Nitrous Oxide Emissions in Pineapple Cultivation on a Tropical Peat Soil
Abstract
:1. Introduction
2. Materials and Methods
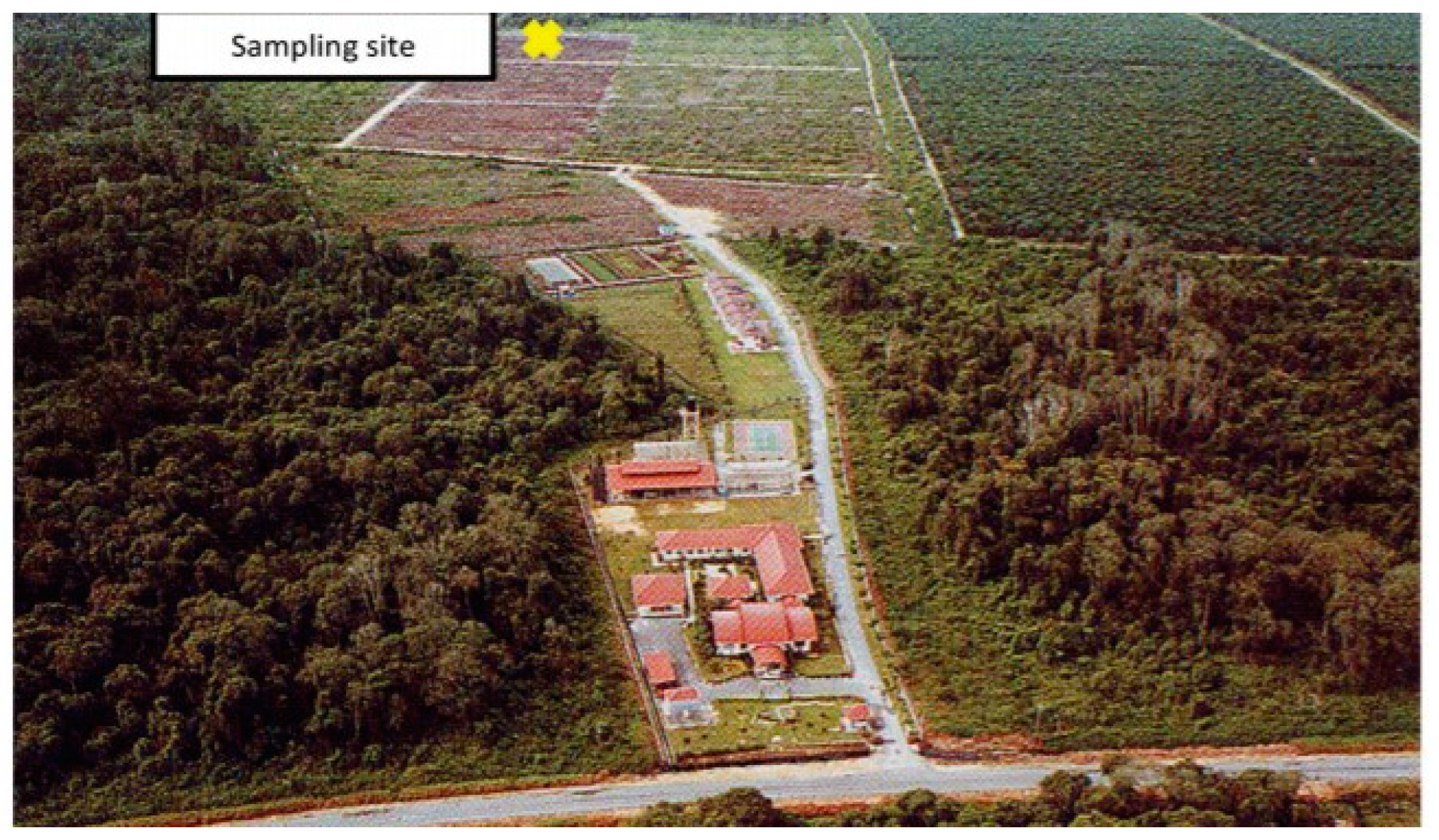
2.1. Information about the Experimental Area

2.2. Greenhouse Gases Emission Measurements
2.2.1. Soil Nitrous Oxide Horizontal Emission Measurements
2.2.2. Soil Nitrous Oxide Vertical Emission Measurements
2.2.3. Nitrous Oxide Flux Calculation
2.2.4. Statistical Analysis
3. Results and Discussions
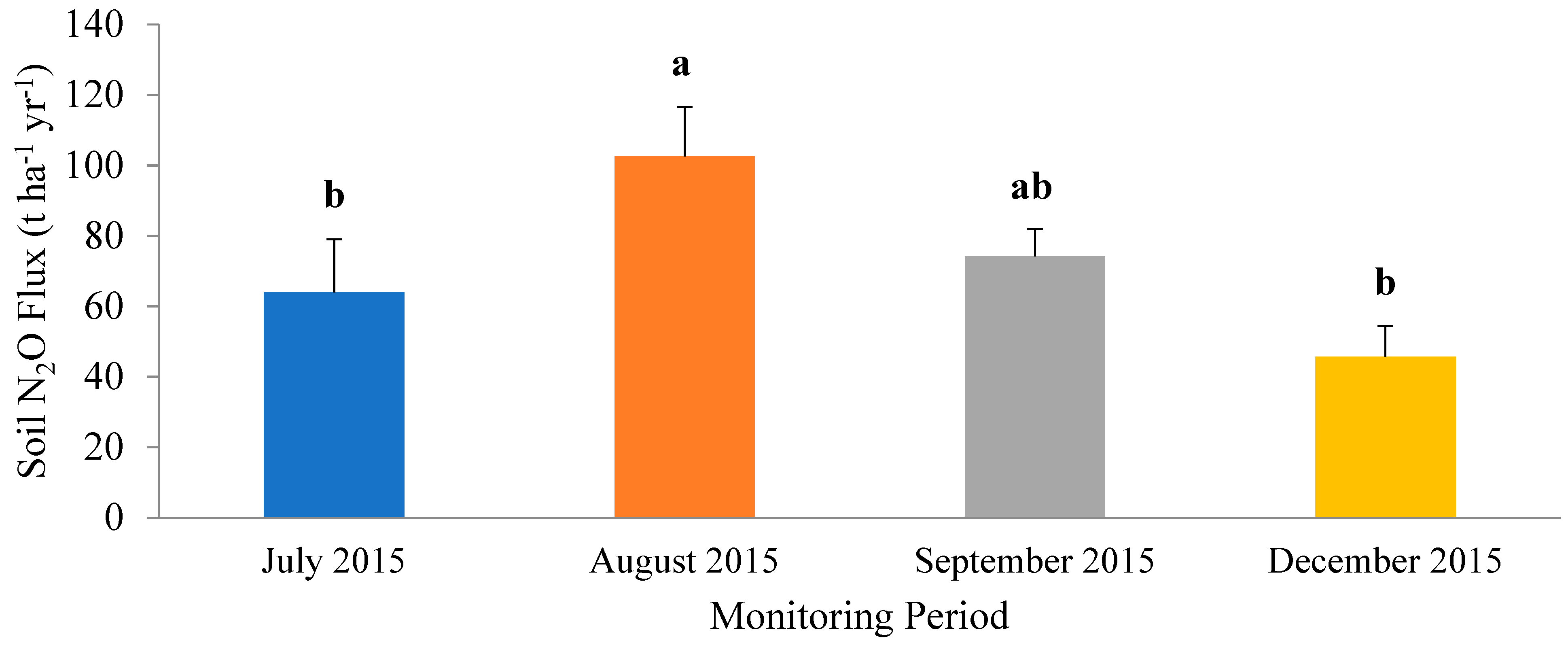
3.1. Horizontal Movement of Nitrous Oxide
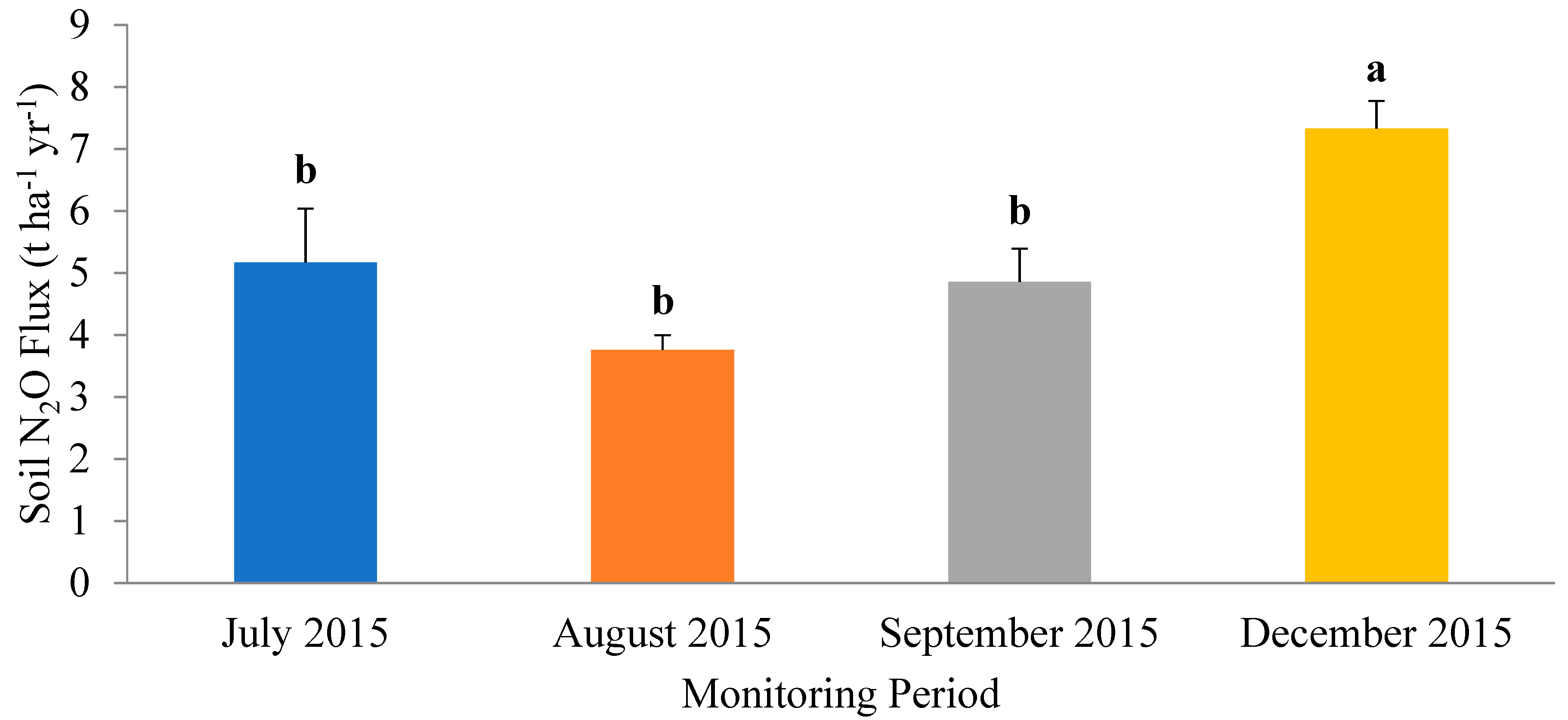
3.2. Vertical Movement of Nitrous Oxide
3.3. Summary on the Movements of the Nitrous Oxide out of the Peat Soil
3.4. Limitation of Greenhouse Gases Emissions Using Closed Chamber Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooijer, A.; Page, S.; Canadell, J.G.; Silvius, M.; Kwadijk, J.; Wösten, H.; Jauhiainen, J. Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 2010, 7, 1505–1514. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.B.; Jamaludin, J. Land clearing techniques employed at MARDI Peat Research Station, Sessang, Sarawak, and their immediate impacts. In Impact of Land Clearing on Peat Ecosystems: A Case Study of MARDI Peat Research Station, Sessang, Sarawak, Malaysia; Ismail, A.B., Ong, S.K., Mohd Hanif, M.J., Umi Kalsom, M.S., Eds.; MARDI: Serdang, Malaysia, 2007; pp. 1–8. [Google Scholar]
- Leng, L.Y.; Ahmed, O.H.; Jalloh, M.B. Brief review on climate change and tropical peatlands. Geosci. Front. 2019, 10, 373–380. [Google Scholar] [CrossRef]
- Hajon, S.K.; Mos, H.; Jantan, N.; Haniff, M.H. Classification of tropical peat in Malaysia. Oil Palm Bull. 2018, 76, 1–7. [Google Scholar]
- Dolmat, M.T. Technology for Planting Oil Palm on Peat; Malaysian Palm Oil Board: Selangor, Malaysia, 2005; pp. 1–76. [Google Scholar]
- Kang, S.; Post, W.F.; Nichols, J.A.; Wang, D.; West, T.O.; Bandaru, V.; Izaurralde, R. Marginal lands: Concept, assessment and management. J. Agric. Sci. 2013, 5, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Couwenberg, J. Greenhouse Gas Emissions from Managed Peat Soils: Is the IPCC reporting guidance realistic? Mires Peat 2011, 8, 1–10. [Google Scholar]
- Farmer, J.; Matthews, R.; Smith, J.U.; Smith, P.; Singh, B.K. Assessing existing peatland models for their applicability for modelling greenhouse gas emissions from tropical peat soils. Curr. Opin. Environ. Sustain. 2011, 3, 339–349. [Google Scholar] [CrossRef]
- Huat, B.B.K.; Kazemian, S.; Prasad, A.; Barghchi, M. State of an art review of peat: General perspective. Int. J. Phys. Sci. 2011, 6, 1988–1996. [Google Scholar]
- Department of Agriculture Malaysia. Crop Statistics Booklet (Food Crop Sub-Sector); DOA: Putrajaya, Malaysia, 2018; p. 49.
- Joosten, H.; Tapio-Biström, M.-L.; Tol, S. Peatlands—Guidance for climate change mitigation through conservation, rehabilitation and sustainable use. In Mitigation of Climate Change Agriculture Series 5, 2nd ed.; FAO and Wetlands: Rome, Italy, 2012; p. 114. [Google Scholar]
- Paramananthan, S. Tropical lowland peats: To conserve or develop them? In Proceedings of the International Palm Oil Sustainability Conference, Sabah, Malaysia, 13–15 April 2008. [Google Scholar]
- Bakar, I.A. Strategies for Mitigation of Carbon Emission in Peatland; MARDI Press: Serdang, Malaysia, 2010; pp. 1–35. [Google Scholar]
- Ahmed, O.H.; Husni, M.H.A.; Anuar, A.R.; Hanafi, M.M. Sustainable Production of Pineapples on Tropical Peat Soils; UPM Press: Selangor, Malaysia, 2013. [Google Scholar]
- Jassal, R.S.; Black, T.A.; Roy, R.; Ethier, G. Effect of nitrogen fertilization on soil CH4 and N2O fluxes, and soil and bole respiration. Geoderma 2011, 162, 182–186. [Google Scholar] [CrossRef]
- Chen, H.; Mothapo, N.V.; Shi, W. The significant contribution of fungi to soil N2O production across diverse ecosystems. Appl. Soil Ecol. 2014, 73, 70–77. [Google Scholar] [CrossRef]
- Reth, S.; Graf, W.; Gefke, O.; Schilling, R.; Seidlitz, H.K.; Munch, J.C. Whole-year-round observation of N2O profiles in soil: A lysimeter study. Water Soil Air Poll. Focus 2008, 8, 129–137. [Google Scholar] [CrossRef]
- Maljanen, M.; Martikkala, M.; Koponen, H.T.; Virkajärvi, P.; Martikainen, P.J. Fluxes of nitrous oxide and nitric oxide from experimental excreta patches in boreal agricultural soil. Soil Biol. Biochem. 2007, 39, 914–920. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Silvennoinen, H.; Hämäläinen, R.; Limin, S.; Raison, R.J.; Vasander, H. Nitrous oxide fluxes from tropical peat with different disturbance history and management. Biogeoscience 2012, 9, 1337–1350. [Google Scholar] [CrossRef] [Green Version]
- International Atomic Energy Agency. Manual on Measurement of Methane and Nitrous Oxide Emisssions from Agriculture, IAEA-TECDOC-674; IAEA: Vienna, Austria, 1992. [Google Scholar]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O: N2 Production in Temperate Grasslands: Processes, Measurements, Modelling and Mitigating Negative Impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef]
- Uchida, Y.; Von Rein, I.; Akiyama, H.; Yagi, K. Contribution of nitrification and denitrification to nitrous oxide emissions in Andosol and from Fluvisol after coated urea application. Soil Sci. Plant Nutrition 2013, 59, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Van Beek, C.L.; Pleijter, M.; Kuikman, P.J. Nitrous oxide emissions from fertilized and unfertilized grasslands on peat soil. Nutrient Cycl. Agroecosyst. 2010, 89, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Kasimir-Klemedtsson, A.; Klemedtsson, L.; Berglund, K.; Martikainen, P.; Silvola, J.; Oenema, O. Greenhouse Gas Emissions from Farmed Organic Soils: A Review. Soil Use Manag. 1997, 13, 245–250. [Google Scholar] [CrossRef]
- Melling, L.; Goh, K.J.; Beauvais, C.; Hatano, R. Carbon Flow and Budget in a Young Mature Oil Palm Agrosystem on Deep Tropical Peat. In Proceedings of the International Symposium and Workshop on Tropical Peatland, Yogyakarta, Indonesia, 27–29 August 2007. [Google Scholar]
- Jauhiainen, J.; Hoojier, A.; Page, S.E. Carbon Dioxide Fluxes in an Acacia Plantation on on Tropical Peatland. Biogeosci. Discuss. 2011, 8, 8269–8302. [Google Scholar]
- Jauhiainen, J.; Silvennoinen, H.; Hämäläinen, R.; Kusin, K.; Limin, S.; Raison, R.J.; Vasander, H. Nitrous Oxide Fluxes from Tropical Peat with different Disturbance History and Management. Biogeosci. Discuss. 2011, 8, 5423–5450. [Google Scholar]
- Hadi, A.; Inubushi, K.; Purnomo Razie, F.; Yamakawa, K.; Tsuruta, H. Effects of Land-Use Change on Nitrous Oxide (N2O) Emission from Tropical Peatlands. Chem.-Global Change Sci. 2000, 2, 347–358. [Google Scholar] [CrossRef]
- Takakai, F.; Morishita, T.; Hashidoko, Y.; Darung, U.; Kuramochi, K.; Dohong, S.; Hatano, R. Effects of Agricultural Land-Use Change and Forest Fire in N2O Emission from Tropical Peatlands, Central Kalimantan, Indonesia. Soil Sci. Plant Nutrition 2006, 52, 662–674. [Google Scholar] [CrossRef]
- Klemedtsson, L.; Ernfors, M.; Björk, R.G.; Weslien, P.; Rütting, T.; Crill, P. Reduction of greenhouse gas emissions by wood application to a Picea abis (L.) Karst. Forest on a drained organic soil. Eur. J. Soil Sci. 2010, 61, 734–744. [Google Scholar] [CrossRef]
- Liimatainen, M.; Martikainen, P.J.; Maljanen, M. Why granulated wood ash decreases N2O production in boreal acidic peat soil? Soil Biol. Biochem. 2014, 79, 140–148. [Google Scholar] [CrossRef]
- Page, S.E.; Morrison, R.; Malins, C.; Hoojier, A.; Rieley, J.O.; Jauhiainen, J. Review of Peat Surface Greenhouse Gas Emissions from Oil Palm Plantations in Southeast Asia; (ICCT White Paper 15); International Council on Clean Transportation: Washington, DC, USA, 2011; pp. 40–45. [Google Scholar]
- Germer, J.; Sauerborn, J. Estimation of the impact of oil palm plantation establishment on greenhouse gas balance. Environ. Devel. Sustain. 2008, 10, 697–716. [Google Scholar] [CrossRef]
- International Panel on Climate Change. IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2006. [Google Scholar]
- Kløve, B.; Sveistrup, T.E.; Hauge, A. Leaching of nutrients and emission of greenhouse gases from peatland cultivation at Bodin, Northern Norway. Geoderma 2010, 154, 219–232. [Google Scholar] [CrossRef]
- Maljanen, M.; Komulainen, V.-M.; Hytönen, J.; Martikainen, P.J.; Laine, J. Carbon dioxide, nitrous oxide and methane dynamics in boreal organic agricultural soils with different soil characteristics. Soil Biol. Biochem. 2004, 36, 1801–1808. [Google Scholar] [CrossRef]
- Ismail, A.B. Towards wise use of tropical peatland: From agriculture perspective. In Proceedings of the International Symposium and Workshop on Tropical Peatland, Kuching, Sarawak, Malaysia, 19–22 August 2008; pp. 129–141. [Google Scholar]
- Inubushi, K.; Furukawa, Y.; Hadi, A.; Purnomo, E.; Tsuruta, H. Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere 2003, 52, 603–608. [Google Scholar] [CrossRef]
- Hadi, A.; Inubushi, K.; Furukawa, Y.; Purnomo, E.; Rasmadi, M.; Tsuruta, H. Greenhouse gas emissions from tropical peatlands of Kalimantan, Indonesia. Nutrient Cycling Agroeco. 2005, 71, 73–80. [Google Scholar] [CrossRef]
- Raziah, M.L.; Alam, A.R. Status and impact of pineapple technology on mineral soil. Econ. Technol. Manag. Rev. 2010, 5, 11–19. [Google Scholar]
- Selamat, M.M. Biologi tanaman dan keperluan persekitaran. In Penanaman Nanas—Nanas Makan Segar Dan Nanas Kaleng; Mohammed Selamat, M., Ed.; MARDI: Serdang, Malaysia, 1996; pp. 7–16. [Google Scholar]
- Mohammed Selamat, M.; Abdul Rahman, H. Amalan kultur. In Penanaman Nanas—Nanas Makan Segar Dan Nanas Kaleng; Mohammed Selamat, M., Ed.; MARDI: Serdang, Malaysia, 1996; pp. 24–34. [Google Scholar]
- Ritchie, R.J.; Bunthawin, S. Photosynthesis in pineapple (Ananas comosus [L.] Merr) measured using PAM (Pulse Amplitude Modulation Fluorometry). Tropical Plant Biol. 2010, 3, 193–203. [Google Scholar] [CrossRef]
- Couwenberg, J.; Hooijer, A. Towards Robust Subsidence-Based Soil Carbon Emission Factors for Peat Soils in South-East Asia, with Special Reference to Oil Palm Plantations. Mires and Peat 12: Art. 1. 2013. Available online: http://www.mires-and-peat.net/pages/volumes/map12/map1201.php (accessed on 10 March 2021).
- Melling, L.; Henson, I. Greenhouse gas exchange of tropical peatlands—A review. J. Oil Palm Res. 2011, 23, 1087–1095. [Google Scholar]
- Daud, A. Economic Valuation of Pineapple Cultivation on Peat Soil at the Integrated Agricultural Development Area, Samarahan, Sarawak. Ph.D. Thesis, University Putra Malaysia, Serdang, Malaysia, 2009. [Google Scholar]
- Ahmed, O.H.; Jeffary, A.V.; Luta, W.; Choo, L.N.L.K. Tropical Peat Soil and Transportation of Greenhouse Gases; Universiti Putra Malaysia Press: Serdang, Malaysia, 2018; pp. 1–120. [Google Scholar]
- Ekono. Report on energy use of peat. Contribution to U.N. In Proceedings of the Conference on New and Renewable Sources of Energy, Nairobi, Kenya, 10–21 August 1981. [Google Scholar]
- Ahmed, O.H.; Liza Nuriati, L.K.C. Greenhouse Gas Emission & Carbon Leaching in Pineapple Cultivation on Tropical Peat Soil; Universiti Putra Malaysia Press: Serdang, Malaysia, 2015. [Google Scholar]
- Norman, J.M.; Kucharik, C.J.; Gower, S.T.; Baldocchi, D.D.; Crill, P.M.; Rayment, M.; Savage, K.; Striegl, R.G. A Comparison of Six Methods for Measuring Soil- Surface Carbon Dioxide Fluxes. J. Geophys. Res. 1997, 102, 28771–28777. [Google Scholar] [CrossRef]
- Crill, P.M. Seasonal Patterns of Methane Uptake and Carbon Dioxide Release by a Temperate Woodland Soil. Global Biogeochem. Cyc. 1991, 5, 319–334. [Google Scholar] [CrossRef]
- Zulkefli, M.; Liza Nuriati, L.K.C.; Ismail, A.B. Soil CO2 Flux from Tropical Peatland under Different Land Clearing Techniques. J. Trop. Agric. Food Sci. 2010, 38, 131–137. [Google Scholar]
- Wid’en, B.; Lindroth, A. A Calibration System for Soil Carbon Dioxide-Efflux Measurement Chambers. Soil Sci. Soc. Am. J. 2003, 67, 327–334. [Google Scholar]
- SAS. SAS/STAT Software, 2nd ed.; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- Liza Nuriati, L.K.C. Greenhouse Gas Emission Partitioning and Carbon Leaching in Drained Tropical Peatland, Saratok, Sarawak, Malaysia. Master’s Thesis, Universiti Putra Malaysia, Serdang, Malaysia, 2014. [Google Scholar]
- Zulkefli, M.; Liza Nuriati, L.K.C.; Ismail, A.B.; Jamaludin, J. Soil carbon loss under different land clearing techniques and agriculture systems on tropical peatland. In Proceedings of the International Symposium and Workshop on Tropical Peatland: Peat Development—Wise Use and Impact Management, Kuching, Malaysia, 19–22 August 2008; pp. 376–381. [Google Scholar]
- Abdul, H.; Kazuyuki, I.; Yuichiro, F.; Erry, P.; Muhammad, R.; Haruo, T. Greenhouse gas emissions from tropical peatlands of Kalimantan, Indonesia. Nutrient Cycling Agrosyst. 2005, 71, 73–80. [Google Scholar]





| Symbol | Description |
|---|---|
| H1 | Completely undecomposed peat. |
| H2 | Almost entirely undecomposed peat. |
| H3 | Very slightly decomposed peat. |
| H4 | Slightly decomposed peat. |
| H5 | Moderately decomposed peat. |
| H6 | Moderately highly decomposed peat. |
| H7 | Highly decomposed peat. |
| H8 | Very highly decomposed peat. |
| H9 | Practically fully decomposed peat. |
| H10 | Completely decomposed peat. |
| Season | Month | Sampling Time | |
|---|---|---|---|
| Dry | July 2015 August 2015 | Early Morning I | 06:00 a.m.to 06:35 a.m. |
| Afternoon | 12:00 p.m. to 12:35 p.m. | ||
| Evening | 18:00 p.m.to 18:35 p.m. | ||
| Midnight | 00:00 a.m. to 12:35 a.m. | ||
| Early Morning II | 06:00 a.m.to 06:35 a.m. | ||
| Wet | September 2005 December 2015 | Early Morning I | 06:00 a.m.to 06:35 a.m. |
| Afternoon | 12:00 p.m. to 12:35 p.m. | ||
| Evening | 18:00 p.m.to 18:35 p.m. | ||
| Midnight | 00:00 a.m. to 12:35 a.m. | ||
| Early Morning II | 06:00 a.m.to 06:35 a.m. | ||
| Soil Temperature | ||||
| Month | July 2015 | August 2015 | September 2015 | December 2015 |
| Variable | ||||
| Soil N2O emission | r = −0.04 p = 0.89 | r = −0.09 p = 0.76 | r = 0.15 p = 0.60 | r = −0.07 p = 0.82 |
| Soil Temperature | ||||
| Month | July 2015 | August 2015 | September 2015 | December 2015 |
| Variable | ||||
| Soil N2O emission | r = 0.14 p = 0.55 | r = −0.03 p = 0.89 | r = 0.27 p = 0.25 | r = 0.11 p = 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeffary, A.V.; Ahmed, O.H.; Heng, R.K.J.; Choo, L.N.L.K.; Omar, L.; Musah, A.A.; Abdu, A. Nitrous Oxide Emissions in Pineapple Cultivation on a Tropical Peat Soil. Sustainability 2021, 13, 4928. https://doi.org/10.3390/su13094928
Jeffary AV, Ahmed OH, Heng RKJ, Choo LNLK, Omar L, Musah AA, Abdu A. Nitrous Oxide Emissions in Pineapple Cultivation on a Tropical Peat Soil. Sustainability. 2021; 13(9):4928. https://doi.org/10.3390/su13094928
Chicago/Turabian StyleJeffary, Alicia Vanessa, Osumanu Haruna Ahmed, Roland Kueh Jui Heng, Liza Nuriati Lim Kim Choo, Latifah Omar, Adiza Alhassan Musah, and Arifin Abdu. 2021. "Nitrous Oxide Emissions in Pineapple Cultivation on a Tropical Peat Soil" Sustainability 13, no. 9: 4928. https://doi.org/10.3390/su13094928







