Vibrational Communication of Scolypopa australis (Walker, 1851) (Hemiptera: Ricaniidae)—Towards a Novel Sustainable Pest Management Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
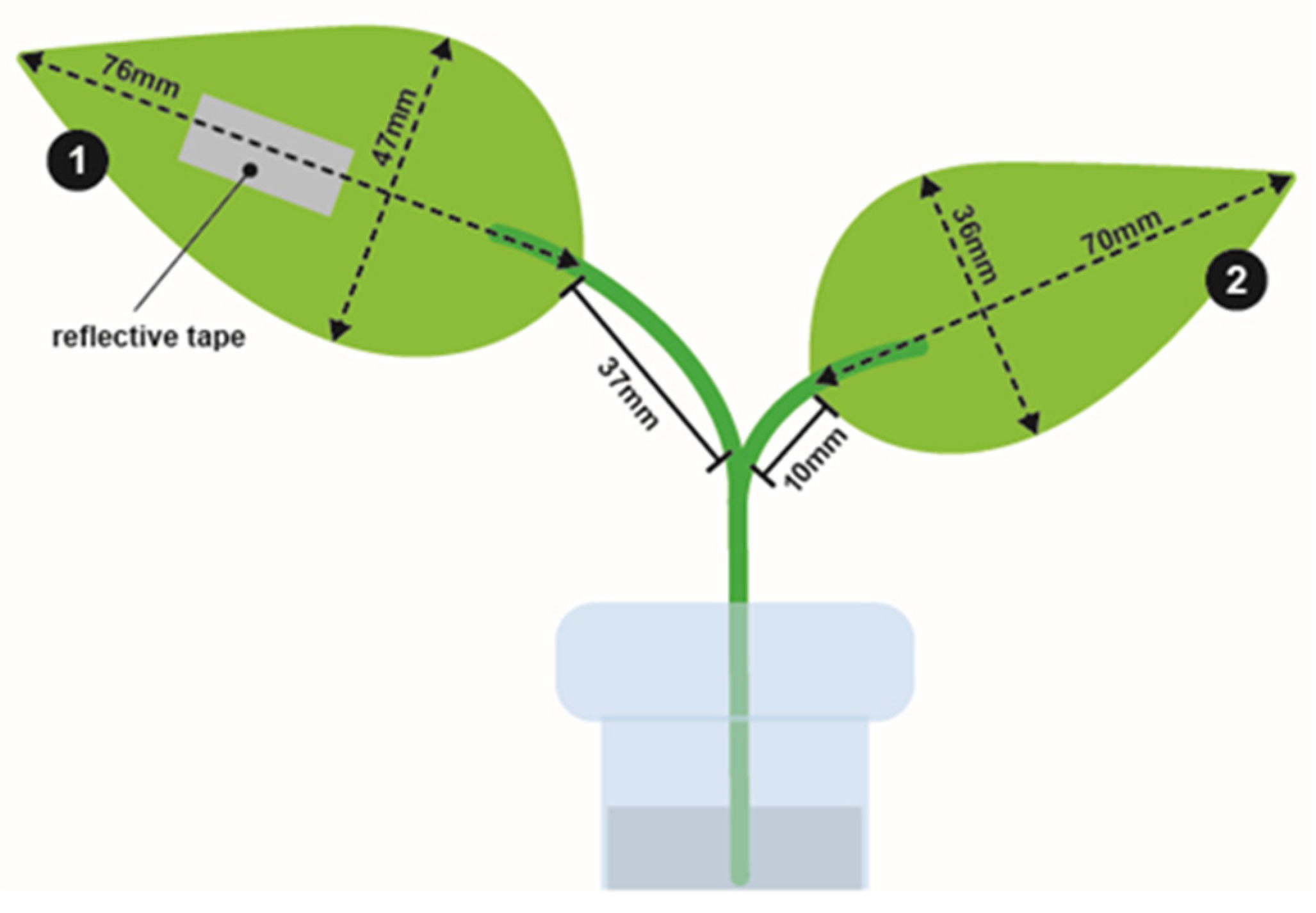
2.2. Experiments
2.3. Data Analysis
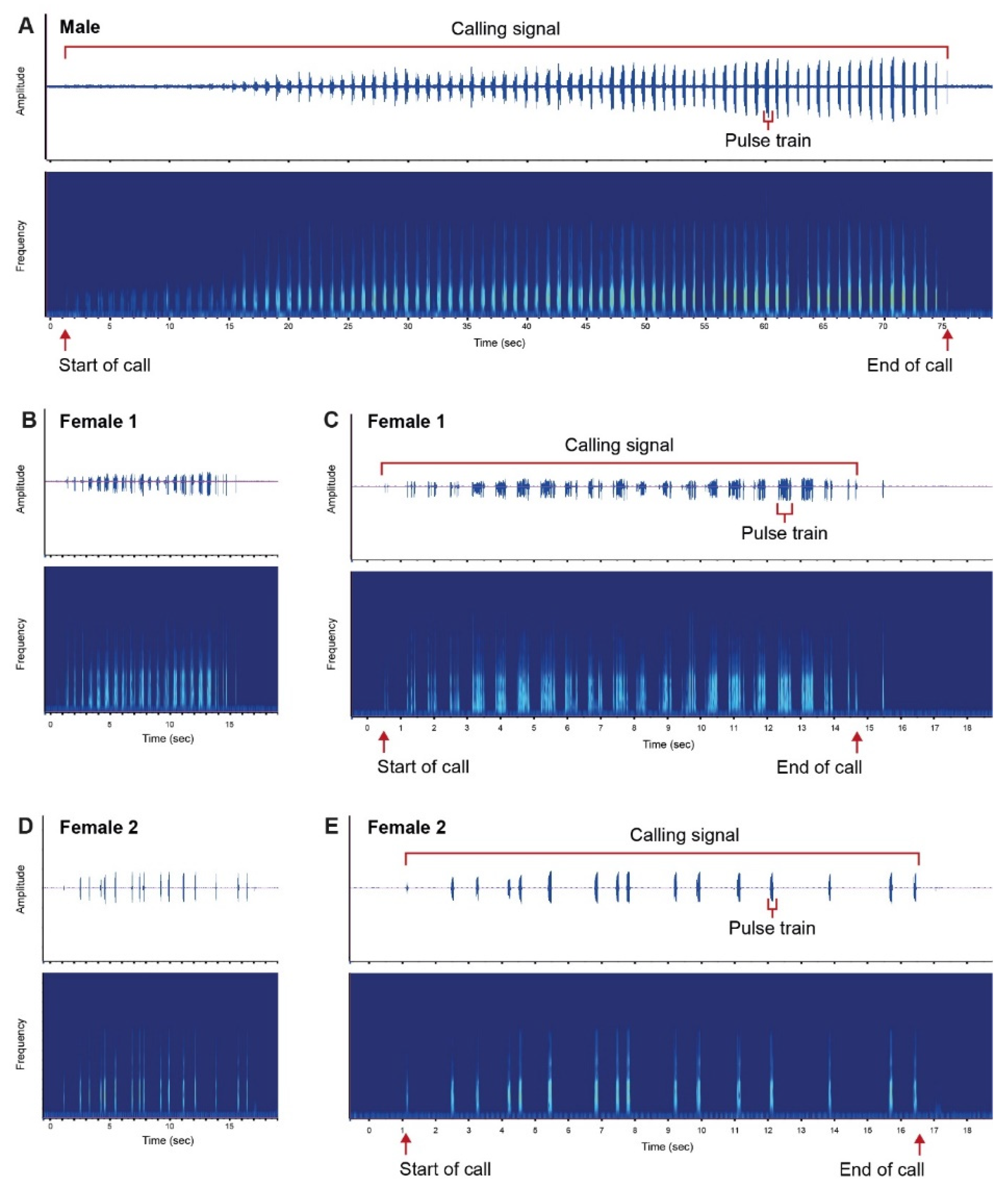
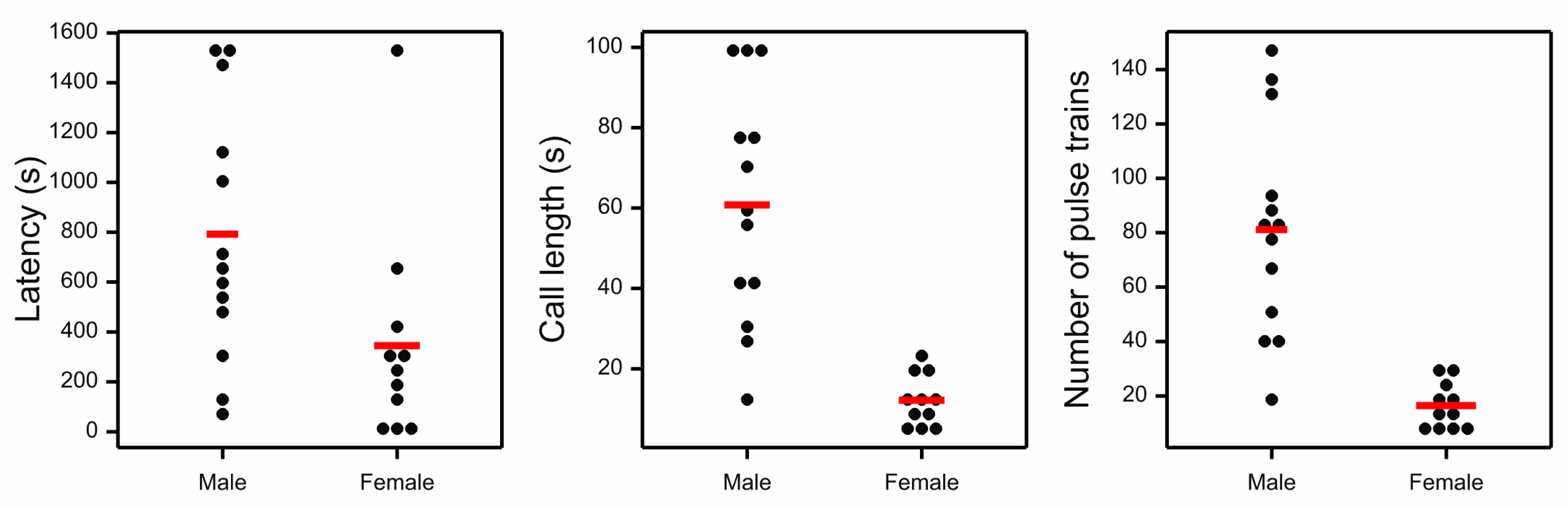
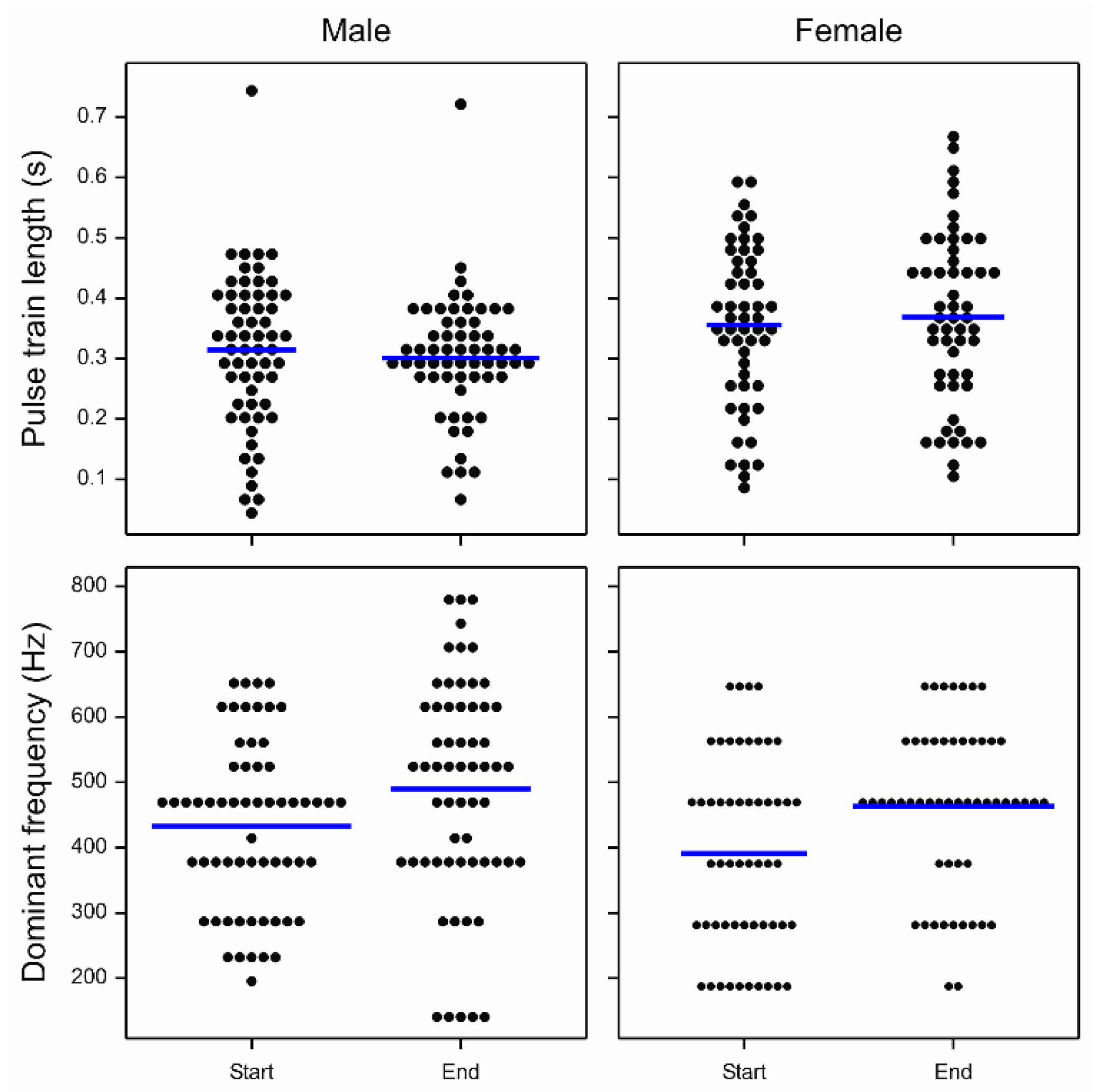
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, V.; Barker, A. Evaluating the Impact of Sooty Mould in New Zealand: Retrospective Report by Scarlatti for Zespri. 2020; p. 17. [Google Scholar]
- Tomkins, A.R.; Holland, P.T.; Thomson, C.; Wilson, D.J.; Malcolm, C.T. Residual life of spinosad on kiwifruit–Biological and chemical studies. N. Z. Plant Prot. 1999, 52, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Holland, P.T.; McGhie, T.K.; Malcom, C.P. Residual life of pesticides on kiwifruit. In Proceedings of the New Zealand Weed and Pest Control Conference, Christchurch, New Zealand, 14–16 August 1984; pp. 136–141. [Google Scholar]
- Hill, P.S.M.; Lakes-Harlan, R.; Mazzoni, V.; Narins, P.M.; Virant-Doberlet, M.; Wessel, A. Biotremology: Studying Vibrational Behavior; Springer: New York, NY, USA, 2019. [Google Scholar]
- Mazzoni, V.; Polajnar, J.; Baldini, M.; Rossi Stacconi, M.V.; Anfora, G.; Guidetti, R.; Maistrello, L. Use of substrate-borne vibrational signals to attract the Brown Marmorated Stink Bug, Halyomorpha halys. J. Pest Sci. 2017, 90, 1219–1229. [Google Scholar] [CrossRef]
- Polajnar, J.; Maistrello, L.; Ibrahim, A.; Mazzoni, V. Can vibrational playback improve control of an invasive stink bug? In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer: New York, NY, USA, 2019; pp. 375–398. [Google Scholar]
- Polajnar, J.; Eriksson, A.; Virant-Doberlet, M.; Mazzoni, V. Mating disruption of a grapevine pest using mechanical vibrations: From laboratory to the field. J. Pest Sci. 2016, 89, 909–921. [Google Scholar] [CrossRef]
- Mazzoni, V.; Lucchi, A.; Čokl, A.; Prešern, J.; Virant-Doberlet, M. Disruption of the reproductive behaviour of Scaphoideus titanus by playback of vibrational signals. Entomol. Exp. Appl. 2009, 133, 174–185. [Google Scholar] [CrossRef]
- Mankin, R.W. Vibrational trapping and interference with mating of Diaphorina citri. In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer: New York, NY, USA, 2019; pp. 399–413. [Google Scholar]
- Mazzoni, V.; Nieri, R.; Eriksson, A.; Virant-Doberlet, M.; Polajnar, J.; Anfora, G.; Lucchi, A. Mating disruption by vibrational signals: State of the field and perspectives. In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer: New York, NY, USA, 2019; pp. 331–354. [Google Scholar]
- Korinšek, G.; Tuma, T.; Virant-Doberlet, M. Automated vibrational signal recognition and playback. In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer: New York, NY, USA, 2019; pp. 149–173. [Google Scholar]
- Zespri. Available online: https://www.zespri.com/en-NZ/Sustainability-Our-Environment (accessed on 6 December 2021).
- A European Green Deal. Available online: https://bit.ly/38rj2sM (accessed on 6 December 2021).
- Broughton, W.B. Method in bioacoustic terminology. In Acoustic Behaviour of Animals; Busnel, R.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1963; p. 324. [Google Scholar]
- Alexander, R.D. Acoustical communication in arthropods. Annu. Rev. Entomol. 1967, 12, 495–526. [Google Scholar] [CrossRef]
- Avosani, S.; Franceschi, P.; Ciolli, M.; Verrastro, V.; Mazzoni, V. Vibrational playbacks and microscopy to study the signalling behaviour and female physiology of Philaenus spumarius. J. Appl. Entomol. 2021, 145, 518–529. [Google Scholar] [CrossRef]
- Mazzoni, V.; Prešern, J.; Lucchi, A.; Virant-Doberlet, M. Reproductive strategy of the Nearctic leafhopper Scaphoideus titanus Ball (Hemiptera: Cicadellidae). Bull. Entomol. Res. 2009, 99, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Nieri, R.; Mazzoni, V.; Gordon, S.D.; Krugner, R. Mating behavior and vibrational mimicry in the glassy-winged sharpshooter, Homalodisca vitripennis. J. Pest Sci. 2017, 90, 887–899. [Google Scholar] [CrossRef]
- Avosani, S.; Daher, E.; Franceschi, P.; Ciolli, M.; Verrastro, V.; Mazzoni, V. Vibrational communication and mating behavior of the meadow spittlebug Philaenus spumarius. Entomol. Gen. 2020, 40, 307–321. [Google Scholar] [CrossRef]
- Mazzoni, V.; Lucchi, A.; Ioriatti, C.; Virant-Doberlet, M.; Anfora, G. Mating behavior of Hyalesthes obsoletus (Hemiptera: Cixiidae). Ann. Entomol. Soc. Am. 2010, 103, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Derlink, M.; Abt, I.; Mabon, R.; Julian, C.; Virant-Doberlet, M.; Jacquot, E. Mating behavior of Psammotettix alienus (Hemiptera: Cicadellidae). Insect Sci. 2018, 25, 148–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhelj, A.; De Groot, M.; Pajk, F.; Simčič, T.; Virant-Doberlet, M. Energetic cost of vibrational signalling in a leafhopper. Behav. Ecol. Sociobiol. 2015, 69, 815–828. [Google Scholar] [CrossRef]
- Eberhard, W.G. Copulatory courtship and cryptic female choice in insects. Biol. Rev. 1991, 66, 1–31. [Google Scholar] [CrossRef]
- Virant-Doberlet, M.; Žežlina, I. Vibrational communication of Metcalfa pruinosa (Hemiptera: Fulgoroidea: Flatidae). Ann. Entomol. Soc. Am. 2007, 100, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Gerard, P.J. Aspects of the Ecology of Scolypopa australis (Walker) (Homoptera:Ricaniidae) and Its Parasite Centrodora scolypopae (Valentine) (Hymenoptera: Aphelinidae). Ph.D. Thesis, The University of Waikato, Hamilton, New Zealand, 1985. [Google Scholar]
- Mankin, R.; Hagstrum, D.; Guo, M.; Eliopoulos, P.; Njoroge, A. Automated applications of acoustics for stored product insect detection, monitoring, and management. Insects 2021, 12, 259. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sullivan, N.J.; Avosani, S.; Butler, R.C.; Stringer, L.D. Vibrational Communication of Scolypopa australis (Walker, 1851) (Hemiptera: Ricaniidae)—Towards a Novel Sustainable Pest Management Tool. Sustainability 2022, 14, 185. https://doi.org/10.3390/su14010185
Sullivan NJ, Avosani S, Butler RC, Stringer LD. Vibrational Communication of Scolypopa australis (Walker, 1851) (Hemiptera: Ricaniidae)—Towards a Novel Sustainable Pest Management Tool. Sustainability. 2022; 14(1):185. https://doi.org/10.3390/su14010185
Chicago/Turabian StyleSullivan, Nicola Jayne, Sabina Avosani, Ruth C. Butler, and Lloyd D. Stringer. 2022. "Vibrational Communication of Scolypopa australis (Walker, 1851) (Hemiptera: Ricaniidae)—Towards a Novel Sustainable Pest Management Tool" Sustainability 14, no. 1: 185. https://doi.org/10.3390/su14010185
APA StyleSullivan, N. J., Avosani, S., Butler, R. C., & Stringer, L. D. (2022). Vibrational Communication of Scolypopa australis (Walker, 1851) (Hemiptera: Ricaniidae)—Towards a Novel Sustainable Pest Management Tool. Sustainability, 14(1), 185. https://doi.org/10.3390/su14010185






