, F.A.; Anuar, M.F.M.; Zaibon, S.; Jusop, S. The Physico-Chemical and Mineralogical Characterization of Mg-Rich Synthetic Gypsum Produced in a Rare Earth Refining Plant. Sustainability 2021, 13, 4840. https://doi.org/10.3390/su13094840
Ayanda FA, Anuar MFM, Zaibon S, Jusop S. The Physico-Chemical and Mineralogical Characterization of Mg-Rich Synthetic Gypsum Produced in a Rare Earth Refining Plant. Sustainability. 2021; 13(9):4840. https://doi.org/10.3390/su13094840
Chicago/Turabian StyleAyanda, Fatai Arolu, Mohd Firdaus Mohd Anuar, Syaharudin Zaibon, and Shamshuddin Jusop. 2021. "The Physico-Chemical and Mineralogical Characterization of Mg-Rich Synthetic Gypsum Produced in a Rare Earth Refining Plant" Sustainability 13, no. 9: 4840. https://doi.org/10.3390/su13094840
APA StyleAyanda, F. A., Anuar, M. F. M., Zaibon, S., & Jusop, S. (2021). The Physico-Chemical and Mineralogical Characterization of Mg-Rich Synthetic Gypsum Produced in a Rare Earth Refining Plant. Sustainability, 13(9), 4840. https://doi.org/10.3390/su13094840






