Abstract
Currently, setting the obligation to use respiratory protective equipment with a level of protection of at least FFP2 in public transport and commercial and shopping centers are considered within the European Union. Many new products appeared on the European market within these specifications, and many symbols have been used. The paper deals with the meaning of selected respiratory protection based on respirators symbols and markings (which appeared massively, especially after the pandemic situation in the spring of 2020), these symbols not being uniformly understood and well communicated. We also mention and discuss some of the problems related to setting the conditions of public procurement, which affect respirators. Thus, this review is an “informative guide” in facilitating the understanding and use of full knowledge of the most appropriate respiratory protective devices in various situations.
1. Introduction
A very frequent and partly mysterious topic of the recent past, present, and certainly the near future, are the problems associated with the spread of a new type of coronavirus, namely SARS-CoV-2, which causes COVID-19. Coronavirus is the name for four genera of viruses of the subfamily Coronavirinae (namely, Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus). The name of this virus was derived from the characteristic arrangement of the surface structures of the lipid envelope, which strikingly resembles the shape of the solar corona [1,2].
Regarding human protection against this virus, hygiene of the hands and wearing a mask are recommended. Asia has a significant advantage over Europe in using masks. People have been using mouth coverings and respirators for many years. An important milestone in their behavior is the SARS epidemic [3]. However, the inhabitants of Asian cities also wear these types of protective equipment as protection against air pollution. Therefore, in addition to medical masks, Asian manufacturers also offer aids (respirators) designed against smog and dust. However, different properties are expected from each such aid [4].
Currently, mainly Chinese or Vietnamese protective equipment is supplied on the European market in bulk. These are products of all possible categories. It is the existence of various types of protective equipment, together with the lack of experience of customers and retailers, that contribute to the current opacity of the respirator market. It was not about any details, but about fundamental problems concerning especially the designation of individual types of respirators [5].
Some people work in an area where it is compulsory to ensure proper respiratory protection. Qualitatively speaking, existing sectors are associated with the need to protect against the effects of biological or chemical contamination [6]. Coronavirus most often affects the mucous membranes of the upper and lower respiratory tract. The incubation period is reported to be 7–14 days. One week after the infection, the manifestations of the disease are fevers that do not subside. They may be accompanied by discomfort, joint and muscle pain, and signs of coughing soon [7].
The symptoms of coronavirus infection are very similar to those of the common flu. There is a dry cough, difficulty breathing, fever and tiredness. This way, the disease can manifest itself for up to 14 days from the moment the virus enters the human body. Severe pneumonia threatens only minimally. The most at-risk group are the elderly, who are in poor health, similar to the flu [8]. Men are more often infected with coronavirus than women. Most people who succumbed to the virus were 60–89 years old [9]. However, the disease is usually only mild, but the possibility of death of the victim should not be underestimated. The virus gradually disappears on its own. Not only in political, personal, and private discussions, and especially in media, many half-truths and professionally inaccurate or misleading information about using filtering half masks (respirators) and face masks (medical and nonmedical masks) have recently appeared, as a result, of which not only professional but also the general public asks many legitimate questions.
At present, many European countries have introduced or are considering introducing the obligation to wear filtering facepiece (FFP) respirators in public transport or in department stores and shopping centers. This situation calls for a clear explanation of what type of respirator will be appropriate. At the same time, it is important to compare to what extent the purchase price will provide the customer with an adequate level of protection.
For a basic understanding of the problem, we state that respirators and medical masks differ primarily regarding their intended use [10]. Respirators are primarily intended to protect the respiratory system of their user from airborne particles in the vicinity—microorganisms (bacteria, viruses, mold spores), fine dust, and toxic solid or liquid particles. On the other hand, medical masks serve mainly to prevent the spread of particles from their users to the environment. Simply, respirators prevent the penetration of microorganisms from the outside in, and thus protect the user primarily. In contrast, medical masks prevent the penetration of microorganisms from the inside out and thus primarily protect the surroundings (infected or potentially infected) of the user [11,12].
Filter half-masks for respiratory protection, which are simply called respirators, are subject to various regulatory standards worldwide. These standards specify certain required physical properties and performance characteristics for respirators to comply with a particular standard or norm [13,14].
There are 9 categories of particulate air respirator classification considered by the US National Institute for Occupational Health and Safety (NIOSH), as follows: N (95, 99, and 100), P (95, 99, and 100), and R (95, 99 and 100). The significance of the letters is:
- N—“not oil resistant” (i.e., those respirators, which are not usable in the environment containing oil droplets);
- R—”somewhat resistant to oil”;
- P—“strongly resistant to oil” (i.e., those respirators that are usable as protection against oily/non-oily aerosols).
The numerical values 95, 99 and 100 show the minimum filtering efficiency, expressed in percentages, respectively 95, 99 and 99.97% (in the case of numerical value 100) [15].
FFRs are classified according to the European standard (EN 149:2001) into 3 classes: FFP1 (minimum filtration efficiencies/yield of 80%), FFP2 (yield 94%) and FFP3 (yield 99%) [14]. It can be observed that FFP2 respirators are almost equivalent to N95 FFRs, thus becoming recommended (both in the US and in other countries) in the prevention of airborne infectious diseases. In the UK, however, FFP3 respirators are the only acceptable FFP class for the executive and safety and health (HSE), offering the highest level of protection against infectious aerosols [16].
During pandemics or emergencies, government institutions, health authorities, and manufacturers often refer to these standards when issuing recommendations for respirators, stating, for example, that a section of the population should use an “N95, FFP2 or another equivalent” respirator [17]. In everyday life, however, it turns out that not everyone is familiar with the information provided. In practice, this means that most of the general population cannot correctly distinguish and determine what specific level of protection provided is associated with the relevant product designation and standard (norm) according to which it was certified and marketed. The paper deals with the meaning of selected respiratory protection based on respirators symbols and markings, which appeared massively, especially after the pandemic situation in the spring months of 2020. These symbols are not uniformly understood and well communicated.
In the actual pandemic frame and circumstances, the “mask” has been implemented as a widely used keyword, a psychological impact symbol, and a mandatory accessory. Considering the medical staff, who used this “object” before the pandemic context as a component part of the protection/work equipment in the everyday hospital activity (and even more so by those who now work in the forefront of public health), there are still some pertinent questions regarding the real protection it offers, the optimal type of mask for the general population, as well as the need to wear it (where when by whom).
This narrative review elaborately discusses the type of masks, their importance, their acceptability in the different regions, especially in the context of the Czech Republic and provides comparative evaluation between the different trademarks or regional marking based on their material, components, their importance in different settings. The information was taken, synthesized and structured from numerous specialized articles, technical standards and recommendations of the World Health Organization (WHO), as well as of other specialized European and International institutions. Correlations between data and the discussions presented in this review are valuable for healthcare workers, the administrative and public health systems, and the general public.
3. Electrostatic Versus Mechanical Filtration
In the filtration system of respirators for the face mask, electrostatic media are often used [30]. It recommends for this purpose the increased permissibility for respiration, the low economic cost, and the high filtration efficiency [31]. Electrostatic filter media contain fibers that form the so-called polarity concentration zones, corresponding to each electrical charge. This charge/polarity is developed during the process of the web (tribocharging—when different fibers are rubbing together like resin wool) and fiber formation in a corona charge [31]
The basic problem with conventional respirators is that their functionality is based on electrostatic filtration. Upon contact with liquid or air humidity, the charge of the filter discharges quickly, and its efficiency is significantly reduced. To keep the user protected, the respirator must be removed from the face after approximately 30 min [32].
When the filter dries, the effectiveness will increase again. It is well-known that a person wearing a respirator is not encouraged to continue wearing a sweat-saturated facial mask filter, simply because the filtration theory states that penetration through the filtration medium is potentiated by moisture, respectively when the fibers are wet, and the empty spaces are filled with liquid [33]. Some manufacturers solve this problem by adding a layer of activated carbon, which absorbs moisture and thus extends the life and efficiency of the filter. However, in an effort not to devalue their goods in the eyes of customers, they often prefer to give other reasons for using carbon in the form of activated carbon [34].
On the other hand, the efficiency of nanofiber filters is based on the mechanical principle. It is considered that the term nanofibers refers to those fibers that have a diameter < 1 micron [35]. This type of extremely fine fibers causes significant increases in filtration efficiency at rather small (even immeasurable) decreases in permeability. Data published as research results as well as case studies prove an increased channel life of the nanofiber channel media and also an enhanced contaminate holding capacity [36]. Some of the most important qualities found in the nature of the materials from which nanofibers are made can be exemplified as follows: porosity, nanofiber width, as well as the homogeneity of nanofiber layers. Moreover, nowadays, eliminating microscopic organisms, disordered particles, and infectious pathogens from both air and water are correlated with the continuously increasing number of respiratory diseases (especially in crowded industrial centers) and also with the threats of various pandemics [37].
Therefore, they work well even in contact with air humidity. It should be considered that the human breath itself contains water vapors and is thus wet. In the scope of a conventional respirator, this means that a person reduces the level of protection through the normal process of putting the respirator on and breathing. Nanofibers, on the other hand, keep the filtration at a high level due to the density of the material. As far as nanofiber respirators are concerned, the filtration efficiency is even increased because the filter is gradually clogged with trapped particles. This process is not quick enough to reduce breathability, considering as well that air molecules size is approximately 300× < than the size of the SARS-CoV-2 virus; implicitly, it can be concluded that air circulation will be the last to suffer [38].
The negative effect of air humidity can be manifested, especially in winter when water can freeze on the surface of nanofibers and thus clog the active pores. Due to this process, the loss of filtration efficiency can occur. Some types of nanofibers can also swell due to the humidity of the air. This increases their volume and thus increases the pressure loss. In this case, however, there will be no loss of filtration efficiency because the number of pores of the nanofibers will be kept at the same level [39]. Published data show as extremely promising those filters made of nanofibers having their diameter < 1 μm and with an increased specific surface area [40,41].
Fine fibers in size of 1–8 microns can be obtained in a unique process of spinning polymer fibers, quenching—drawing the air and forming the web. The process is known as melt-blowing (MB) and is considered one of the nonwoven commercial technologies [42]. During this process, the polymer is fed/inserted into an extruder to be melted and then pushed through a filter to finally reach the spinning head. By using high-speed hot air, at this moment, the melt is drawn into filaments and forms (on the webformer) nonwovens [43], having fiber with variable diameters (1–10 µm) and an average diameter of 1–2 µm. MB fabric is well-known for the high barrier properties and for its specific high surface area/unit of weight [43]. Over time, many commercial technologies have been developed to obtain ultra-fine nonwoven materials by melting, trying various techniques to produce finer fibers. For this purpose, a 2009 study used ultrasonic waves in MB, obtaining the reduction of the diameter of prepared bicomponent polyethylene terephthalate (PET) + polyamide (PA) 6 melt-blown webs to 2.11 µm (from 3.62 µm) [44]. In the last decade, innovative mold configurations were tested and verified, as well as the optimal processing conditions to decrease the fiber size to 300–500 nm. Furthermore, several new techniques/methods have been initiated, developed and published to optimize the characteristics, added value and performance given by using these MB fibers [44,45].
Results obtained proved that the reusability of MB filter (which contains nanofiber filter and belongs to the N95 face mask); some parameters that must be considered and mentioned here are as follows: airflow rate, filtration efficiency, specific surface, morphological properties, etc., all of them being evaluated after two different types of cleaning treatments [45]. Compared to the NF filter, which retains its good filtration efficiency, the MB filtering performance decreases after the treatment with ethyl alcohol. Therefore, it can be concluded that the NF filter can be reused and is good enough even after its treatment. This fact can result in a viable solution in solving the current shortage of face masks in many countries, also increasing the safety of medical or auxiliary staff directly involved in contact with coronavirus-infected patients [46,47].
If the user wants to protect himself against viruses with a conventional respirator, which uses polypropylene electrostatic melt-blown as a filter, it would be necessary to choose a product of FFP3 class, certified according to European standard EN149:20001 + A1:2009 [14].
However, it should be emphasized that respirators are manufactured primarily as personal protective equipment. For this reason, they are tested mainly for the capture of solid or liquid particles and not microorganisms. Their use with (against) viruses was not considered. Due to this, EU standards do not require such functionality from them.
The fact that a conventional FFP3 respirator also protects against viruses is rather a “wish” based on the following assumption: If the product catches paraffin oil, one of the tested substances, it does not release the virus during the tests. In this context, it can be inferred that besides capturing the oil fluid, the filter also retains microorganisms. They move through the air in droplets caused by coughing, talking, and breathing. In practice, most FFP3 respirators retain virus due to the density of the melt-blown layers. However, this is realized at the expense of breathability, in other words, at the expense of increasing the pressure drop, as the filter must be disproportionately strong and dense [48].
To breathe as normally as possible during common activities through respirators of classes FFP2 and 3, they are usually equipped with a so-called exhalation valve, which is designed and realized to facilitate the evacuation of accumulated gases inside the face mask [49].
When used with (against) viruses and bacteria, it becomes an Achilles’ heel. Pathogens can get inside through it in a few tens of minutes or a few hours. The function of the valve is not to filter but to remove air humidity.
By using a valve, the respirator is kept dry and thus contributes to prolonging the efficiency of the electrostatic filtration. At the same time, it increases the user’s comfort. It is true that the higher the class of protective devices without a valve, the harder it is to breathe through it. The valve also helps to release warm air from the inside area of the respirator and is, therefore, used primarily in operations with hot and humid environments, such as smelters or glassworks [21].
The FFP3 respirator with an exhalation valve serves well to protect against fine solid particles (dust, smog, or pollen) that should not enter the user’s airways. However, being used as protective work equipment, they are not designed to protect the environment from being contaminated by the user. At first sight, this may be a negligible detail. However, it is a serious problem with their concomitant use with (against) coronavirus [10,11,12,13].
It is certainly no coincidence that the relevant standards define a medical protective device (mouth coverings) as a tool that is primarily intended to protect others from its wearer. Therefore, doctors and other hospital staff use mouth coverings in order not to spread their own droplets among patients. They are used for prevention in cases when the healthcare provider is ill, not being aware of that fact, meaning that he does not have symptoms yet. In fact, however, he can already be infectious and thus endanger people with often weakened immunity [50].
Nonetheless, even respirators without an exhalation valve have many disadvantages. They are not very breathable (difficult to breathe), they are not user-friendly (due to weight, stiffness, and inflexibility), they are difficult to store due to their dimensions, and their price is relatively high. In contrast, nanofiber breathing protective equipment can trap particles mechanically. Moreover, they work on the principle of a miniature sieve, up to the size of micrometer units, being cheaper than FFP3 class respirators and demonstrating their effectiveness regardless of humidity. Due to the extreme density of nanofibers, the filter can be much thinner than with melt-blown, so it is much easier to breathe through nanofiber masks. Therefore, they do not need a risky exhalation valve, which guarantees higher safety and reliability [12,13,14].
The general public is often asked why nanofiber masks do not have the FFP3 class, although they filter particles and microorganisms more efficiently than respirators using in a working environment. The reason is quite simple. Due to the mentioned thinness of the nanofiber filter, test paraffin oil penetrates through it. This protective work equipment has a shortcoming; it does not matter in virus protection. For this reason, the FFP2 category is sufficient for nanofiber products, in this case, supplemented by separately performed virus and bacterial detection tests. The discrepancy between the effectiveness of nanofibers and the officially lower protection class FFP also stems from the obsolescence of standards. They were created several decades ago when nanofibrous materials had not yet been worked on. Standards, therefore, do not take sufficient account of new materials and technologies that better combine efficiency with user comfort.
4. Specifications of Respirator Standards
There are many products of the respirator type on the market that are differently labeled in terms of respirator standards (Figure 1). Respirator standards, the labeling of products of the respirator type, are based on various norms and standards according to which they are certified, and, based on their conformity, they are placed on the market. Their overview is given in Table 2 [11,19,47,51,52].

Figure 1.
Example of respirator standard labeling according to different standards; (a) designation N95; (b) designation FFP2; (c) N100; (d) FFP3.

Table 2.
Specifications of respirator standards and corresponding norms (standards).
The basic differences of the above standards are given in Table 3 and Table 4. The indication of the filtration capacity (filtration efficiency of the material or the filter’s efficiency) means how much x% of all particles having a diameter of 0.3 µm or more is retained [14,24,29,47,53,54]. The same applies to data on the overall effectiveness of protection.

Table 3.
Overall, minimum filtering protection efficiency and respirator filtering capacity standards.

Table 4.
Comparison of filtering efficiency (expressed as a percentage) with respirator labeling standards according to the norms.
Respiratory resistance data are also essential information about the quality of the respirator. The respiratory resistance of the respirator is tested by inhalation and exhalation [52,53]. Respiratory breathing resistance of respirators must not exceed the established inhalation values [14,47,54] given in Table 5. The maximum permissible resistance exhalation within 160 L/min is 3.0/300 mbar/Pa.

Table 5.
FFPs classification according to their maximum permissible resistance data for inhalation.
From a regulatory point of view, respirators in the European Union (EU) are ordered among the so-called personal protective equipment of category III according to the European Regulation 2016/425 on personal protective equipment [55]. In the case of respirators that provide two-way protection, meaning those that protect not only the person wearing it but also the user’s surroundings, it is possible to apply the new Regulation (EU) 2017/745 on medical devices [56]. In the detailed study of Regulation (EU) 2016/425 of the European Parliament and of the Council on personal protective equipment and European standard EN 149 + A1 Respiratory protective devices—Filtering half masks to protect against particulates—Requirements, testing and marking [57] related to the testing of filtering half masks—respirators are mentioned, but many very interesting pieces of information can be found in Section 3.
There is also the possibility that respirators of the EN 149:2001 + A1:2009 classification marked FFP2 have been designed to protect against the effects of coronavirus, a virus called SARS CoV-2 [14,54]. During the state of emergency declared by the Czech Republic government in the spring of 2020 and subsequently in the autumn, there was a notified body (notified person), which in the conditions of the Czech Republic is usually represented by the Occupational Safety Research Institute (number 1204) for the respirators assessment. Certificates released in the spring were usually valid for three months. These certificates were not EU certificates proving examination according to Regulation (EU) 2016/425, module B [55]. For the certified product, only selected properties related to its protective function were verified; based on these properties, it can be stated that they ensure an adequate level of health and safety protection following the essential requirements set out in Regulation (EU) 2016/425 [55]. The notified body (notified person) mentions on the certificates that their validity is limited to three months from the date of release and that the Occupational Safety Research Institute must be contacted at the latest after its expiry, and the remaining tests should be completed [58]. The respirator tested in this way was labeled, for example, as follows: BreaSAFE® ANTI-COVID-19/FFP2 [57]. In practice, this means that FFP2-certified respirators can be used in environments where harmful and mutagenic substances move in the air. Nanomaterials used for respirator production are nanomembranes, which capture at least 94% of all solid particles in the air up to 0.6 μm [59,60,61,62]. Respirators marked in this way have a measured uptake of 98.5%, which means that BreaSAFE ANTI COVID respirators also have a tested uptake of viruses and bacteria of 97–99% [63].
To follow European rules, the respirator must be certified by a “testing laboratory” or otherwise notified body before being placed on the European market [64]. The manufacturer must mark the respirator with the standard number, EN 149 + A1, whereas, for older products, only EN 149 was indicated. In addition, the manufacturer must indicate the protection class and affix the CE marking to the product, together with the four-digit number of the notified body (notified person), which regularly inspects the product [65]. The respirator must also be marked NR if it is intended for single-use, for about 8 h, or R for repeated use. The respirator must also be accompanied by instructions, which must be included for each product.
For respirators, the filtration efficiency of the material (Table 3), from which the respirator is made, is tested. Data on inhalation and exhalation resistance are also essential (Table 5). The basic problem is that a material that filters well and thus absorbs aerosols of pollutants very well is not very breathable in terms of respiratory resistance (significant pressure loss). This problem often occurs with filter materials of nanofiber sorption materials. The tests also verify the CO2 concentration in the under-mask space. There must be no respiratory effect. It results that in the case of respirators, it is not sufficient to demonstrate the filtration effect of the material from which they are made. It is possible that respirators that do not have all the necessary properties are on the market or are entering the market.
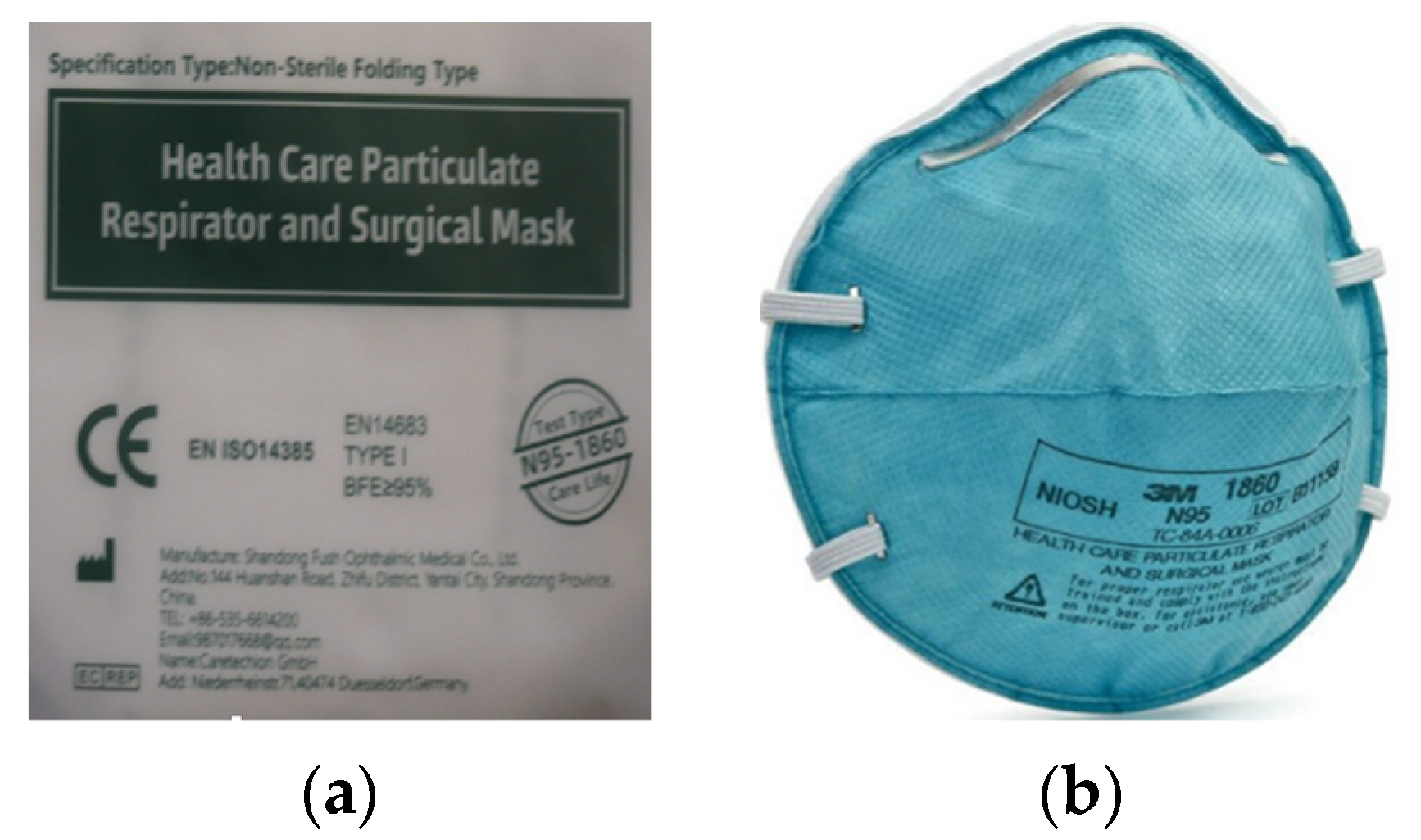
The relatively confusing situation with respirators labeling was further complicated by the supply of respirators, which were subsequently distributed to selected groups of the population from the Czech Republic in the autumn of 2020 (Figure 2a).
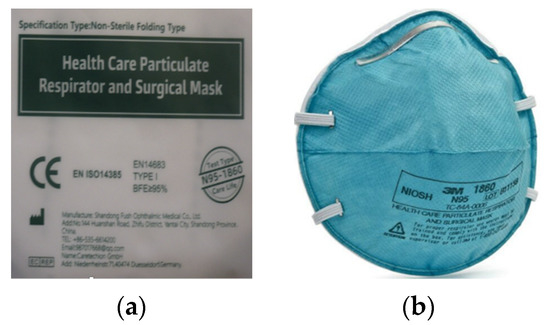
Figure 2.
Examples of respirator information and labeling: (a) respirator sent to selected groups of the population of the Czech Republic in the autumn of 2020; (b) specification of the designation “Test type N95-1860”.
This particular respirator (Figure 2a) cannot be considered a respirator but a mask or a surgical mask. This statement is based on the text in Figure 2a, which states EN 14683 + AC medical face masks—requirements and test methods. This standard applies to masks that belong to the category “medical devices” and, therefore, does not deal with assessing the quality of respirators at all. The fact that it is primarily a mask can be inferred from the “BFE”. When assessing medical masks according to EN 14683 + AC, the test includes a bacterial filtration efficiency (BFE) test, a breathability test and a splash resistance test. Furthermore, this standard stipulates the test of microbial purity according to the standard EN ISO 11737-1: 2018 and evaluating the biocompatibility of the material according to EN ISO 10993-1: 2009. Furthermore, the respirator in Figure 2a was not provided with information in Czech and did not bear the correct marking of conformity with European CE standards. The CE letters on the respirator packaging are listed but most likely expressed as the “China Export” mark. It is very similar and very easily interchangeable with the CE mark as indicated by the notified body (notified person). The EN 14385 standard, which sets requirements for stationary sources of air pollution, was also incorrectly marked. The respirator also lacks the designation NR or R, and therefore, it is not clear whether it is intended for single or repeated use. Figure 2a at the bottom right shows the text “Test type N95-1860”, which, when compared to the text shown in Figure 2b, refers to the method of testing performed according to the American standard defined by the NIOSH-42CFR84 corresponding to the N95 respirator standard. Based on this information, it can be concluded that the breathability was tested and not the mask [47,51,52,53,54,55,56,57].
It can, therefore, be said that this example can very easily be included in the category “How not to be confused when buying or choosing a respirator”. In this case, the contracting authority has been confused with the entire evaluation procedure, which is relatively significant for the state contract. To these problems, we add that the usage of a mask contributes to the protection of the wearer, but the manufacturer does not guarantee this protection. The problem with masks lies in the insufficient sealing line, as air penetrates around the nose and face. This sometimes undermines the good filtration efficiency of the used material. Tests do not verify the breathability of the material. It should be noted that although no standardized testing is required for homemade masks and other temporary respiratory protective equipment, a separate standard requiring medical parameters applies to medical masks. Another relevant difference between masks vs. respirators is the opening of the pores in filtering material that is roughly 10× the size of respirators [66].
5. Data Arising from the Specification of Respiratory Standards
Analyzing available data, the following considerations must be considered. Respirators corresponding to the Chinese standard GB2626-2006Kn (labeled KN95) are intended primarily for industrial applications, while the European standard EN 149-2001 + A1 is intended for half-masks. The assessment of respirators and their mutual comparison according to these norms differ, and several established EN 149-2001 + A1 norms are not fully applicable.
The European equivalent FFP2 is comparable to N95 and KN95, which means that the opposite is true; standard N95 respirators are comparable to the FFP2 level.N95s respirators are a subset of the N95 respirators. While standard N95 respirators, like KN95 respirators, are intended for industrial workers, N95s (“N95-surgical”) respirators are specifically designed for healthcare professionals. The main difference, as opposed to N95 respirators, is their resistance to body fluids and the need for the United States Food and Drug Administration (USFDA) approval [66]. Under American law, these respirators are Class 2 medical devices. Thus, N95s respirators are similar to GB 19083-2010, level 1, while standard N95 respirators are similar to KN95 respirators according to the GB 2626-2006 norm.
The FFP3 European standard is comparable to N100. The N95 label (94% filtering capacity) corresponds to FFP2 or P2, i.e., 94% filtering capacity. The N99 label (99% filtering capacity) corresponds to FFP3, i.e., 99% filtering capacity. The N100 label (99.97% filtering capacity) corresponds to P3, i.e., 99.95% filtering capacity. While the N95/N100 specifications are a bit higher than FFP, it does not mean that the respirators are actually better [67]. In testing the filtration efficiency of respirators, very fine paraffin oil aerosols are used (Table 1).
Other countries have their own standards with more or less similar technical requirements for respirators. The most commonly known is the American NIOSH-42C FR84 standard (Table 2). This standard distinguishes between filters of types N, R, and P according to whether and how much they repel oily particles (type N respirators are not resistant to oily particles, type R respirators are resistant to oily particles for up to 8 h, and type P respirators are very resistant to oily particles) [68].
The filter type designation is followed by the efficiency, similar to respirators according to the GB 2626-2006 norm. Manufacturers often sell the same respirator in the USA and in Europe, whereas in the USA, they have the rating N95/N100 and in Europe, FFP2/FFP3 only because these are the accepted rating specifications in the applicable regions.
The above standards specify only the minimum percent of particulates that the respirator’s filter or capture, as shown in the following example. If a respirator is FFP2 rated, it will filter (capture) at least 94% of particulates with a diameter of 300 nm or greater. In practice, however, it will filter out (capture) particulates to an extent somewhere between 94% and 99%. Given that this result shifts a specific respirator into a qualitatively different dimension in terms of its ability to capture particulate matter, the exact indication is very likely to be given by the manufacturer in bold type in the product description to indicate that although it is certified (marketed) according to one or another norm, in use, it can move into higher (into the highest) categories.
High-efficiency respirators for particulate sizes of 300 nm (N95/FFP2 or better) can filter particles up to the size of the coronavirus (approximately 100 nm). The differences between FFP2 and FFP3 are modest, FFP labeling being in accord with European norm EN 149 + A1. The N95 or KN95 labels are according to other international norms (Table 2). Face masks cannot be labeled FFP2 or FFP3 because these indicators are reserved solely for respirators [69].
Chinese respirators designated KN95, Korean respirators designated AS/NZ P2 Class 1, and Japanese DS respirators can understandably be considered “equivalent” to US NIOSH N95 respirators and European FFP2 respirators for filtering non-oil-based particulates, such as particles generated by fires, air pollution PM2.5 (particulates smaller than 2.5 μm), volcanic eruptions, or bioaerosols (e.g., viruses).
Respirators labeled FFP1, FFP2, and FFP3 according to the new Czech standard ČSN EN 149:2001 correspond to those designations formerly under the old standard EN 149:1991 P1, P2, and P3 [70]. The P1 filter corresponds to the efficiency of FFP1 and protects against nontoxic solid particulates up to a maximum concentration of 5 × NPK-P (maximum permissible concentration of chemical substances or dust in the work area, as established by Law No. 361/2007, Czech Law Coll. [71], and expressing such a concentration of chemical substances, in which there is no damage to health)/PEL (permissible exposure limits of substances in the air of workplaces according to Law No. 361/2007 Czech Law Coll.). The P2 filter corresponds to FFP2 efficiency and protects against moderately toxic particles, including fibrous substances (including asbestos) up to 10 × NPK-P/PEL. The P3 filter corresponds to the efficiency of FFP3 and protects against toxic substances, including viruses, spores, bacteria, radioactive substances, and carcinogens up to 50 × NPK-P/PEL [71].
FFP3 respirators protect against the “SARS coronavirus” virus according to Amendment 7 to Law No. 361/2007, Czech Law Coll., in which it is classified as a group-3 biological agent [71]. These agents can cause dire human illnesses and present a serious danger to employees, and from the point of view of the possibility of spreading in the environment outside the workplace, although effective prophylaxis or treatment of a possible disease usually is available. This virus may pose a limited risk of infection to workers because it is not normally transmitted through the air, and therefore, workplace requirements are sufficient when working with a group-2 biological agent (i.e., those that can cause human illnesses and can be hazardous to workers). However, these are unlikely to spread to the environment outside the workplace, as effective prophylaxis or treatment of a possible disease is usually available.
An ordinary face mask is not sufficient to protect against any virus because it is manufactured from a material, which does not prevent the penetration of viral particles (and infection), face masks being suitable, for example, in a dusty environment.
Technical norms and standards establishing the basic parameters to which respirators must comply can differ slightly according to the global perspective. In the EU, compliance with the basic technical requirements for category III personal protective equipment under Czech Government Regulation 2016/425 on Personal Protective Equipment is demonstrated, inter alia, through using the harmonized technical standard EN 149:2001 + A1:2009 Respirators—Filtration Half-masks for Particle Protection—Requirements, Testing, and Labeling.
In China, compliance is demonstrated according to standards that set requirements for respirators, i.e., GB 19083-2010, Technical Requirements for Protective Masks for Medical Use, and GB 2626-2006 Respiratory Protective Devices—Respirators for Air Purification without Power Supply, the former being designed for health, the latter for the industry.
The required protection is provided only by a respirator with an FFP3 degree of protection, or FFP2 as the case may be. There is a possibility that an FFP2 respirator may be used to protect against the COVID-19 disease caused by the new SARS-cov-2 coronavirus. The tested respirator must be labeled ANTI-COVID-19/FFP2. Due to the technologies used, based on nanomembranes made of nanofibers, the labeled respirators have a measured capture rate of 98.5%. For example, BreaSAFE ANTI-COVID respirators have a tested capture rate of 97–99% for viruses and bacteria. For this reason, respirators labeled this way are adequate and, in relation to the purchase price, the best option for protection against coronavirus.
When comparing individual respirators, it is not only important that they are following the norms and levels of protection for which they were manufactured, but also how high the minimum required filtration efficiency they have. If the respirator falls into class FFP2 or GB 19083-2010, this does not mean that it has an efficiency of exactly 95%, but that its efficiency ranges from 95% to 98.99%. A respirator with an efficiency of 98.9%, for example, will technically fall into the class of FFP2 or GB 19083-2010 level 1 type respirators, but in practice, it will already be in principle comparable to respirators of FFP3 or GB 19083-2010 level 2 type, shifting to the lower limit of the next efficiency zone (i.e., Approximately 99%).
The Chinese norm GB2626-2006 distinguishes between respirators with KN and KP type filters. The KN type filter restrains only non-oily particles; the KP type filter restrains out both oily and non-oily particles. The number after the filter designation indicates the filtration percentage. This standard applies to full face masks, disposable and reusable half masks. There exist variants with and without a valve. Thus, a KN95 respirator filters out non-oily particles with an efficiency rate of at least 95%. KN100/KP100 respirators have an efficiency of at least 99.97%. It results that a KN95 respirator always has efficiency at the FFP2 level (which has a minimum filtering efficiency of 94%), and some may even reach the FFP3 level.
The GB 19083-2010 technical standard adjusts the respirator requirements for healthcare professionals and represents a very high standard of user protection. In terms of technical requirements, it is in many ways similar to GB 2626-2006. However, it does not distinguish between KN and KP filter types. As it is intended only for the medical field, the requirements for the filtration of oily particles have been replaced by the requirements for protection against body fluids. It should also be noted that respirators tested according to this standard do not have an exhalation valve, which is undesirable in healthcare. They also distinguish between three levels of efficiency, but the lowest level 1 stipulates at least 95% efficiency, level 2 requires at least 99% efficiency, and level 3 corresponds to a KN100 respirator—the efficiency must be at least 99.97%. If respirators meet the requirements of this standard, this means that their filtering efficiency is at least comparable to FFP2 type respirators. When they fall into level 2 or 3, they provide protection similar to, or even higher than, FFP3 type respirators.
Face masks or respirators of lower protection classes (FFP1, KN90/KP90) are suitable for community use due to their seal. When properly seated and used, they provide an even higher level of protection than standard medical and nonmedical face masks.
“Best practice” would be FFP3 class respirators and respirators meeting the requirements of GB 19083-2010 levels 2 and 3, which have a filtration efficiency above 99% (GB 19083-2010 level 3 respirators provide an even higher level of protection than FFP3 type respirators) (Table 3 and Table 4). Valve-fitted respirators, even those that meet the FFP3 respirator standard, do not protect the user’s environment from contamination. They are not suitable for protection against coronavirus infection [72].
People should not trust products labeled as “nanomasks”. In some cases, it has been found that they do not contain any nanofiber fabrics nor any other nanoobjects that would technologically increase their filtering efficiency [73]. A certain type of “fake news” can be determined via whether the manufacturer or seller uses the correct terminology. In detecting viruses and bacteria (respirators), the term “filtering efficiency” should be used instead of the term absorption efficiency or its modification. We are concerned here with the capture of particulates that are not chemical substances.
Before choosing a respirator, users should always familiarize themselves with local regulations and respiratory protection requirements and/or consult professionally trained personnel and seek guidance on proper respirator selection from local public health authorities.
6. Some Aspects of Public Procurement in the Field of Respirators
It is interesting that one of the most frequently resolved situations at the Public Prosecutor’s Office of the Czech Republic within the COVID 19 pandemic was purchasing protective equipment intended not only for health professionals but also for the general public. The text presented in this section primarily provides information based on the laws valid in the Czech Republic. Given that the Czech Republic is a part of the EU structure and it must keep European rules and laws, it is very likely that it can be assumed that the experience with production practice will be very similar within other EU countries.
When awarding public contracts, many unsuitable tender conditions appeared in the Czech Republic in particular. These conditions often appeared in tender documents. Given the application of the rules in force in the EU, it is reasonable to assume that similar problems have occurred in other EU countries. It is logical that the number of respirator suppliers meeting the conditions of certification under EU law has increased significantly compared to the beginning of the so-called first wave of pandemic situation. Experience has shown that there is no longer any reason to define respiratory requirements with reference to alternative standards operating in other parts of the world that are similar to EU standards, even though these have not always been comparable requirements [47,48]. Furthermore, during the resolution of the consequences of the first wave of the COVID 19 pandemic, it turned out that the alternative parameters tend to specify the maximum price of the FFP2 respirator.
Representatives of the production practice state that very often there was an error in defining the tender conditions by combining the reference with the European standard or its adoption by the Czech technical standard ČSN EN 149:2001 + A1:2009 [57]. This standard introduces the designation of protection class FFP2 and at the same time a reference to the designation KN95, which is an expression of protection class, but according to the Chinese standard GB 2626-2019.
As mentioned above, the following applies to regulating personal protective equipment, including FFP2 respirators:
- -
- Respirators belong to the so-called regulated products falling, according to Regulation (EU) 2016/425 [56] of the European Parliament and of the Council on personal protective equipment, into the third, thus the highest risk category, and as such must “meet” all necessary functional parameters prescribed by standard EN 149. These parameters must be complied with, although, due to the sudden pandemic, this obligation has been temporarily relaxed by the Commission of Recommendation (EU) 2020/403 on conformity assessment and market surveillance procedures related to the threat of coronavirus COVID-19;
- -
- The same requirements are not set for obtaining protection classes FFP2 and KN95, although these are approximately the same levels of protection within different standards.
It was found that the requirement for the level of protection of KN95 according to the Chinese standard was also problematic in terms of its proportionality and non-discriminatory nature. It was obvious that its determination was not justified by the overall purpose of acquiring the subject of performance, a respirator manufactured following the regulations in force in the EU. A similar situation applies, for example, to the N95 respirator category, which is based on United States standards. Even in this case, it is not a requirement stemming from an EU standard [74].
If the contracting authority imposes a requirement in the tender conditions that the FFP2 respirator “comply” with the EN 149 standard (in the relevant protection class, here FFP2), this is objectively proportionate and non-discriminatory, as it can be justified by the existence of Regulation (EU) 2016/425 on personal protective equipment. However, the requirement of KN95 (or N95) cannot be justified by reference to EU legislation and is, therefore, set “beyond the scope” of the purpose of supply and, as such, is objectively unfounded [75].
The situation with the release of certificates was also problematic. If the tenderer has submitted a certificate declaring compliance with the EN 149 standard and is issued by a notified body, this is evidence of compliance with the conditions for participation. Alternatively, it was a document certifying compliance with the standard in combination with a satisfactory test of the sample, which allowed the bidder to decide on his choice in the subsequent conclusion of the contract. However, it is important to note that this does not mean that respirators that do not comply with EN 149 may be supplied during the performance of the contract. In addition to technical regulations, according to which only respirators complying with the EN 149 standard can be supplied to the EU market, there is also competitive regulation in the area of public procurement, which also affects the period of implementation of the public contract after signing the contract [76].
According to the Czech Republic valid laws, the contracting authorities may not allow a substantial change in the obligation under the contract. Therefore, if the contract defines respirators by reference to standard EN 149, which can be strongly recommended, the respirators supplied must actually comply with this standard. In the event of proof to the contrary, by accepting them, the contracting authority would act in conflict with the applicable legislation and thus be exposed at least to a fine for the offense.
Therefore, if the contracting authority finds out through its external or internal control mechanisms that, despite the corresponding certificates or samples delivered before signing the contract, the respirators supplied actually do not meet the EN 149 standard, it should demand immediate compensation from the supplier (proper performance) or use the relevant legal provisions. These provisions allow withdrawing from the contract directly, in the text of the law, even if it is not explicitly stipulated in the contract. However, the entrustment of the contracting authority’s contractual guarantees in compliance with the EN 149 requirement is strongly recommended, not only in connection with the possibility of withdrawing from the contract but also with contractual penalties or other contractual instruments at least compensate the contracting authority. These are, in particular, the costs associated with temporary delivery, costs of in-depth testing of continuously selected random samples, which have proved noncompliance with the requirements and the preferences. Finally, it can also be a preventive action to transfer towards the supplier the motivation for prevention and economic interest in detecting unsatisfactory respirators before the contracting authority.
In some cases, it may be an illegal determination of technical conditions by describing the parameters of a particular respirator design of one supplier. Such a supplier, which itself complies with the EN 149 standard, cannot be promoted to its level in terms of liability for other suppliers involved in a public contract. The parameters of one specific manufactured respirator cannot be a model for the tender conditions, as it would be a textbook example of indirect discrimination. However, it is necessary to mention that it is very probable that the contracting authority would proceed in this way rather out of ignorance.
Below are anonymous citations of frequent alternative parameters that result from folk creativity rather than relying on functional properties relevant for the purpose of the respirator, which is regulated in full-scope by EN 149, without the need (justification) for additional requirements of its technical design or marking.
- -
- “FFP2/KN95 respirator”—the indication of KN95 type is not justified. It is not possible to replace the FFP2 respirator with KN95 and vice versa. Respirators supplied to the Czech and European markets must meet the requirements of standard EN 149 and must, therefore, primarily bear the designation FFP2 and meet this level of protection.
- -
- “Protection class KN95 or N95”—it is not justified to require these protection classes, which are based on Chinese, respectively, American standards. On the territory of the Czech Republic, as an EU member state, the designation is justified only regarding Regulation (EU) 2016/425 of the European Parliament and of the Council on personal protective equipment, standard EN 149. Only this standard specifies the FFP respirator and its requirements.
- -
- “Material: nonwoven fabric, white color”—These requirements do not determine the functionality and effectiveness of the respirator. These are self-serving and rather superficial requirements that do not indicate the quality of the product. Neither the type of material nor its color provides the required functionality of the respirator as defined by standard EN 149. This standard does not specify the types of material in any way.
- -
- “At least four layers”—The number of layers and their specific material composition do not determine the functionality of the respirator. The standard does not define the number of layers or types of materials. These are again self-serving and rather superficial requirements that say nothing about the final quality of the product.
- -
- “CE marking, EU type certificate from a notified body that the notified body has carried out product testing in accordance with Regulation (EU) 2016/425, ČSN EN 149:2001 + A1:2009—according to Articles 7.3., 7.9.2., 7.1., 7.16, 8.4.1”—These are the articles of standard EN 149, compliance with which was required during the period of validity of Recommendation (EU) 2020/403 on conformity assessment and market surveillance procedures in relation to the threat of coronavirus COVID-19, at the time when there was not enough protective equipment on the European market, and it was possible to obtain a so-called “temporary certificate” during the conformity assessment of the product. After the first wave of the pandemic, in June 2020, this exemption was abolished in the Czech Republic with the so-called “temporary certificate”. It was no longer possible to grant or apply for it. At present, it is necessary to meet all the requirements of the EN 149 standard.
- -
- “Minimum filtration efficiency of 95%”—This parameter is based on the Chinese standard GB 2626 for KN95 type respirators. It has already been stated that standard EN 149 similarly defines a maximum particle penetration of 6%, a filtration efficiency of at least 94%. A higher value of this parameter (plus only 1%) alone will not provide better protection for respirator users. This parameter alone says nothing in isolation about the respirator’s ability to ensure higher functionality and user safety. The application of this parameter is especially problematic in the case of long-term protection. Among other things, the EN 149 standard requires the maintenance of maximum penetration of 6% even at the end of an eight-hour work shift (test 7.9.2—Penetration of filter material). In the context of the EN 149 standard, it is, therefore, necessary to respect all its requirements without giving priority to more easily perceived values.
Many inappropriate tender conditions also emerged during the organization of tenders, focusing on requirements concerning the maximum price of an FFP2 respirator or other personal protective equipment or medical devices.
It can be argued that the maximum purchase price per manufactured piece, which is a logical aspect of the manufacturer’s activities, is completely against the requirement to achieve maximum quality while meeting all requirements of the very strict standard EN 149. At the same time, it is also in conflict with the requirements of the contracting authority, which, on the contrary, requires the lowest bid price. Indeed, if an economic operator offers a value higher than the value corresponding to the actual market price of FFP2 respirators (complying with the EN 149 standard), it will not normally be placed first in the valuation (only for the consideration of the price) and his work related to the preparation of the offer is useless. In a price-only competition, suppliers are motivated enough to try to offer the lowest price possible. Due to the nature of the business activities, a professional supplier naturally monitors the current price level on the market. These activities are done within large deliveries of contracting authorities or within retail.
Considering the above information, it is clear that there is no relevant reason to set an upper limit. Rather than offering benefits in the procurement procedure, using this may raise doubts about the real purpose of such a requirement. In addition, the requirement not to exceed the maximum price is justified in cases where only the part of the item forming the assessed price is limited. This applies in particular to hourly rates for services of indefinite scope, which are to be added to a fixed price for services of closed scope. In the case of evaluating a precisely determined quality (according to the EN 149 standard) and evaluating the price per piece, the purpose of stating the maximum price is completely inefficient.
However, if the contracting authority seeks to prevent selecting a supplier who will not be able to supply FFP2 respirators in the required volume and quality (EN 149 standard) at the offered price, establishing extremely low tender prices shall be considered. In this case, on the other hand, it is possible to set a minimum price limit, which will indicate a possible suspicion of the contracting authority leading, for example, to the possibility of asking for clarification of the reason for its determination in the form of evidence. Regarding quality assessment, it is probably sufficient for the contracting entity to consistently insist on quality testing and verification of compliance with EN 149. Moreover, if the contracting authority wants to inform suppliers in advance that compliance with the EN 149 standard is essential, regardless of the attractiveness of the low-price offer, it is necessary to use legal procedures and prefer not to limit the maximum bid price. This should be subject to free competition between suppliers, following the spirit of the evaluation rules.
The price information is also essential. It should be emphasized that the “maximum” value per 1 piece of respirator is often linked to the expected value and the expected quantity of the scope of delivery. However, the contracting authority is obliged to follow the law to determine the expected value of the delivery regarding current and relevant information. The correct determination of the expected value of 1 respirator has, among other things, a fundamental impact on determining the public procurement regime, whether it is a small contract, or a subthreshold contract or an above-limit contract, which is associated with the obligation to proceed primarily in open or restricted procedures with longer deadlines, etc.
If the contracting authority determines the expected value in confidence based on a completely unrealistic estimate of the price of FFP2 respirators, which will meet the conditions set out in EN 149, it is likely to commit an artificial reduction in the value of the public contract, and in breach of public procurement law. This situation occurs when the tender conditions of other contracting authorities are taken over, or in the case of a mere subjective wish, that the value of 1 piece of respirator does not exceed the maximum permissible limit of the purchase price in some way.
Regarding the above and discussed reasons, it is possible to recommend the following form of tender conditions. These could be primarily intended to process specific text parts according to the general customs and the formal structure of the tender documentation. At the same time, it is recommended that more extensive documentation be required before the contract is signed. Alternatively, it is also possible to supplement them following the procedures laid down by law. This approach would not be a purely formal strict requirement, at least unless it is an “emergency” procurement regime in the case of an urgent and thus lower supply volume. It is, therefore, necessary for the contracting authority to set the technical conditions so that respirators must meet at least all requirements of the EN 149:2001 + A1:2009 standard, which are set for protection class FFP2. The supplier will prove this requirement in the offer primarily by submitting a certificate (CE) or an EU certificate. This certificate must be issued by a notified body. The certificate verifies compliance with this standard. Furthermore, it is necessary for the supplier to submit a test report at the moment of the contracting authority’s call towards the selected supplier. To avoid doubt, the contracting authority shall specify in advance that respirators meeting only some of the requirements of the above standard will not be considered as an equivalent solution in the sense of the specified requirements. There will not be considered, for example, respirators that meet only the requirements for protection class N95 or protection class KN95 according to the Chinese standard GB 2626-2019, those that do not a priori meet all the requirements of the standard EN 149:2001 + A1:2009. Furthermore, it is appropriate to require the submission of respirator samples to demonstrate that the requirements of the technical conditions are met. To avoid a deterrent effect in the case of short deadlines for the submission of tenders, it may be appropriate to require them to be submitted only upon invitation. However, this requirement is always at the individual discretion, for example, due to the urgency and size of the delivery. Furthermore, it is possible to recommend that the business conditions should be prepared in the form of a specific draft contract or a framework agreement, the annex to which will be the definition of these technical conditions. Furthermore, it would be appropriate to ensure coherence with the qualification requirement for the submission of a sample, which will entitle to random testing and possible return or withdrawal from the contract. It is also appropriate to provide penalties for non-delivery of products of the quality required by the technical conditions. Furthermore, it would be appropriate to submit a test report issued by a testing institute, which will serve as objective evidence of noncompliance with the requirements of the standard.
7. Examples of Some Rough/Uncompliant/Dispired Respirators
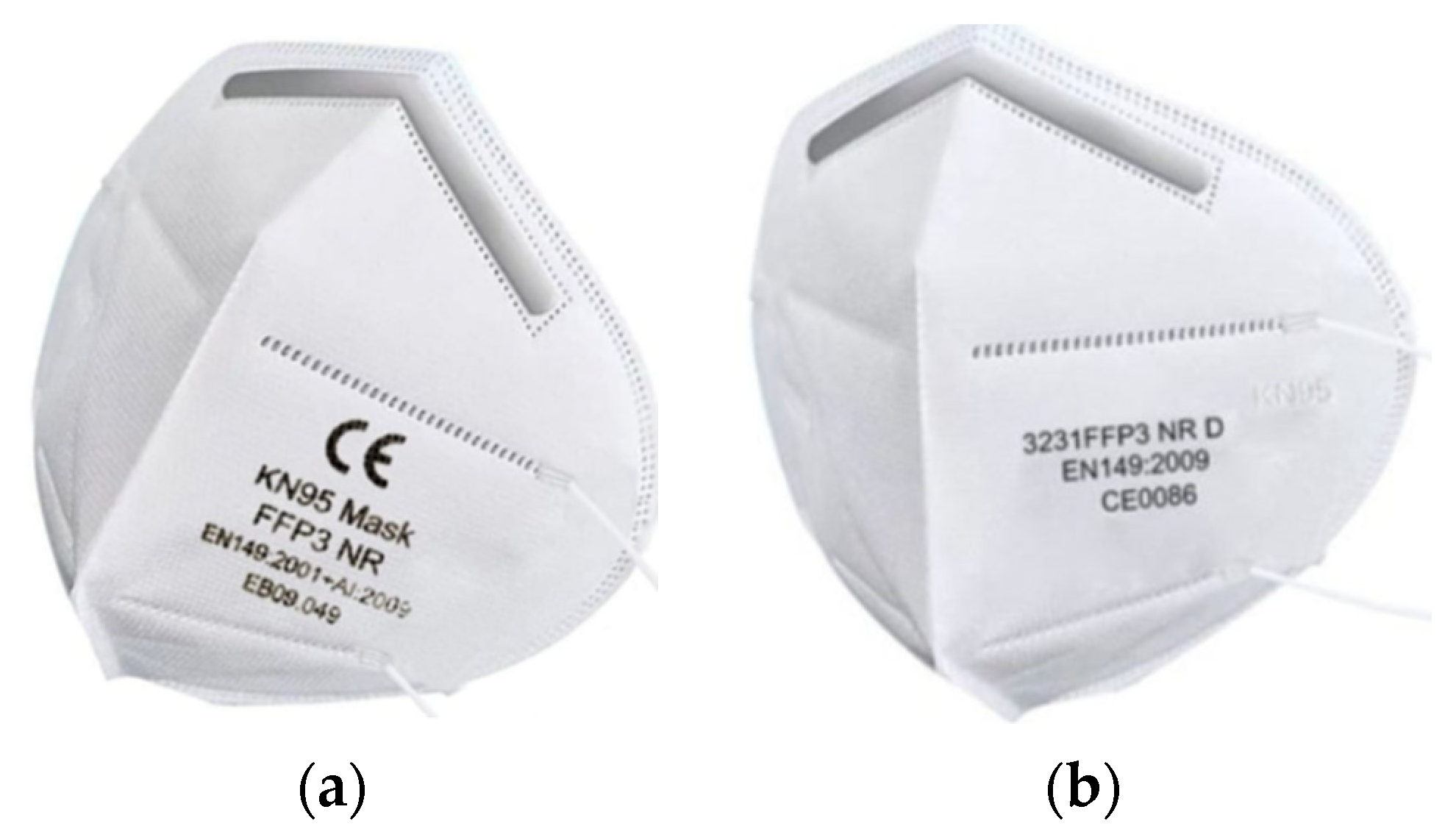
The efforts of manufacturers and probably also distributors of respirators and technological products based on nanomaterials are very often driven by effort and profit maximization. When most European countries have decided to rethink their approach to protecting the respiratory organs of the general population from the effects of the COVID-19 pandemic, there are many respirators on the market that are likely to only pretend to be declared quality EN 149.
Figure 3a shows an example of a respirator that declares its filtration and protection capabilities according to two different standards. The first line shows the CE marking corresponding to the designation of the notified body (notified person). However, the four-digit numeric code is not given. The second line of the nameplate shows the filtration efficiency KN95, which corresponds to the Chinese standard defined by the norm GB2626-2006. According to this standard, filtration efficiency of at least 95% is declared. The third line contains the data FFP3, which corresponds to the European standard EN 149-2001 + A1. According to this information, the user could rightly expect an efficiency of at least 99.95%. This expectation is more intense because the number of the European standard is given, while the Chinese one is not mentioned. Figure 3b shows the letter and number code KN95 on the right side of the respirator. Even in this case, the data FFP3 is mentioned. This mark corresponds to the European standard EN 149-2001 + A1. However, the designation of the standard for this type of respirator is questionable because it is not given in full. Thus, for both types of respirators, there is conflicting information regarding the level of provided protection. It is very likely that too much effort to maximize profits has led the manufacturer of the respirators in the picture to artificially raise the level of protection from FFP2 to FFP3. If the manufacturers indicated an FFP2 level that corresponds to the declared KN95 level according to the Chinese standard, the potential buyer of these respirators would probably not know anything.
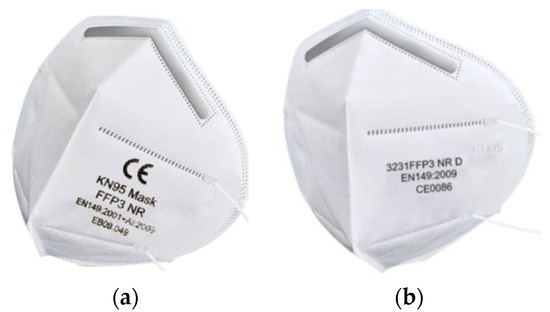
Figure 3.
Examples of controversial information provided to respirators: (a) respirator certified according to two standards with different results; (b) respirator certified according to the standard without a particular supplement.
8. Conclusions
Million respirators and masks are being used in Europe to weather the storm of the coronavirus epidemic. They come from various manufacturers in the world. None of the commercially available respirators provide 100% protection to the user, not even in combination with other protective equipment, but using respiratory protective equipment is one of the relevant preventive measures, which helps in reducing the spread of respiratory diseases, including COVID-19. The correct selection/choice of the face mask to meet the various requirements (exposure to existing hazards agents in the air, work environment and tasks of the mask wearer, as well as the specific characteristics of each individual) has an overwhelming importance in proper exposure control. This review provides exactly the data necessary for correct and documented information so that the choice of the mask being made in full knowledge of the facts.
Author Contributions
All authors have equal contributions to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study is available in used literature and on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Veils and Respirators Not Only against Coronavirus + Preventive Measures against Infection. Available online: https://1url.cz/XzfCF (accessed on 23 February 2021).
- Kabir, M.T.; Uddin, M.S.; Hossain, M.F.; Abdulhakim, J.A.; Alam, M.A.; Ashraf, G.M.; Bungau, S.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Aleya, L. nCOVID-19 Pandemic from Molecular Pathogenesis to Potential Investigational Therapeutics. Front. Cell Dev. Biol. 2020, 8, 616. [Google Scholar] [CrossRef]
- Perrotta, D.; Grow, A.; Rampazzo, F.; Cimentada, J.; Del Fava, E.; Gil-Clavel, S.; Zaghenil, E. Behaviors and attitudes in response to the COVID-19 pandemic: Insights from a cross-national Facebook survey. medRxiv 2020, 20096388. [Google Scholar] [CrossRef]
- Brook, R.D.; Newby, D.E.; Rajagopalan, S. The Global Threat of Outdoor Ambient Air Pollution to Cardiovascular Health: Time for Intervention. JAMA Cardiol. 2017, 2, 353–354. [Google Scholar] [CrossRef]
- Filtered Inhalation. How the Mouthpieces, Veils, and Respirators Performed in the Big Test. Available online: https://1url.cz/yzmH7 (accessed on 23 February 2021).
- Otrisal, P.; Friess, K.; Urban, M.; Bungau, S.; Tit, D.M.; Mosteanu, D.E.; Melicharik, Z.; Bungau, C.; Aleya, L. Barrier properties of anti-gas military garments, considering exposure to gas organic compounds. Sci. Total Environ. 2020, 714, 136819. [Google Scholar] [CrossRef]
- Negrut, N.; Codrean, A.; Hodisan, I.; Bungau, S.; Tit, D.M.; Marin, R.; Behl, T.; Banica, F.; Diaconu, C.C.; Nistor-Cseppento, D.C. Efficiency of antiviral treatment in COVID-19. Exp. Ther. Med. 2021, 21, 648. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Bungau, S.; Kumar, A.; Uddin, M.S.; Kumar, C.; Pal, G.; Sahil; Shrivastava, K.; Zengin, G.; et al. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020, 257, 118075. [Google Scholar] [CrossRef] [PubMed]
- Worl Health Organization (WHO). Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 22 April 2021).
- Feng, S.; Shen, C.; Xia, N.; Song, W.; Fan, M.; Cowling, B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020, 8, 434–436. [Google Scholar] [CrossRef]
- Brosseau, L.; Ann, R.B. N95 Respirators and Surgical Masks. Centers for Disease Control and Prevention, 2009. Available online: https://blogs.cdc.gov/niosh-science-blog/2009/10/14/n95/ (accessed on 21 April 2021).
- Forouzandeh, P.; O’Dowd, K.; Pillai, S.C. Face masks and respirators in the fight against the COVID-19 pandemic: An overview of the standards and testing methods. Saf. Sci. 2021, 133, 104995. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials. ASTM F2101-07 Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials, Using a Biological Aerosol of Staphylococcus Aureus; American Society for Testing and Materials: West Conshohocken, PA, USA, 2007. [Google Scholar]
- European Committee for Standardization; BSI. Respiratory Protective Devices—Filtering Half Masks to Protect Against Particles—Requirements, Testing, Marking; BS EN 149:2001 + A1:2009; European Committee for Standardization: Brussels, Belgium; BSI: London, UK, 2001. [Google Scholar]
- National Institute for Occupational Safety and Hygiene (NIOSH). NIOSH Guide to the Selection and Use of Particulate Respirators Certified Under 42 CFR 84; (DHHS (NIOSH) Publication no. 96-101); National Institute for Occupational Safety and Hygiene (NIOSH): Cincinnati, OH, USA, 1996.
- Coia, J.E.; Ritchie, L.; Adisesh, A.; Makison Booth, C.; Bradley, C.; Bunyan, D.; Carson, G.; Fry, C.; Hoffman, P.; Jenkins, D.; et al. Healthcare Infection Society Working Group on Respiratory and Facial Protection. Guidance on the use of respiratory and facial protection equipment. J. Hosp. Infect. 2013, 85, 170–182. [Google Scholar] [CrossRef]
- CDC. Coronavirus Disease 2019 (COVID-19): Steps to Prevent Illness. Available online: https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html (accessed on 21 April 2021).
- Czech Researchers are Testing How Masks from T-Shirts and Handkerchiefs Catch Coronavirus. Available online: https://1url.cz/xzfnR (accessed on 27 February 2020).
- Moravová, V.; Král, J.; Kubátová, I.; Martinovský, A. Srovnávací Analýza Respirátorů dle Norem EN 149:2001+A1:2009 GB 19083-2010 GB 2626-2006, 1st ed.; Porta Medica s.r.o.: Prague, Czech Republic, 2020; pp. 1–18. [Google Scholar]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef]
- Zhou, S.S.; Lukula, S.; Chiossone, C.; Nims, R.W.; Suchmann, D.B.; Ijaz, M.K. Assessment of a respiratory face mask for capturing air pollutants and pathogens including human influenza and rhinoviruses. J. Thorac. Dis. 2018, 10, 2059–2069. [Google Scholar] [CrossRef]
- Gheorghe, G.; Ilie, M.; Bungau, S.; Stoian, A.M.P.; Bacalbasa, N.; Diaconu, C.C. Is there a Relationship between COVID-19 and Hyponatremia? Medicina 2021, 57, 55. [Google Scholar] [CrossRef]
- Pusta, C.T.J.; Bungau, S.; Buhas, C.L.; Popa, A.R.; Vesa, C.M.; Buhas, B.A.; Bardaca Urducea, C.; Tit, D.M.; Abdel-Daim, M.; Judea, A.S. Experimental study upon the virulence of infectious microbial agents involved in violent deaths presenting septic states. Rev. Chim. 2019, 70, 2720–2726. [Google Scholar] [CrossRef]
- The American Society of Agricultural and Biological Engineers (ASABE). Available online: https://1url.cz/izIs3 (accessed on 31 October 2020).
- Rybka, A.; Gavel, A.; Meloun, J.; Tichá, L.; Pejchal, J. Decontamination of High-risk Biological Agents—Spraying Challenges. Sci. Lett. 2019, 88, 195–200. [Google Scholar] [CrossRef]
- Soult, A. Colloids and Suspensions. 2019. Available online: https://chem.libretexts.org/@go/page/155673 (accessed on 22 April 2021).
- How to Wear Mandatory Nano Masks and Respirators? Above All, it Must Seal and “Pulsate” with the Breath. Available online: https://1url.cz/vzyiS (accessed on 24 February 2021).
- WHO. Coronavirus Disease (COVID-19): How is It Transmitted? Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted (accessed on 22 April 2021).
- Chen, C.-C.; Huang, S.H. The Effects of Particle Charge on the Performance of an Electret Filtering Facepiece. Am. Ind. Hyg. Assoc. J. 1998, 59, 227–233. [Google Scholar] [CrossRef]
- OSTI.GOV, U.S. Department of Energy Office of Scientific and Technical Information. Technical Report: Electrostatic Air Filters Generated by Electric Fields. Available online: https://www.osti.gov/biblio/6391664-6it4gP/ (accessed on 23 April 2021).
- Brown, R.C. Air Filtration and Integrated Approach to the Theory and Applications of Fibrous Filters; Pergamon Press: Tarrytown, NY, USA, 1993. [Google Scholar]
- Larson, D.L. Collection Efficiency Comparison of N95 Respirators Saturated with Artificial Perspiration. ACS Chem. Health Saf. 2020, 27, 299–307. [Google Scholar] [CrossRef]
- Bungău, C.C.; Prada, I.F.; Prada, M.; Bungău, C. Design and Operation of Constructions: A Healthy Living Environment-Parametric Studies and New Solutions. Sustainability 2019, 11, 6824. [Google Scholar] [CrossRef]
- Respirators Versus Nanofiber Masks: What About Protection? Available online: https://1url.cz/tznyC (accessed on 29 January 2021).
- Huang, Z.M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef]
- Khude, P. Nanofibers for High Efficiency Filtration. J. Mater. Sci. Eng. 2017, 6, 1000399. [Google Scholar] [CrossRef]
- Lee, B.U. Minimum Sizes of Respiratory Particles Carrying SARS-CoV-2 and the Possibility of Aerosol Generation. Int. J. Environ. Res. Public Health 2020, 17, 6960. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, V.; Ouyang, M.; Graham, K. Polymeric nanofibres in high efficiency filtration applications. Filtration 2006, 6, 286–293. [Google Scholar]
- Lee, J.K.; Ahn, Y.C.; Park, S.K.; Kim, G.T.; Hwang, Y.H.; Lee, C.G.; Shin, H.S. Development of high efficiency nanofilters made of nanofibers. Curr. Appl. Phys. 2006, 6, 1030–1035. [Google Scholar]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef]
- Feng, J. Preparation and properties of poly(lactic acid) fiber melt blown non-woven disordered mats. Mater. Lett. 2017, 189, 180–183. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, S.W.; Joshi, M.K.; Kim, C.S. Fabrication and characterization of silver nanoparticle-incorporated bilayer electrospun-melt-blown micro/nanofibrous membrane. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 514–520. [Google Scholar] [CrossRef]
- Pu, Y.; Zheng, J.; Chen, F.; Long, Y.; Wu, H.; Li, Q.; Yu, S.; Wang, X.; Ning, X. Preparation of Polypropylene Micro and Nanofibers by Electrostatic-Assisted Melt Blown and Their Application. Polymers 2018, 10, 959. [Google Scholar] [CrossRef]
- Sun, G.W.; Song, J.; Xu, L.; Wang, X.H. Numerical modelling of microfibers formation and motion during melt blowing. J. Text. Inst. 2018, 109, 300–306. [Google Scholar] [CrossRef]
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K., II; Cha, J.H.; Kim, I.S. Reusability comparison of melt-blown vs. Nanofiber face mask filters for use in the coronavirus pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241. [Google Scholar] [CrossRef]
- ICC. European Standard Uni EN 149:2001+A1:2009 FACEMASKS. Available online: https://icc-iso.org/index.php/en/certificates/58-maskat-en (accessed on 22 April 2021).
- Lee, S.A.; Hwang, D.C.; Li, H.Y.; Tsai, C.F.; Chen, C.W.; Chen, J.K. Particle Size-Selective Assessment of Protection of European Standard FFP Respirators and Surgical Masks against Particles-Tested with Human Subjects. J. Healthc. Eng. 2016, 2016, 8572493. [Google Scholar] [CrossRef]
- Roberge, R.J.; Kim, J.H.; Coca, A. Protective facemask impact on human thermoregulation: An overview. Ann. Occup. Hyg. 2012, 56, 102–112. [Google Scholar] [PubMed]
- Matuschek, C.; Moll, F.; Fangerau, H.; Fischer, J.C.; Zänker, K.; van Griensven, M.; Schneider, M.; Kindgen-Milles, D.; Trudo Knoefel, W.; Lichtenberg, A.; et al. Face masks: Benefits and risks during the COVID-19 crisis. Eur. J. Med. Res. 2020, 25, 32. [Google Scholar] [CrossRef]
- Standards Australia. Standards Catalogue. Available online: https://www.standards.org.au/standards-catalogue/sa-snz/publicsafety/sf-010/as-slash-nzs--1716-2012 (accessed on 23 April 2021).
- 3M™ Aura™ Particle Respirators 9300+Gen3 Series: Technical Data Sheet. Available online: https://1url.cz/wzIi5 (accessed on 29 October 2020).
- C2020. 3M™ Particulate Filter Half Masks (Respirators), Type 8233E: Technical Data Sheet. Available online: https://1url.cz/DzIib (accessed on 29 October 2020).
- British Standard. Respiratory Protective Devices—Filtering Half Masks to Protect against Particles—Requirements, Testing, Marking. Available online: http://www.nobelcert.com/DataFiles/FreeUpload/EN%20149-2001%20plus%20A1-2009.pdf (accessed on 23 April 2021).
- European Union. Regulation (EU) 2016/425 of the European Parliament and of the Council of 9 March 2016 on Personal Protective Equipment and Repealing Council Directive 89/686/EEC. Off. J. Eur. Union 2016, 59, 98. Available online: https://eur-lex.europa.eu/eli/reg/2016/425/oj (accessed on 23 April 2021).
- European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj (accessed on 23 April 2021).
- ACT Testing Technology Co., Ltd. ČSN EN 149+A1 (83 225): Respiratory Protective Devices—Filtering Half Masks to Protect Against Particles—Requirements, Testing, Marking; ACT Testing Technology Co., Ltd.: Guangzhou, China, 2009; p. 32. [Google Scholar]
- FFP2 Respirator, Ultra-Thin, Maximally Breathable, Czech Manufacturer, Certificate. Available online: https://1url.cz/XzIkj (accessed on 30 December 2020).
- BreaSAFE® ANTI-COVID-19 | 3 pcs. Available online: https://1url.cz/dzIkp (accessed on 30 December 2020).
- Otrisal, P.; Obsel, V.; Buk, J.; Svorc, L. Preparation of Filtration Sorptive Materials from Nanofibers, Bicofibers, and Textile Adsorbents without Binders Employment. Nanomaterials 2018, 8, 564. [Google Scholar] [CrossRef]
- Prikryl, R.; Otrisal, P.; Obsel, V.; Svorc, L.; Karkalic, R.; Buk, J. Protective Properties of a Microstructure Composed of Barrier Nanostructured Organics and SiOx Layers Deposited on a Polymer Matrix. Nanomaterials 2018, 8, 679. [Google Scholar] [CrossRef]
- Bărdacă Urducea, C.; Nechifor, A.C.; Dimulescu, I.A.; Oprea, O.; Nechifor, G.; Totu, E.E.; Isildak, I.; Albu, P.C.; Bungău, S.G. Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method. Nanomaterials 2020, 10, 2349. [Google Scholar] [CrossRef]
- FFP2 Respirators with Nanomembrane. Available online: https://1url.cz/PzIgM (accessed on 2 January 2021).
- Respiratory Protection Information. Available online: https://1url.cz/mzIij (accessed on 28 February 2021).
- Masks and Respirators. Available online: https://1url.cz/pzCQ8 (accessed on 30 January 2020).
- Neupane, B.B.; Mainali, S.; Sharma, A.; Giri, B. Optical microscopic study of surface morphology and filtering efficiency of face masks. PeerJ 2019, 7, e7142. [Google Scholar] [CrossRef]
- N95 Respirators, Surgical Masks, and Face Masks. Available online: https://1url.cz/TzIkc (accessed on 31 January 2021).
- Lepelletier, D.; Grandbastien, B.; Romano-Bertrand, S.; Aho, S.; Chidiac, C.; Géhanno, J.F.; Chauvin, F.; French Society for Hospital Hygiene and the High Council for Public Health. What face mask for what use in the context of COVID-19 pandemic? The French guidelines. J. Hosp. Infect. 2020, 105, 414–418. [Google Scholar] [CrossRef]
- Rengasamy, S.; Eimer, B.; Shaffer, R. Comparison of Nanoparticle Filtration Performance of NIOSH-approved and CE-Marked Particulate Filtering Facepiece Respirators. Ann. Occup. Hyg. 2009, 53, 117–128. [Google Scholar] [CrossRef] [PubMed]
- How to Choose the Right Type of Filter Half Mask—Respirator? Available online: https://1url.cz/TzIi8 (accessed on 30 January 2021).
- Government Regulation No. 361/2007 Coll. Government Order Laying Down Conditions for the Protection of Health at Work. Available online: https://1url.cz/7zIwK (accessed on 30 January 2021).
- Institute of Medicine (US) Committee on Personal Protective Equipment for Healthcare Personnel to Prevent Transmission of Pandemic Influenza and Other Viral Respiratory Infections: Current Research Issues. Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Personnel; Larson, E.L., Liverman, C.T., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- There are Fake Nanotracks on the Market. The Veil from the T-Shirt has the Same Effect, the Test Revealed. Available online: https://1url.cz/JzIg4 (accessed on 2 November 2020).
- Mottay, L.; Le Roux, J.; Perumal, R.; Esmail, A.; Timm, L.; Sivarasu, S.; Dheda, K.S. KN95 filtering facepiece respirators distributed in South Africa fail safety testing protocols. Afr. Med. J. 2020, 9, 13162. [Google Scholar] [CrossRef]
- O’Kelly, E.; Arora, A.; Pirog, S.; Ward, J.; Clarkson, P.J. Comparing the fit of N95, KN95, surgical, and cloth face masks and assessing the accuracy of fit checking. PLoS ONE 2021, 16, e0245688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Emery, A.R.; Tannyhill, R.J., 3rd; Zheng, H.; Wang, J. Masks or N95 Respirators During COVID-19 Pandemic-Which One Should I Wear? J. Oral Maxillofac. Surg. 2020, 78, 2114–2127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).