Scale Change and Correlation of Plant Functional Characteristics in the Desert Community of Ebinur Lake
Abstract
:1. Introduction
2. Materials and Methods
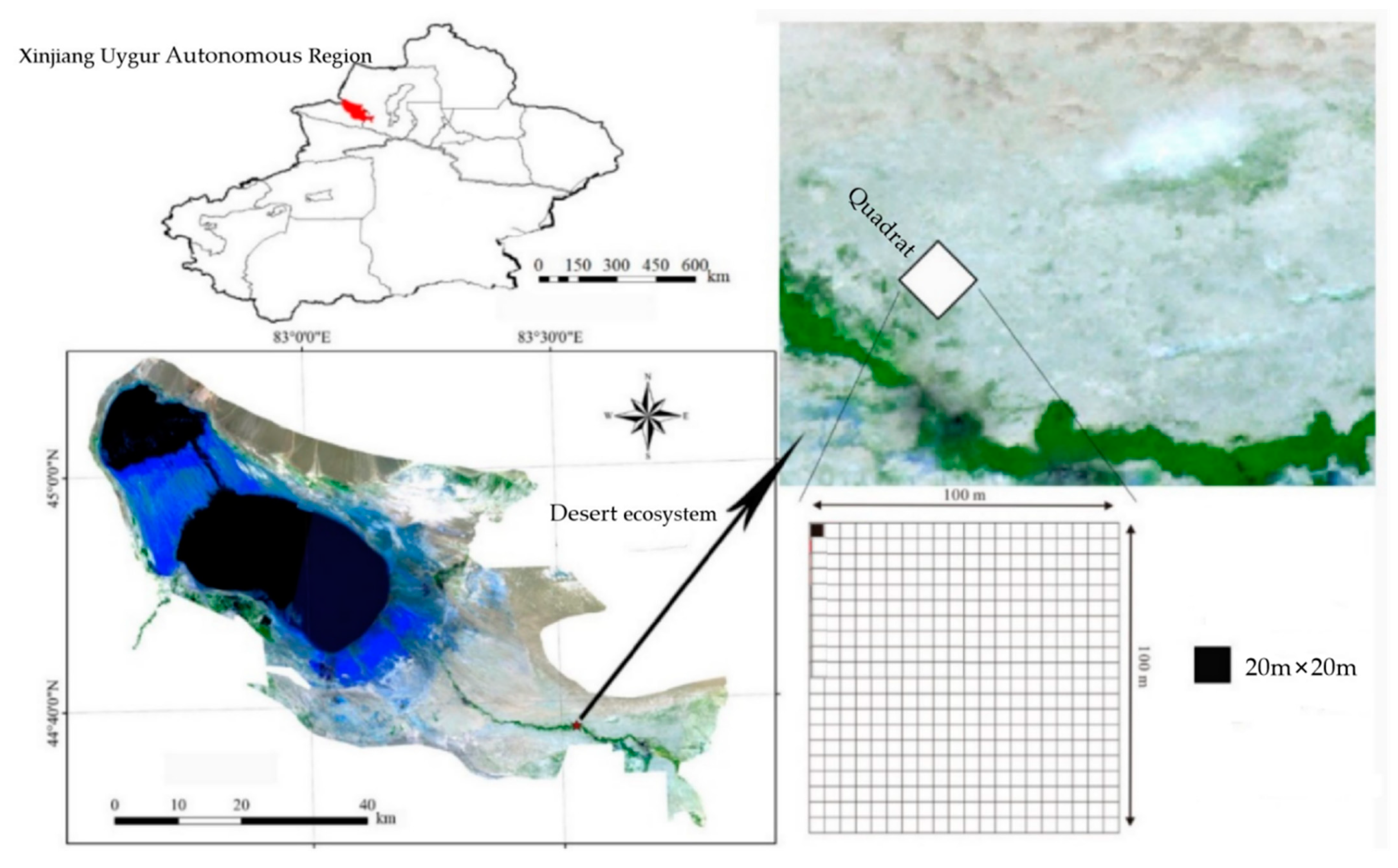
2.1. Sampling Site Setting and Investigation
2.1.1. Description of the Study Area
2.1.2. Plant Community Investigation
2.2. Statistical Analysis
2.2.1. Selection and Determination of Functional Traits
2.2.2. Trait-Gradient Analysis.
3. Result and Analysis
3.1. Differences in Plant Functional Traits of Various Species
3.2. Changes of Plant Functional Traits between and within Communities
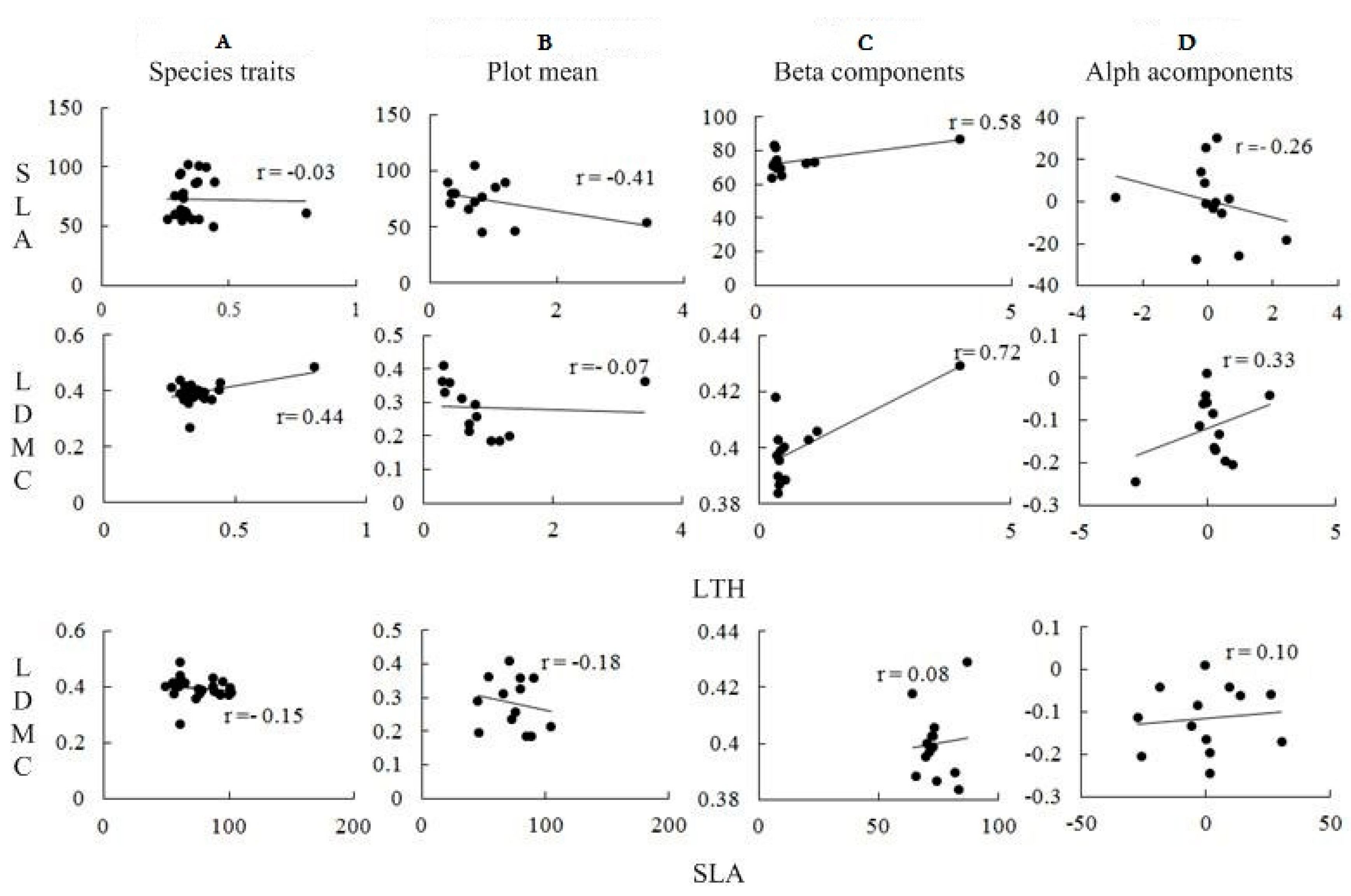
3.3. Correlation Analysis of Plant Functional Traits
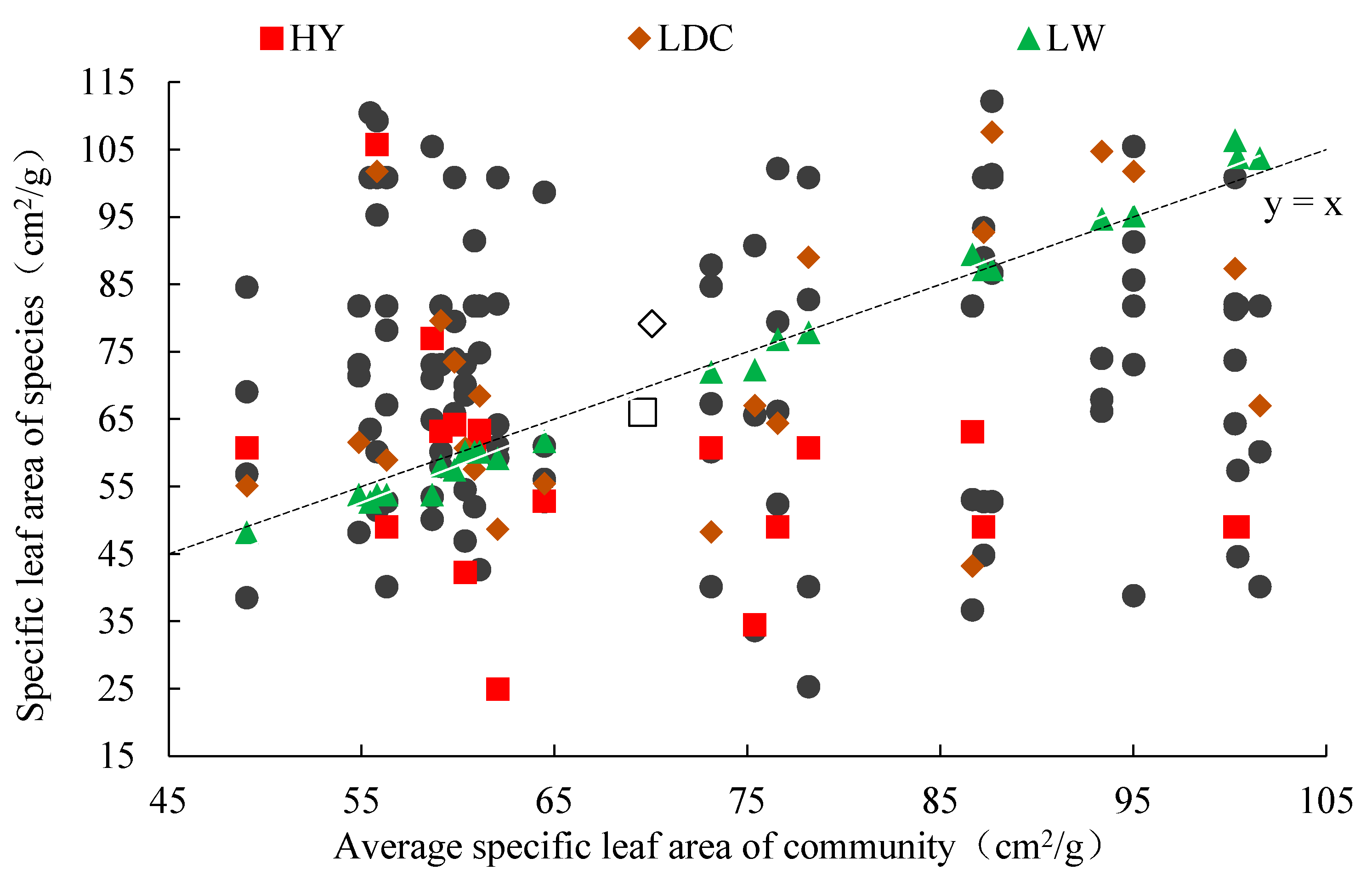
3.4. Sample Average Trait Value/Species Functional Traits and Correlations between α and β Components
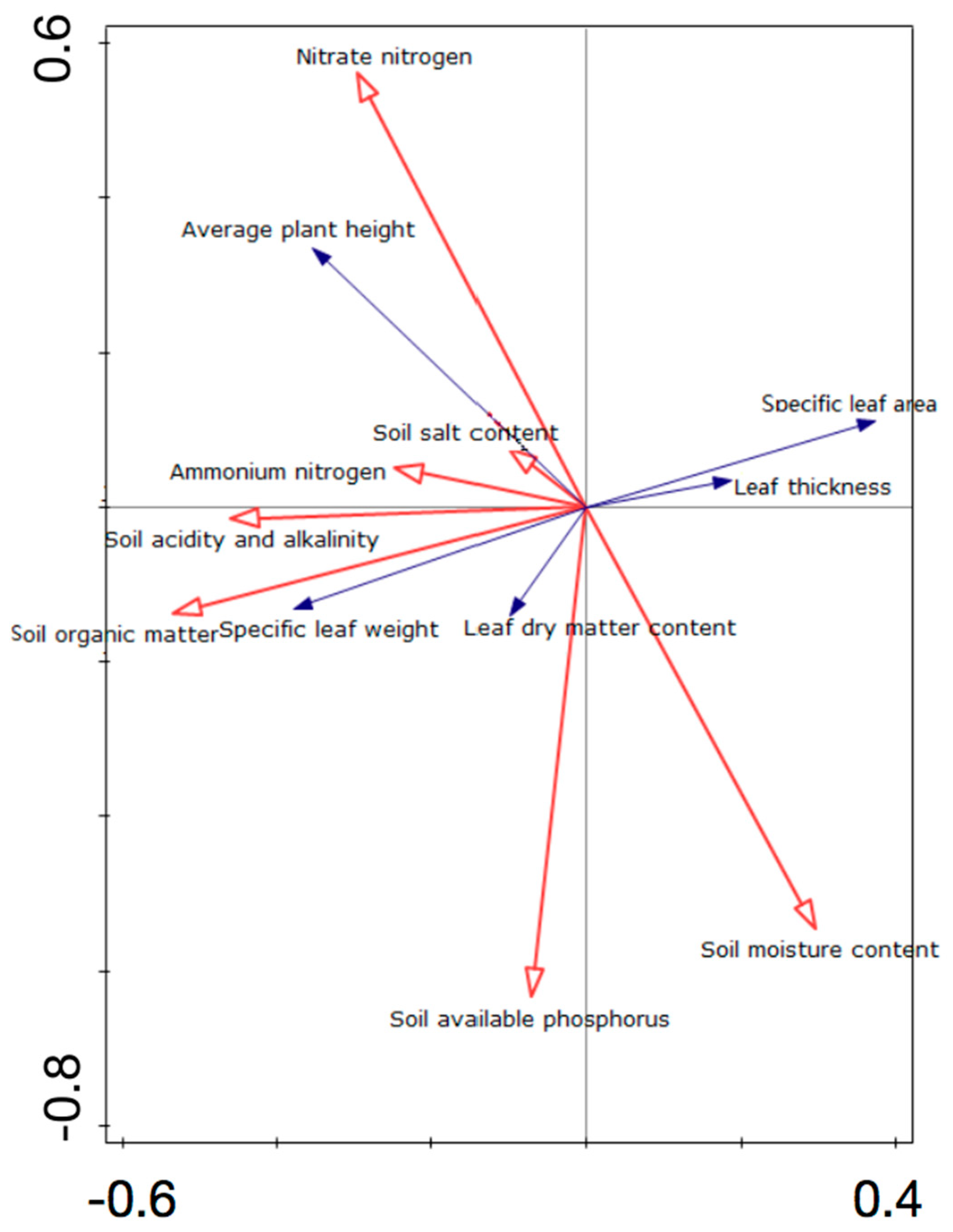
3.5. Redundancy Analysis (RDA) Ordination of Different Plant Functional Traits and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ficken, C.D.; Rooney, R.C. Linking plant conservatism scores to plant functional traits. Ecol. Indic. 2020, 115, 106376. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin, F.S.; Cornelissen, J.H.C.; Díaz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.-L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Chang. Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Valiente, J.A.; Ros, F.V.; Aranda, I. Exploring the impact of neutral evolution on intrapopulation genetic differentiation in functional traits in a long-lived plant. Tree Genet. Genomes 2014, 10, 1181–1190. [Google Scholar] [CrossRef]
- Marcisz, K.; Colombaroli, D.; Jassey, V.E.J.; Tinner, W.; Kołaczek, P.; Gałka, M.; Karpińska-Kołaczek, M.; Słowiński, M.; Lamentowicz, M. Can we predict the response of ecosystems to environmental change based on the traits of species in a long-time scales? A high-resolution palaeoecological study from two Sphagnum-dominated peatlands from Poland. In Proceedings of the International Symposium on Testate Amoebae, Ilhabela, Brazil, 12–14 September 2016. [Google Scholar]
- Schick, A.; Porembski, S.; Hobson, P.R.; Ibisch, P.L. Classification of key ecological attributes and stresses of biodiversity for ecosystem-based conservation assessments and management. Ecol. Complex. 2019, 38, 98–111. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, Z.M.; Miao, L.P. Source of variation of plant functional traits in the Yanhe river watershed:the influence of environment and phylogenetic background. Acta Ecol. Sin. 2013, 33, 6543–6552. [Google Scholar] [CrossRef]
- Xi, X.Q.; Zhao, Y.J.; Liu, Y.G.; Wang, X.; Gao, X.M. Variation and correlation of plant functional traits in karst area of central Guizhou Province, China. Chin. J. Plant Ecol. 2011, 35, 1000–1008. [Google Scholar] [CrossRef]
- Westoby, M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Ding, M.; Wen, Z.; Zheng, Y. Scale change and dependence of plant functional traits in hilly areas of the loess region, Shaanxi Province, China. Acta Ecol. Sin. 2014, 34, 2308–2315. [Google Scholar]
- Liu, R.; Liang, S.; Huang, D.; Huang, C.; Jiang, Y. Variation in functional traits of woody species across scales in a riparian zone of Lijiang River, Southwest China. Acta Ecol. Sin. 2019, 39, 8038–8047. [Google Scholar]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Soudant, A.; Boucher, F.; Saccone, P.; Lavorel, S. Intraspecific functional variability: Extent, structure and sources of variation. J. Ecol. 2010, 98, 604–613. [Google Scholar] [CrossRef]
- Ferrarini, A.; Dai, J.; Bai, Y.; Alatalo, J.M. Redefining the climate niche of plant species: A novel approach for realistic predictions of species distribution under climate change. Sci. Total Environ. 2019, 671, 1086–1093. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Martinez-Vilalta, J.; Retana, J. Intraspecific variability in functional traits matters: Case study of Scots pine. Oecologia 2014, 175, 1337–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Zak, M.R. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Gross, N.; Börger, L.; Soriano-Morales, S.I.; Le Bagousse-Pinguet, Y.; Quero, J.L.; García-Gómez, M.; Valencia-Gómez, E.; Maestre, F.T. Uncovering multiscale effects of aridity and biotic interactions on the functional structure of Mediterranean shrublands. J. Ecol. 2013, 101, 637–649. [Google Scholar] [CrossRef]
- Lippman, Z.B.; Semel, Y.; Zamir, D. An integrated view of quantitative trait variation using tomato interspecific introgression lines. Curr. Opin. Genet. Dev. 2007, 17, 545–552. [Google Scholar] [CrossRef]
- Tang, Q. Variation of Plant Functional Traits in Subtropical Evergreen Deciduous Broad-Leaved Mixed Forest; China Academy of Forestry Sciences: Beijing, China, 2016. [Google Scholar]
- Niu, K.; Liu, Y.; Shen, Z.; He, F.; Fang, J. Community assembly: The relative importance of neutral theory and niche theory. Biology 2009, 17, 579. [Google Scholar]
- Escudero, A.; Valladares, F. Rait-based plant ecology: Moving towards a unifying species coexistence theory. Oecologia 2016, 180, 919–922. [Google Scholar] [CrossRef]
- Ding, M. Component Decomposition and Scale Variation of Plant Functional Traits in Loess Hilly Region; Northwest A & F University: Yang Ling, China, 2014. [Google Scholar]
- Yan, Y.; Zhang, Q.; Buyantuev, A.; Liu, Q.; Niu, J. Plant functional β diversity is an important mediator of effects of aridity on soil multifunctionality. Sci. Total. Environ. 2020, 726, 138529. [Google Scholar] [CrossRef]
- Hill, M.J.; Heino, J.; White, J.C.; Ryves, D.B.; Wood, P.J. Environmental factors are primary determinants of different facets of pond macroinvertebrate alpha and beta diversity in a human-modified landscape. Biol. Conserv. 2019, 237, 348–357. [Google Scholar] [CrossRef]
- Qie, Y.; Jiang, L.; LV, G.; Yang, X.; Teng, D.; Wang, H. Response of leaf functional traits of temperate desert plants to soil water and salt. Acta Ecol. Sin. 2018, 27, 2000–2010. [Google Scholar]
- M’hammed, B.; Souad, N.; Haroun, C. Vegetation traits are accurate indicators of how do plants beat the heat in drylands: Diversity and functional traits of vegetation associated with water towers in the Sahara Desert. Ecol. Indicators 2020, 114, 106364. [Google Scholar]
- Ulrich, W.; Zaplata, M.K.; Winter, S.; Fischer, A. Spatial distribution of functional traits indicates small scale habitat filtering during early plant succession. Perspect. Plant Ecol. Evol. Syst. 2017, 28, 58–66. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; He, N.; Ye, Z.; Chen, C.; Zang, R.; Feng, Y.; Lu, Q.; Li, J. Plant functional traits regulate soil bacterial diversity across temperate deserts. Sci. Total Environ. 2020, 715, 136976. [Google Scholar] [CrossRef]
- Hou, J.; Zhu, H.; Fu, B.; Lu, Y.; Zhou, J. Functional traits explain seasonal variation effects of plant communities on soil erosion in semiarid grasslands in the Loess Plateau of China. Catena 2020, 194, 104743. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Chen, L.; Mo, B. Response of deep soil moisture to land use and afforestation in the semi-arid Loess Plateau, China. J. Hydrol. 2012, 475, 111–122. [Google Scholar] [CrossRef]
- Gong, X.; Lv, G. Diversity of desert plant communities and niche of dominant species in dugaylin desert of Ebinur Lake Basin. Biodiversity 2017, 25, 34–45. [Google Scholar]
- He, X.; Qin, L.; Lv, G.; Yang, J.; Gong, Y.; Yang, X. Surface energy budget characteristics of wetland in arid desert area of Ebinur Lake Basin in Xinjiang. J. Ecol. 2017, 36, 309–317. [Google Scholar]
- Zhang, X.; Li, Y.; He, X.; Lv, G. Effects of water and salt changes on the relationship between functional diversity and species diversity of desert plants. J. Ecol. 2019, 38, 2354–2360. [Google Scholar]
- Zhang, X.; Li, Y.; He, X.; Lv, G. Response of desert plant functional traits and diversity to soil water and salt changes. Acta Zool. Sin. 2019, 39, 1541–1550. [Google Scholar]
- Song, L. Application of Chlorophyll Fluorescence Kinetics in Researches on Plant Stress Resistance. J. Sichuan For. Sci. Technol. 2013, 34, 69–71. [Google Scholar]
- Jin, T. Study on Drought Adaptability of Artificial Robinia Pseudoacacia Forest in Northern Shaanxi; Graduate School of Chinese Academy of Sciences: Beijing, China, 2011. [Google Scholar]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 2010, 143, 155–162. [Google Scholar] [CrossRef]
- Britz, S.J.; Adamse, P. Uv-B-induced increase in specific leaf weight of cucumber as a consequence of increased starch content. Photochem. Photobiol. 2010, 60, 116–119. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Ackerly, D.D. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 2009, 79, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Ackerly, D.D.; Cornwell, W.K. A trait-based approach to community assembly: Partitioning of species trait values into within- and among-community components. Ecol. Lett. 2010, 10, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.I.N.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, R. Variation of Functional Traits of Woody Plants in the Water Land Ecotone of Lijiang River; Guangxi Normal University: Guilin, China, 2018. [Google Scholar]
- Gong, S.; Wen, Z.; Shi, Y. Response of plant community functional traits to environmental gradient in Yanhe River Basin. Acta Zool. Sin. 2011, 31, 6088–6097. [Google Scholar]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant Ecological Strategies: Some Leading Dimensions of Variation Between Species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Marika, M.; Matty, P.; Berg, I.; Tanya, H. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 2012, 15, 1033–1041. [Google Scholar]
- Pan, Y.; Chen, X.; Jiang, Y.; Shi, C.; Lu, Z.; Huang, Y.; Ni, M.; Qin, C.; Liu, R. Response of leaf functional traits and soil factors to slope aspect in Karst Rocky Mountain of Guilin. Acta Zool. Sin. 2018, 38, 1581–1589. [Google Scholar]
- Anke, K.; Kleyer, M. A parsimonious combination of functional traits predicting plant response to disturbance and soil fertility. J. Veg. Sci. 2010, 19, 681–692. [Google Scholar]
- Dang, H. Study on Multi-Scale Construction Mechanism of Desert Community Based on Characters in Arid Area; Northwest University: Xi’an, China, 2018. [Google Scholar]
- Bestelmeyer, B.T.; Trujillo, D.A.; Tugel, A.J.; Havstad, K.M. A multi-scale classification of vegetation dynamics in arid lands: What is the right scale for models, monitoring, and restoration? J. Arid. Environ. 2006, 65, 296–318. [Google Scholar] [CrossRef]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Xiong, M. Association and Differentiation of Plant Functional Traits in Tropical Cloud Forest in Hainan; Hainan University: Hainan, China, 2015. [Google Scholar]
- Weiher, E.; Freund, D.; Bunton, T.; Stefanski, A.; Lee, T.; Bentivenga, S. Advances, challenges and a developing synthesis of ecological community assembly theory. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2403–2413. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional Traits and Niche-Based Tree Community Assembly in an Amazonian Forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, V.; Violle, C.; Mondy, C.P.; Hoffmann, L.; Muller, S.D. Intraspecific variability and trait-based community assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]


| Index | Definition | Ecological Significance |
|---|---|---|
| Plant height (H) Leaf thickness (LTH) | Refers to the distance from the root neck to the top of the main stem. Thickness of plant leaves in the direction perpendicular to the main nerve surface. | Reflects the plant’s ability to compete for light [34]. Reflects plant resource acquisition and water conservation [35]. |
| Leaf area (SLA) | The ratio of a leaf’s one-sided area to its dry weight. Within the same individual or community, generally plants growing in weaker light have a larger leaf area; the SLA can be used as an index of leaf shading. | Reflects the plant’s ability to obtain resources, and the plant growth and photosynthetic rates [36]. |
| Specific leaf weight (LMA) | Refers to the leaf weight (dry or fresh weight) per SLA, but is usually expressed as dry weight. | Closely related to plant growth and resistance to stress [37]. |
| Leaf dry matter content (LDMC) | Dry leaf weight/saturated fresh leaf weight, or the ratio of dry matter to fresh leaf weight. | Reflects the plant’s ability to obtain resources and resist physical damage [36]. |
| Parameter | Characteristics | ||||
|---|---|---|---|---|---|
| Plant LEAF Thickness (mm) | Average Plant Height (cm) | Heavier than Leaf (g/cm2) | Specific Leaf Area (cm2/g) | Leaf Dry Matter Content (g/kg) | |
| Species characteristic | |||||
| ti, mean | 0.93 | 110.97 | 0.02 | 73.64 | 0.28 |
| ti, min–max | 0.29–3.43 | 4.00–542.63 | 0.01–0.02 | 45.45–104.57 | 0.18–0.41 |
| β, min–max | 0.33–4.01 | 75.60–102.87 | 0.01–0.02 | 64.24–87.23 | 0.38–0.43 |
| ai, min–max | −2.83–2.44 | 97.20–449.52 | −0.01–0.01 | −27.61–30.16 | −0.24–0.04 |
| bi, mean | 0.16 | −0.17 | 0.11 | 0.1 | 0.16 |
| bi, min–max | −4.1 | −5.48–2.71 | −0.92–1.09 | 1.18–1.57 | −1.21–1.03 |
| Community characteristics | |||||
| pj, mean | 0.51 | 95.27 | 0.01 | 72.53 | 0.4 |
| pj, min–max | 0.26–4.01 | 72.13–126.49 | 0.01–0.02 | 49–101.55 | 0.27–0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lv, G.; Jiang, L.; Wang, J. Scale Change and Correlation of Plant Functional Characteristics in the Desert Community of Ebinur Lake. Sustainability 2021, 13, 4983. https://doi.org/10.3390/su13094983
Li H, Lv G, Jiang L, Wang J. Scale Change and Correlation of Plant Functional Characteristics in the Desert Community of Ebinur Lake. Sustainability. 2021; 13(9):4983. https://doi.org/10.3390/su13094983
Chicago/Turabian StyleLi, Hanpeng, Guanghui Lv, Lamei Jiang, and Jinlong Wang. 2021. "Scale Change and Correlation of Plant Functional Characteristics in the Desert Community of Ebinur Lake" Sustainability 13, no. 9: 4983. https://doi.org/10.3390/su13094983
APA StyleLi, H., Lv, G., Jiang, L., & Wang, J. (2021). Scale Change and Correlation of Plant Functional Characteristics in the Desert Community of Ebinur Lake. Sustainability, 13(9), 4983. https://doi.org/10.3390/su13094983




