Effects of the Curing Conditions on the Carbonation Curing Efficiency of Ordinary Portland Cement and a Belite-Rich Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Curing Conditions and Test Methods
3. Results and Discussion
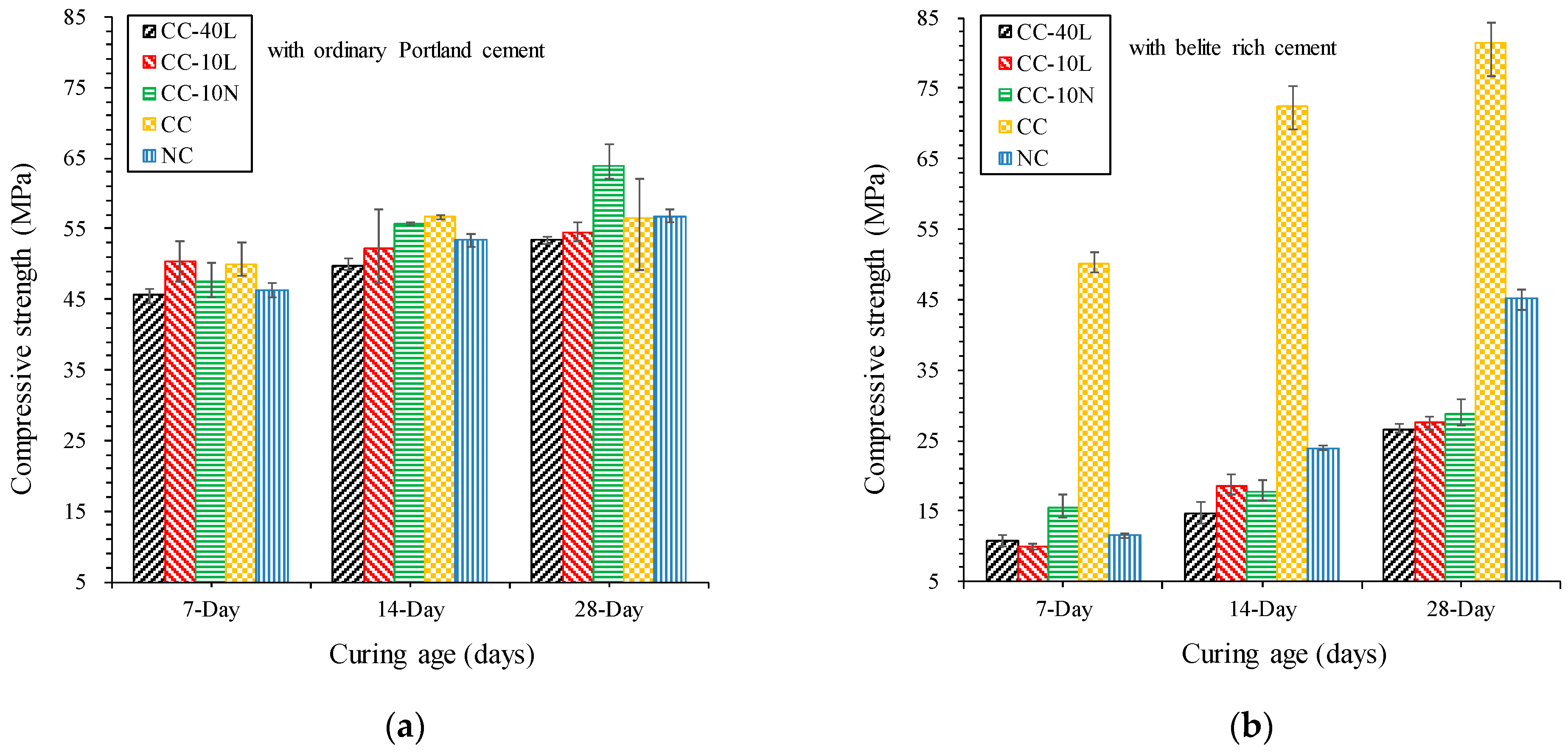
3.1. Compressive Strength
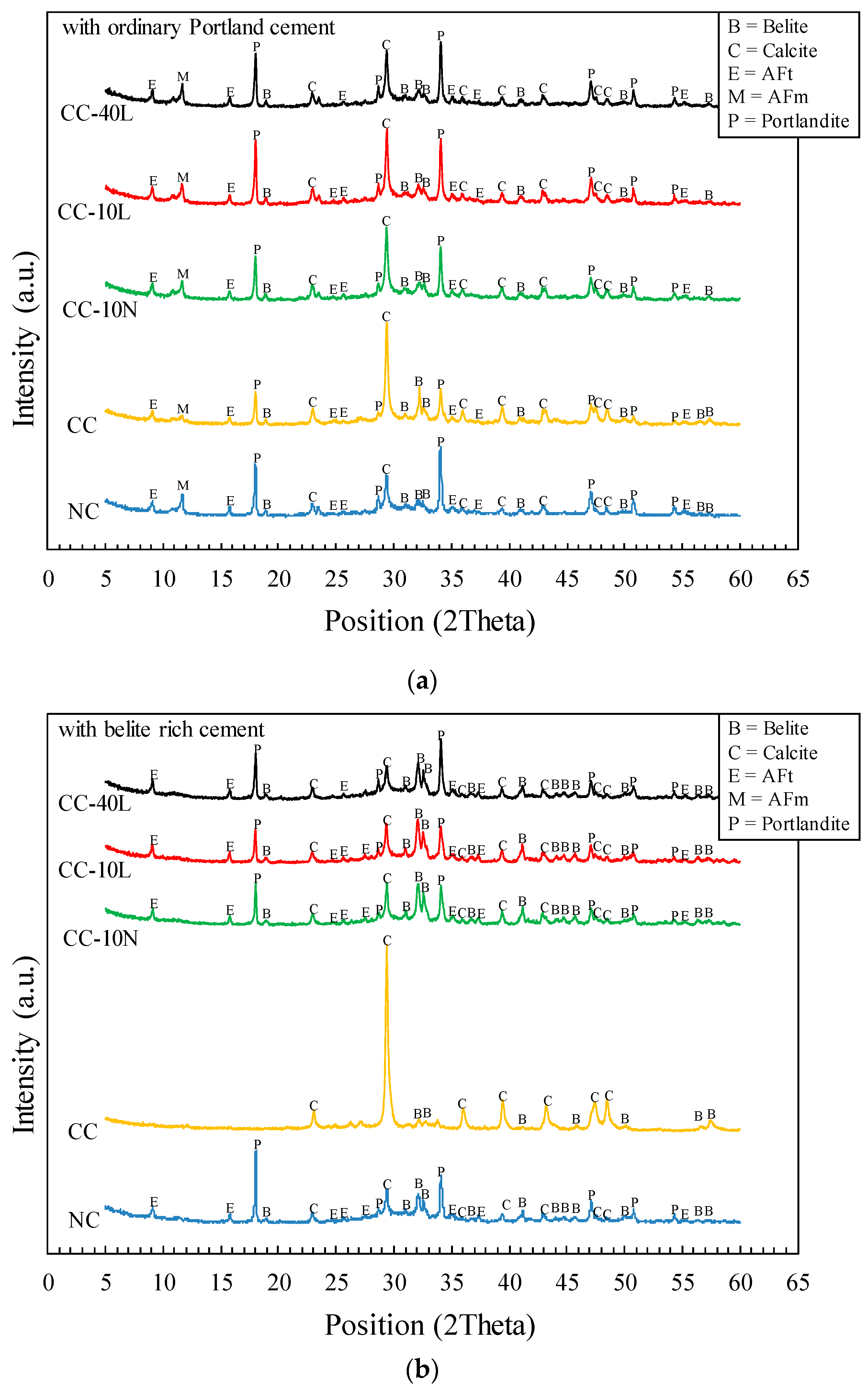
3.2. X-ray Diffraction
3.3. Thermogravimetric Analysis
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhan, B.; Poon, C.; Shi, C. CO2 curing for improving the properties of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2013, 42, 1–8. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X. Effect of carbonation curing regime on strength and microstructure of Portland cement paste. J. CO2 Util. 2019, 34, 74–86. [Google Scholar] [CrossRef]
- Maddalena, R.; Roberts, J.J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- IEA. CO2 Emissions in 2019; IEA: Paris, France, 2019; Available online: https://www.iea.org/articles/global-co2-emissions-in-2019 (accessed on 6 May 2021).
- IEA. Global Status Report for Buildings and Construction 2019; IEA: Paris, France, 2019; Available online: https://www.iea.org/reports/global-status-report-for-buildings-and-construction-2019 (accessed on 6 May 2021).
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production-present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Andersson, R.; Stripple, H.; Gustafsson, T.; Ljungkrantz, C. Carbonation as a method to improve climate performance for cement based material. Cem. Concr. Res. 2019, 124. [Google Scholar] [CrossRef]
- Kelektsoglou, K. Carbon capture and storage: A review of mineral storage of CO2 in Greece. Sustainability 2018, 10, 4400. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Gao, X. Use of Carbonation Curing to Improve Mechanical Strength and Durability of Pervious Concrete. ACS Sustain. Chem. Eng. 2020, 8, 3872–3884. [Google Scholar] [CrossRef]
- Neves Junior, A.; Toledo Filho, R.D.; de Moraes Rego Fairbairn, E.; Dweck, J. The effects of the early carbonation curing on the mechanical and porosity properties of high initial strength Portland cement pastes. Constr. Build. Mater. 2015, 77, 448–454. [Google Scholar] [CrossRef]
- Li, Z.; He, Z.; Chen, X. The performance of carbonation-cured concrete. Materials 2019, 12, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of Hydration of Calcium Silicates by Carbon Dioxide Treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Siddique, S.; Naqi, A.; Jang, J.G. Influence of water to cement ratio on CO2 uptake capacity of belite-rich cement upon exposure to carbonation curing. Cem. Concr. Compos. 2020, 111, 103616. [Google Scholar] [CrossRef]
- Han, S.H.; Jun, Y.; Shin, T.Y.; Kim, J.H. CO2 curing efficiency for cement paste and mortars produced by a low water-to-cement ratio. Materials 2020, 13, 3883. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Berger, R.L.; Breese, J. Accelerated Curing of Compacted Calcium Silicate Mortars on Exposure to CO2. J. Am. Ceram. Soc. 1974, 57, 394–397. [Google Scholar] [CrossRef]
- Kou, S.-C.; Zhan, B.; Poon, C.-S. Use of a CO2 curing step to improve the properties of concrete prepared with recycled aggregates. Cem. Concr. Compos. 2014, 45, 22–28. [Google Scholar] [CrossRef]
- Jeon, I.K.; Qudoos, A.; Kim, H.G. Influence of carbonation curing on hydration and microstructure of magnesium potassium phosphate cement concrete. J. Build. Eng. 2021, 38. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Chen, T. Influence of mineral admixtures on carbonation curing of cement paste. Constr. Build. Mater. 2019, 212, 653–662. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Yu, Z. Effects of environmental factors on concrete carbonation depth and compressive strength. Materials 2018, 11, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiş, C.D. Carbonation-porosity-strength model for fly ash concrete. J. Mater. Civ. Eng. 2004, 16, 91–94. [Google Scholar] [CrossRef]
- Yang, T.; Keller, B.; Magyari, E.; Hametner, K.; Günther, D. Direct observation of the carbonation process on the surface of calcium hydroxide crystals in hardened cement paste using an atomic force microscope. J. Mater. Sci. 2003, 38, 1909–1916. [Google Scholar] [CrossRef]
- Chun, Y.-M.; Naik, T.R.; Kraus, R.N. Carbon dioxide sequestration in concrete in different curing environments. Proc. Coventry Univ. Int. Conf. Sustain. Constr. Mater. Technol. 2007, 18–24. [Google Scholar] [CrossRef]
- Tu, Z.; Guo, M.-Z.; Poon, C.S.; Shi, C. Effects of limestone powder on CaCO3 precipitation in CO2 cured cement pastes. Cem. Concr. Compos. 2016, 72, 9–16. [Google Scholar] [CrossRef]
- Gao, H.; Liao, H.; Wang, M.; Cheng, F. Reinforcing the physicochemical properties of concrete through synergism of CO2 curing and Ca(OH)2 solution drenching. Constr. Build. Mater. 2021, 280. [Google Scholar] [CrossRef]
- Pan, X.; Shi, C.; Farzadnia, N.; Hu, X.; Zheng, J. Properties and microstructure of CO2 surface treated cement mortars with subsequent lime-saturated water curing. Cem. Concr. Compos. 2019, 99, 89–99. [Google Scholar] [CrossRef]
- Chougule, A.R.; Patil, M.B.; Prakash, K.B. Effect on different curing methods on the properties of hvggbfs concrete. Int. J. Civ. Eng. Technol. 2018, 9, 1175–1183. [Google Scholar]
- Yeih, W.; Chang, J.J. The influences of cement type and curing condition on properties of pervious concrete made with electric arc furnace slag as aggregates. Constr. Build. Mater. 2019, 197, 813–820. [Google Scholar] [CrossRef]
- El-Didamony, H.; Sharara, A.M.; Helmy, I.M.; El-Aleem, S.A. Hydration characteristics of β-C2S in the presence of some accelerators. Cem. Concr. Res. 1996, 26, 1179–1187. [Google Scholar] [CrossRef]
- Nishikawa, T.; Suzuki, K.; Ito, S.; Sato, K.; Takebe, T. Decomposition of synthesized ettringite by carbonation. Cem. Concr. Res. 1992, 22, 6–14. [Google Scholar] [CrossRef]
- Hager, I. Colour Change in Heated Concrete. Fire Technol. 2014, 50, 945–958. [Google Scholar] [CrossRef] [Green Version]
- Vance, K.; Falzone, G.; Pignatelli, I.; Bauchy, M.; Balonis, M.; Sant, G. Direct Carbonation of Ca(OH)2 Using Liquid and Supercritical CO2: Implications for Carbon-Neutral Cementation. Ind. Eng. Chem. Res. 2015, 54, 8908–8918. [Google Scholar] [CrossRef]
- Cuesta, A.; Ayuela, A.; Aranda, M.A.G. Belite cements and their activation. Cem. Concr. Res. 2021, 140, 106319. [Google Scholar] [CrossRef]
- Guerrero, A.; Goñi, S.; Macías, A.; Luxán, M.P. Effect of the starting fly ash on the microstructure and mechanical properties of fly ash–belite cement mortars. Cem. Concr. Res. 2000, 30, 553–559. [Google Scholar] [CrossRef]



| Cement | Chemical Composition (wt.%) | Mineral Composition 1 (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | R2O | C3S | C2S | C3A | C4AF | |
| Ordinary Portland cement | 62.5 | 21.0 | 5.9 | 3.2 | 2.1 | 0.8 | 49 | 23 | 10 | 9 |
| Belite-rich cement | 62.5 | 25.3 | 3.1 | 3.6 | 2.0 | 0.5 | 31 | 48 | 3 | 11 |
| No. | Code | Curing Method | Permeating Liquid | Depth 2 |
|---|---|---|---|---|
| 1 | CC-40L | Carbonation curing 1 | Lime-saturated water | 40 mm |
| 2 | CC-10L | Carbonation curing | Lime-saturated water | 10 mm |
| 3 | CC-10N | Carbonation curing | Water | 10 mm |
| 4 | CC | Carbonation curing | - | - |
| 5 | NC | Water curing | Water | >50 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Pei, J.; Siddique, S.; Jang, J.-G. Effects of the Curing Conditions on the Carbonation Curing Efficiency of Ordinary Portland Cement and a Belite-Rich Cement Mortar. Sustainability 2021, 13, 5175. https://doi.org/10.3390/su13095175
Kim H, Pei J, Siddique S, Jang J-G. Effects of the Curing Conditions on the Carbonation Curing Efficiency of Ordinary Portland Cement and a Belite-Rich Cement Mortar. Sustainability. 2021; 13(9):5175. https://doi.org/10.3390/su13095175
Chicago/Turabian StyleKim, Hyeju, Junjie Pei, Salman Siddique, and Jeong-Gook Jang. 2021. "Effects of the Curing Conditions on the Carbonation Curing Efficiency of Ordinary Portland Cement and a Belite-Rich Cement Mortar" Sustainability 13, no. 9: 5175. https://doi.org/10.3390/su13095175
APA StyleKim, H., Pei, J., Siddique, S., & Jang, J.-G. (2021). Effects of the Curing Conditions on the Carbonation Curing Efficiency of Ordinary Portland Cement and a Belite-Rich Cement Mortar. Sustainability, 13(9), 5175. https://doi.org/10.3390/su13095175








