Antioxidant and Antimicrobial Influence on Oyster Mushrooms (Pleurotus ostreatus) from Substrate Supplementation of Calcium Silicate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Samples
2.3. Bioactive Molecules
2.3.1. Organic Acids
2.3.2. Phenolic Compounds
2.4. Bioactivities
2.4.1. Antioxidant Activity
2.4.2. Antimicrobial Activity
2.4.3. Cytotoxicity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Organic Acids and Phenolic Compounds
3.2. Antioxidant Activity and Cytotoxicity in Non-Tumour Cell Line
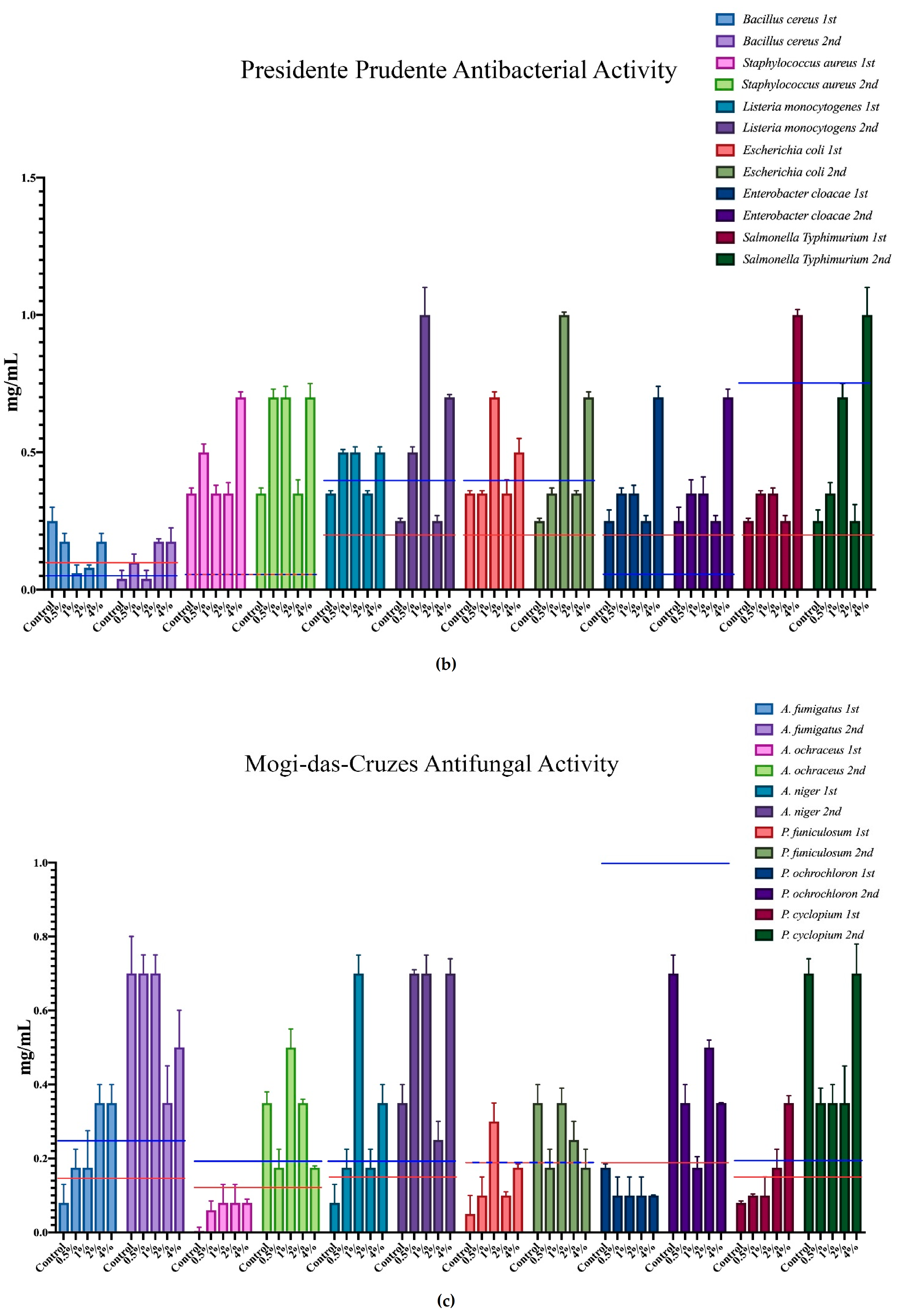
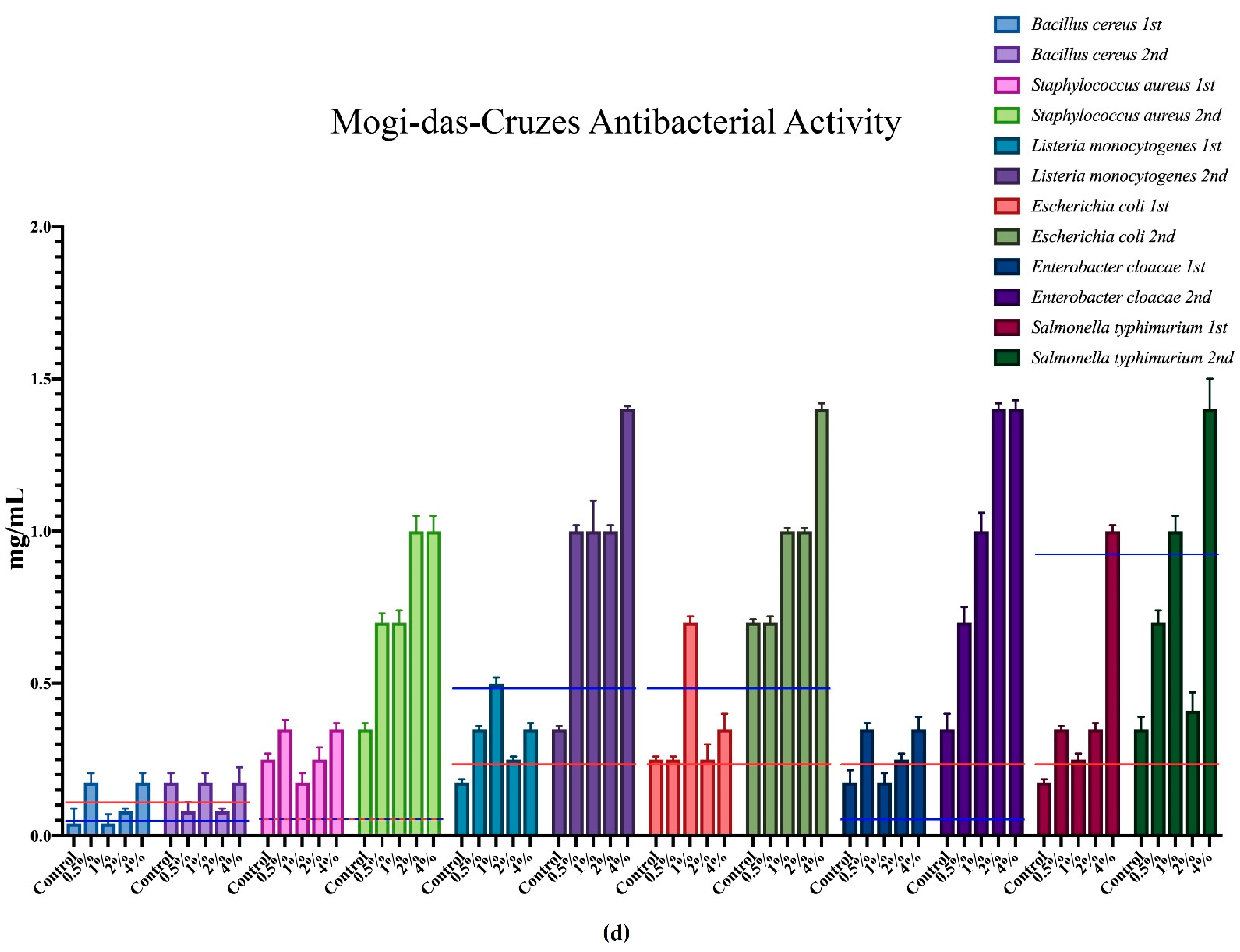
3.3. Antimicrobial Activities
3.4. Linear Discriminant Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angiolillo, L.; Del Nobile, M.A.; Conte, A. The extraction of bioactive compounds from food residues using microwaves. Curr. Opin. Food Sci. 2015, 5, 93–98. [Google Scholar] [CrossRef]
- Naim, L.; Alsanad, M.A.; El Sebaaly, Z.; Shaban, N.; Fayssal, S.A.; Sassine, Y.N. Variation of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) performance subjected to differentdoses and timings of nano-urea. Saudi J. Biol. Sci. 2020, 27, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Nölle, N.; Argyropoulos, D.; Ambacher, S.; Müller, J.; Biesalski, H.K. Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. LWT Food Sci. Technol. 2017, 85, 400–404. [Google Scholar] [CrossRef]
- Thongsook, T.; Kongbangkerd, T. Influence of calcium and silicon supplementation into Pleurotus ostreatus substrates on quality of fresh and canned mushrooms. Food Sci. Technol. Int. 2011, 17, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.A.A.; Carvalho, J.G.; Guimares, P.T.G.; Figueiredo, F.C.; Araújo, A.R. Suprimento do silicato de cálcio e a eficiência nutricional de variedades de cafeeiro. Rev. Bras. Cienc. Solo 2009, 33, 1705–1714. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, R.V.C.; Carocho, M.; Fernandes, Â.; Zied, D.C.; Cobos, J.D.V.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barros, L. Influence of Calcium Silicate on the Chemical Properties of Pleurotus ostreatus var. florida (Jacq.) P. Kumm. J. Fungi 2020, 6, 299. [Google Scholar] [CrossRef] [PubMed]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Talabani, R.M.; Garib, B.T.; Masaeli, R. Bioactivity and physicochemical properties of three calcium silicate-based cements: An in vitro study. BioMed Res. Int. 2020, 2020, 9576930. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, H.K.; Lee, H.N.; Kim, Y.J.; Patel, K.D.; Knowles, J.C.; Lee, J.H.; Song, M. Physical properties and biofunctionalities of bioactive root canal sealers in vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1546–1562. [Google Scholar] [CrossRef]
- Patil, A.; Durgude, A.; Pharande, A.; Kadlag, A.; Nimbalkar, C. Effect of calcium silicate as a silicon source on growth and yield of rice in different acid soils of Karnataka, southern India Socioeconomics. Int. J. Chem. Stud. 2017, 5, 545–549. [Google Scholar]
- Marques, D.J.; Ferreira, M.M.; da Silva Lobato, A.K.; De Freitas, W.A.; Carvalho, J.D.A.; Ferreira, E.D.; Broetto, F. Potential of calcium silicate to mitigate water deficiency in maize. Bragantia 2016, 75, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Parthiban, P.; Chinniah, C.; Baskaran, K.M.; Suresh, K.; Karthick, S. Influence of Calcium Silicate Application on the Population of Sucking Pests of Groundnut (Arachis hypogaea L.). Silicon 2019, 11, 1687–1692. [Google Scholar] [CrossRef]
- Kumara, B.H.; Yogendra, N.D.; Prakash, N.B.; Kumar, A. Effect of calcium silicate and need based nitrogen on pests management in aerobic rice (Oryza sativa L.). Int. J. Plant Prot. 2016, 9, 133–136. [Google Scholar] [CrossRef]
- de Andrade, M.C.N.; Kopytowski Filho, J.; Minhoni, M.T.D.A.; Coutinho, L.N.; Figueiredo, M.B. Productivity, biological efficiency, and number of Agaricus blazei mushrooms grown in compost in the presence of Trichoderma sp. and Chaetomium olivacearum contaminants. Braz. J. Microbiol. 2007, 38, 243–247. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra-Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant: Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Glamočlija, J.; Stojković, D.; Nikolić, M.; Ćirić, A.; Reis, F.S.; Barros, L.; Ferreira, I.C.F.R.; Soković, M. A comparative study on edible Agaricus mushrooms as functional foods. Food Funct. 2015, 6, 1900–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukatani, T.; Suenaga, H.; Shiga, M.; Noguchi, K.; Ishiyama, M.; Ezoe, T.; Matsumoto, K. Comparison of the WST-8 colorimetric method and the CLSI broth microdilution method for susceptibility testing against drug-resistant bacteria. J. Microbiol. Methods 2012, 90, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno [3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Barros, L.; Antonio, A.L.; Barreira, J.C.M.; Bento, A.; Kaluska, I.; Ferreira, I.C.F.R. Analysis of organic acids in electron beam irradiated chestnuts (Castanea sativa Mill.): Effects of radiation dose and storage time. Food Chem. Toxicol. 2013, 55, 348–352. [Google Scholar] [CrossRef]
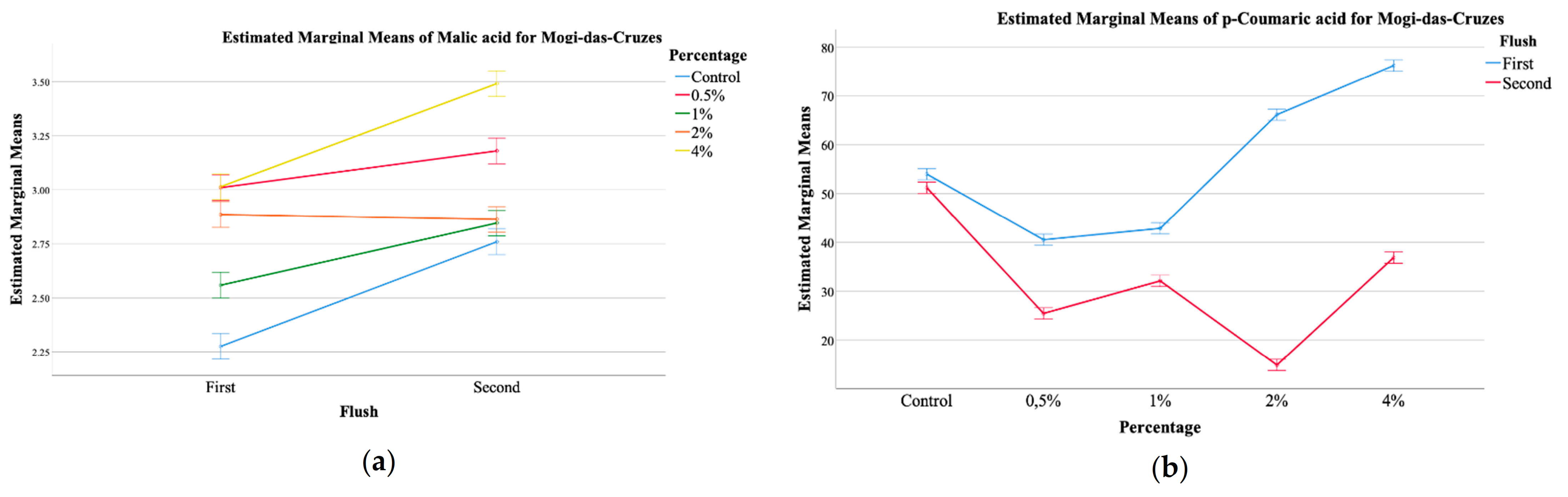



| Mogi-das Cruzes (MC) | |||||||||
| Oxalic Acid (g/100 g) | Malic Acid (g/100 g) | Fumaric Acid (g/100 g) | Total Organic Acids (g/100 g) | Protocatechuic Acid (µg/100 g) | p-coumaric Acid (µg/100 g) | Cinnamic Acid (µg/100 g) | Total Phenolic Acids (µg/100 g) | ||
| Harvest Number (HN) | First | 0.19 ± 0.04 | 2.7 ± 0.3 | 0.250 ± 0.008 | 3.2 ± 0.3 | 116 ± 27 | 56 ± 17 | 36 ± 11 | 208 ± 48 |
| Second | 0.22 ± 0.05 | 3.0 ± 0.3 | 0.257 ± 0.009 | 3.5 ± 0.3 | 177 ± 46 | 32 ± 12 | 30 ± 4 | 239 ± 56 | |
| p-value (n = 15) | t-test | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Calcium Silicate Concentration (CS) | Control | 0.22 ± 0.08 | 2.51 ± 0.3 | 0.251 ± 0.002 | 2.9 ± 0.3 | 137 ± 53 | 52 ± 1 | 26 ± 5 | 216 ± 57 |
| 0.5% | 0.21 ± 0.03 | 3.1 ± 0.1 | 0.25 ± 0.02 | 3.5 ± 0.2 | 167 ± 74 | 33 ± 8 | 37 ± 2 | 237 ± 64 | |
| 1% | 0.16 ± 0.01 | 2.7 ± 0.2 | 0.250 ± 0.003 | 3.1 ± 0.2 | 146 ± 46 | 37 ± 6 | 27 ± 1 | 211 ± 41 | |
| 2% | 0.17 ± 0.02 | 2.87 ± 0.02 | 0.253 ± 0.005 | 3.30 ± 0.04 | 112 ± 16 | 41 ± 28 | 35 ± 12 | 187 ± 56 | |
| 4% | 0.24 ± 0.03 | 3.2 ± 0.3 | 0.259 ± 0.002 | 3.7 ± 0.2 | 170 ± 10 | 56 ± 21 | 40 ± 9 | 266 ± 21 | |
| p-value (n = 6) | THSD test | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HN × CS (n = 30) | p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
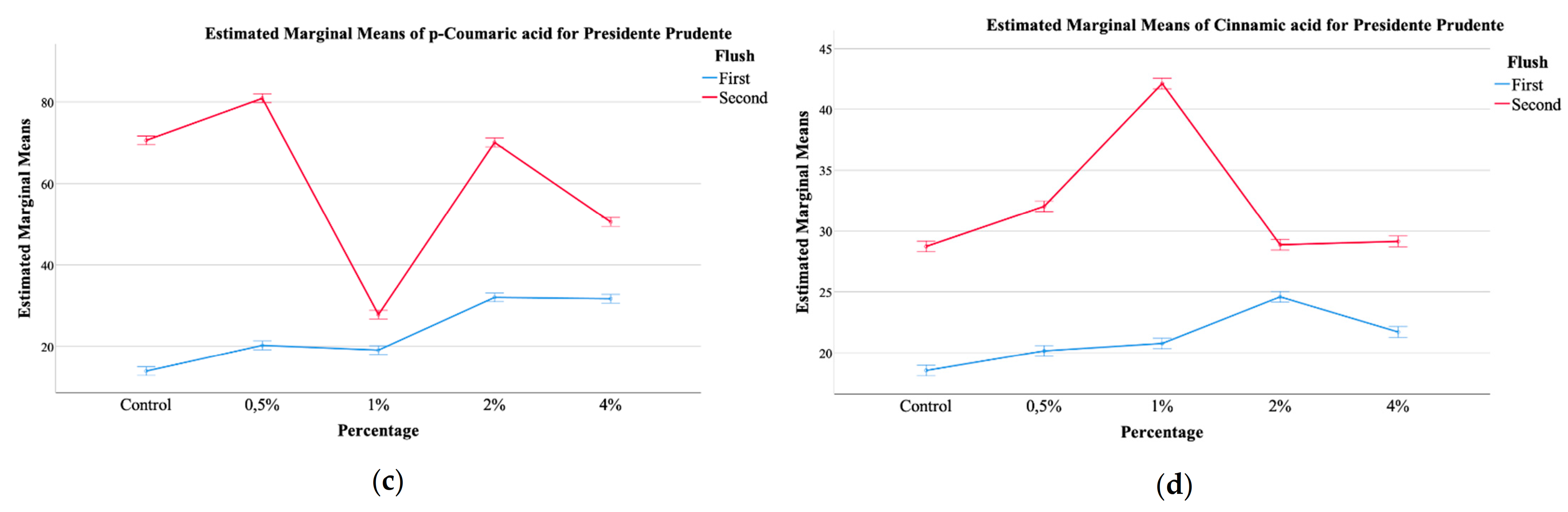
| Presidente Prudente (PP) | |||||||||
| Harvest Number (HN) | First | 0.20 ± 0.08 | 3.5 ± 0.8 | 0.28 ± 0.02 | 4.0 ± 0.8 | 140 ± 62 | 23 ± 8 | 21 ± 2 | 185 ± 67 |
| Second | 0.3 ± 0.2 | 2.8 ± 0.4 | 0.27 ± 0.02 | 3.4 ± 0.3 | 193 ± 29 | 60 ± 19 | 32 ± 5 | 285 ± 38 | |
| p-value (n = 15) | t-test | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Calcium Silicate Concentration (CS) | Control | 0.3 ± 0.2 | 2.6 ± 0.2 | 0.27 ± 0.02 | 3.20 ± 0.04 | 113 ± 58 | 42 ± 31 | 24 ± 6 | 179 ± 94 |
| 0.5% | 0.208 ± 0.008 | 4 ± 1 | 0.27 ± 0.04 | 4 ± 1 | 198 ± 41 | 51 ± 33 | 26 ± 6 | 274 ± 81 | |
| 1% | 0.3 ± 0.2 | 3.0 ± 0.6 | 0.278 ± 0.004 | 3.5 ± 0.4 | 190 ± 16 | 23 ± 5 | 31 ± 12 | 245 ± 33 | |
| 2% | 0.25 ± 0.03 | 3.5 ± 0.6 | 0.29 ± 0.01 | 4.1 ± 0.7 | 211 ± 13 | 51 ± 21 | 27 ± 2 | 290 ± 11 | |
| 4% | 0.25 ± 0.05 | 3.0 ± 0.3 | 0.252 ± 0.009 | 3.5 ± 0.3 | 122 ± 41 | 41 ± 10 | 25 ± 4 | 188 ± 55 | |
| p-value (n = 6) | THSD test | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HN × CS (n = 30) | p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Presidente Prudente (PP) | |||
| TBARS (EC50 mg/mL) | OxHLIA (IC50 μg/mL) | ||
| Harvest Number (HN) | First | 0.8 ± 0.3 | 41 ± 23 |
| Second | 0.5 ± 0.2 | 56 ± 38 | |
| p-value (n = 15) | t-test | <0.001 | <0.001 |
| Calcium Silicate Concentration (CS) | Control | 0.7 ± 0.3 | 67 ± 8 |
| 0.5% | 0.8 ± 0.1 | 80 ± 30 | |
| 1% | 0.6 ± 0.1 | 41 ± 12 | |
| 2% | 0.8 ± 0.6 | 55 ± 16 | |
| 4% | 0.4 ± 0.1 | n.a. | |
| p-value (n = 6) | THSD test | <0.001 | <0.001 |
| HN × CS (n = 30) | p-value | <0.001 | <0.001 |
| Mogi-das Cruzes (MC) | |||
| Harvest Number (HN) | First | 0.7 ± 0.1 | 16 ± 32 |
| Second | 0.5 ± 0.2 | 56 ± 51 | |
| p-value (n = 15) | t-test | <0.001 | <0.001 |
| Calcium Silicate Concentration (CS) | Control | 0.4 ± 0.2 | n.a. |
| 0.5% | 0.44 ± 0.05 | n.a. | |
| 1% | 0.6 ± 0.2 | 30 ± 33 | |
| 2% | 0.75 ± 0.02 | 54 ± 60 | |
| 4% | 0.69 ± 0.05 | 96 ± 20 | |
| p-value (n = 6) | THSD test | <0.001 | <0.001 |
| HN × CS (n = 30) | p-value | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, R.V.C.; Carocho, M.; Fernandes, Â.; Pinela, J.; Stojković, D.; Soković, M.; Zied, D.C.; Cobos, J.D.V.; González-Paramás, A.M.; Ferreira, I.C.F.R.; et al. Antioxidant and Antimicrobial Influence on Oyster Mushrooms (Pleurotus ostreatus) from Substrate Supplementation of Calcium Silicate. Sustainability 2021, 13, 5019. https://doi.org/10.3390/su13095019
Cardoso RVC, Carocho M, Fernandes Â, Pinela J, Stojković D, Soković M, Zied DC, Cobos JDV, González-Paramás AM, Ferreira ICFR, et al. Antioxidant and Antimicrobial Influence on Oyster Mushrooms (Pleurotus ostreatus) from Substrate Supplementation of Calcium Silicate. Sustainability. 2021; 13(9):5019. https://doi.org/10.3390/su13095019
Chicago/Turabian StyleCardoso, Rossana V. C., Márcio Carocho, Ângela Fernandes, José Pinela, Dejan Stojković, Marina Soković, Diego Cunha Zied, Juan Diego Valenzuela Cobos, Ana M. González-Paramás, Isabel C. F. R. Ferreira, and et al. 2021. "Antioxidant and Antimicrobial Influence on Oyster Mushrooms (Pleurotus ostreatus) from Substrate Supplementation of Calcium Silicate" Sustainability 13, no. 9: 5019. https://doi.org/10.3390/su13095019









