Occurrence of Linear Alkylbenzene Sulfonates, Nonylphenol Ethoxylates and Di(2-ethylhexyl)phthalate in Composting Processes: Environmental Risks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Biosolid Samples
2.3. Determination of Sludge Characterization Parameters
2.4. Analysis of LAS, NPE and DEHP
2.5. Ecotoxicological Risk Assessment
3. Results
3.1. Characterization Parameters
3.2. Concentrations of Organic Compounds
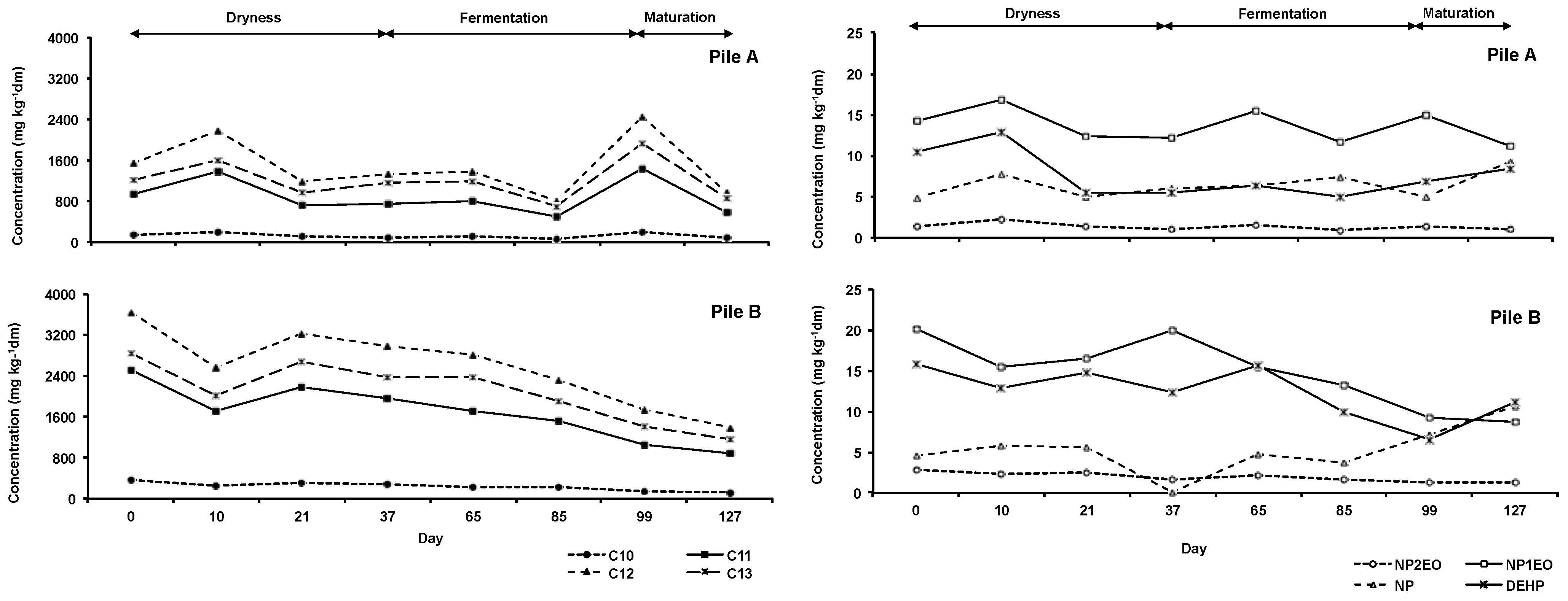
3.3. Occurrence of Organic Pollutants during the Composting Process
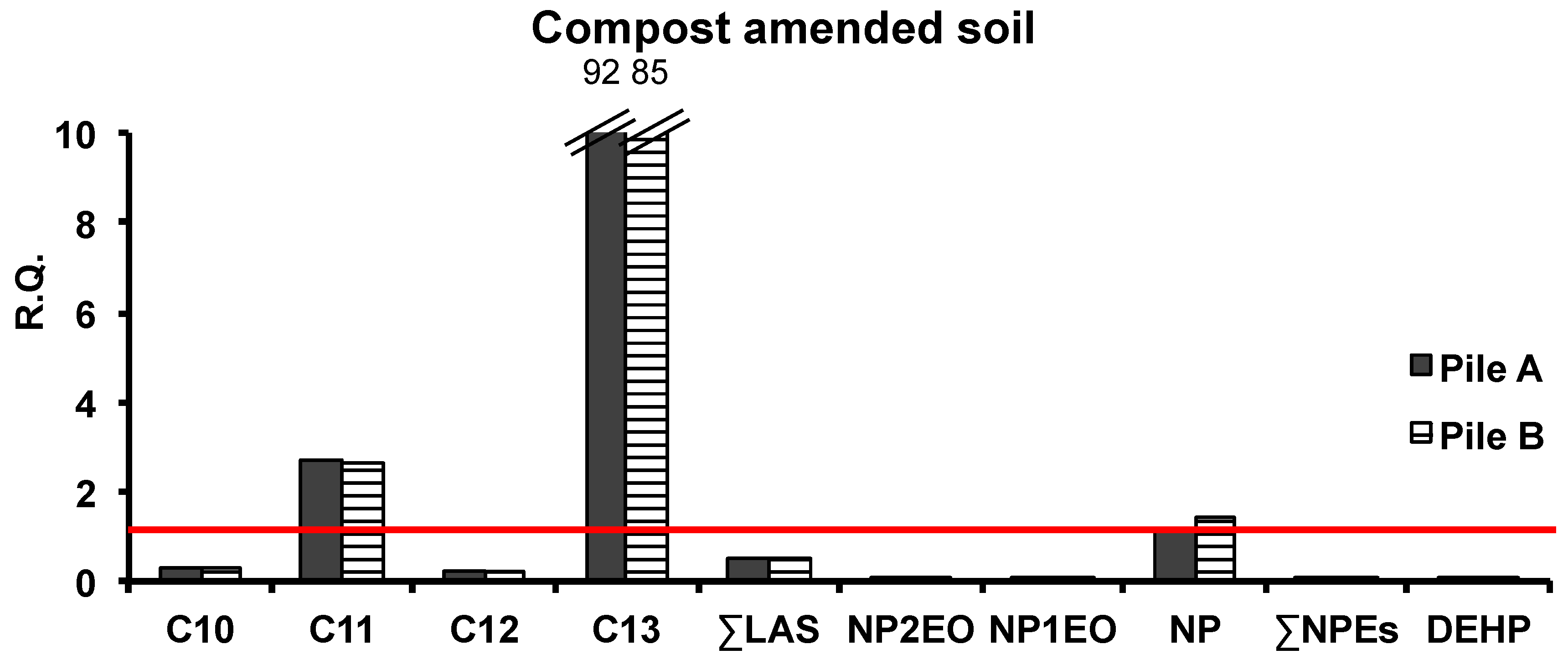
3.4. Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceuticals and their metabolites in sewage sludge and soil: A review on their distribution and environmental risk assessment. Tr. Environ. Anal. Chem. 2021, 30, e00125. [Google Scholar] [CrossRef]
- Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Approaches to the implications of the EU directive on sludge: Analytical methodologies, concentration levels and occurrence of organic pollutants in different types of sewage sludge. In Sewage Sludge Management: From the Past to Our Century; Zorpas, A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 123–137. [Google Scholar]
- Fijalkowski, K. Emerging contaminants in sludge (endocrine disruptors, pesticides, and pharmaceutical residues, including illicit drugs/controlled substances, etc.). Ind. Munic. Sludge 2020, 455–473. [Google Scholar] [CrossRef]
- Francou, C.; Poitrenaud, M.; Houot, S. Stabilization of organic matter during composting: Influence of process and feedstock. Compost Scie Util 2005, 13, 72–83. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge: The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- EU. Working Document on Sludge, Third Draft; EU: Brussels, Belgium, 27 April 2000. [Google Scholar]
- Aparicio, I.; Santos, J.L.; Alonso, E. Limitation of the concentration of organic pollutants in sewage sludge for agricultural purposes: A case study in South Spain. Waste Manag. 2009, 29, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- González, M.M.; Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Degradation and environmental risk of surfactants after the application of compost sludge to the soil. Waste Manag. 2012, 32, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, S.; Tor, A.; Aydin, M.E. Investigation on the levels of heavy metals, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls in sewage sludge samples and ecotoxicological testing. Clean-Soil Air Water 2013, 41, 411–418. [Google Scholar] [CrossRef]
- Clarke, R.M.; Cummins, E. Evaluation of ‘classic’ and emerging contaminants resulting from the application of biosolids to agricultural lands: A Review. Human Ecol. Risk Assess. Int. J. 2015, 7039, 492–513. [Google Scholar] [CrossRef]
- APHA-AWWA-WPCF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- González, M.M.; Santos, J.L.; Aparicio, I.; Alonso, E. Method for the simultaneous determination of the most problematic families of organic pollutants in compost and compost-amended soil. Anal. Bioanal. Chem. 2010, 397, 222–285. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Determination of priority pollutants in aqueous samples by dispersive liquid-liquid microextraction. Anal. Chim. Acta. 2013, 773, 60–67. [Google Scholar] [CrossRef]
- European Community (EC). echnical GUIDANCE Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) no. 1488/94 on Risk Assessment for Existing Substances and Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market, Parts I, II and IV; EUR 20418 EN/1; European Communities: Brussels, Belgium, 2003. [Google Scholar]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Haz. Mat. 2012, 239, 40–47. [Google Scholar] [CrossRef]
- Ying, G.G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Perez, M.C.; Perales-Vargas, J.A.; Nebot-Sanz, E.; Sales-Marquez, D. Effect of the test media and toxicity of LAS on the growth of Isochrysis galbana. Ecotoxicology 2008, 17, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Fenner, K.; Kooijman, C.; Scheringer, M.; Hungerbuhler, K. Including transformation products into the risk assessment for chemicals: The case of nonylphenol ethoxylate usage in Switzerland. Environ. Sci. Technol. 2002, 36, 1147–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, J.E.; Adams, W.J.; Biddinger, G.R.; Robillard, K.A.; Gorsuch, J.W. Chronic toxicity of 14 phthalate esters to Daphnia magna and rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 1995, 14, 1967–1976. [Google Scholar] [CrossRef]
- Feijtel, T.C.J.; Struijs, J.; Matthis, E. Exposure modelling of detergent surfactants – Prediction of 90th-percentile concentrations in the Netherlands. Environ. Toxicol. Chem. 1999, 18, 2645–2652. [Google Scholar]
- During, R.A.; Krahe, S.; Gäth, S. Sorption behaviour of nonylphenol in terrestrial soils. Environ. Sci. Technol. 2002, 36, 4052–4057. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, J.; Sun, H.; Dai, S. Sediment-porewater partition of nonylphenol polyethoxylates: Field measurements from Lanzhou Reach of Yellow River, China. Arch. Environ. Contam. Toxicol. 2008, 55, 173–179. [Google Scholar] [CrossRef]
- Bis (2-ethylhexyl) Phthalate (DEHP). Summary Risk Assessment Report. European Comission. Institute for Health and Consumer Protection Toxicology and Chemical Substance (TCS) European Chemicals Bureau I-21027 Ispra (VA) Italy. Available online: http://echa.europa.eu/documents/10162/060d4981-4dfb-4e40-8c69-6320c9debb01 (accessed on 1 December 2021).
- Alonso Álvarez, E.; Callejón Mochón, M.; .Jiménez Sánchez, J.C.; Ternero Rodríguez, M. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 2002, 47, 765–775. [Google Scholar] [CrossRef]
- Alonso, E.; Aparicio, I.; Santos, J.L.; Villar, P.; Santos, A. Sequential extraction of metals from mixed and digested sludge from aerobic WWTPs sited in the south of Spain. Waste Manag. 2009, 29, 418–424. [Google Scholar] [CrossRef]
- Camacho-Muñoz, D.; Martín, J.; Santos, J.L.; Alonso, E.; Aparicio, I.; De la Torre, T.; Rodríguez, C.; Malfeito, J.J. Effectiveness of three configurations of membrane bioreactors on the removal of priority and emergent organic compounds from wastewater: Comparison with conventional wastewater treatments. J. Environ. Monit. 2012, 14, 1428–1436. [Google Scholar] [CrossRef]
- Lara-Martín, P.A.; González-Mazo, E.; Brownawell, B.J. Multi-residue method for the analysis of synthetic surfactants and their degradation metabolites in aquatic systems by liquid chromatography-time-of-flight-mass spectrometry. J. Chromatogr. A 2011, 1218, 4799–4807. [Google Scholar] [CrossRef] [PubMed]
- Pakou, C.; Kornaros, M.; Stamatelatou, K.; Lyberatos, G. On the fate of LAS, NPEOs and DEHP in municipal sewage sludge during composting. Bioresour. Technol. 2009, 100, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.D.; Wang, T.Y.; Niu, M.J.; Chen, X.J.; Liu, C.L.; Wang, Y.W.; Chen, T.B. Biodegradation of nonylphenol during aerobic composting of sewage sludge under two intermittent aeration treatments in a full-scale plant. Environ. Pollut. 2018, 238, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Moeller, J.; Reeh, U. Degradation of nonylphenol ethoxylates (NPE) in sewage sludge and source separated municipal solid waste under bench-scale composting conditions. Bull. Environ. Contam. Toxicol. 2003, 70, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.W.; Wang, M.-J.; Padgett, E.; Lopez-Real, J.M.; Beck, A.J. Impact of drying and composting procedures on the concentrations of 4-nonylphenols, di-(2-ethylhexyl)phthalate and polychlorinated biphenyls in anaerobically digested sewage sludge. Chemosphere 2007, 68, 1352–1358. [Google Scholar] [CrossRef]
- Poulsen, T.G.; Bester, K. Organic micropollutant degradation in sewage sludge during composting under thermophilic conditions. Environ. Sci. Technol. 2010, 44, 5086–5091. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Chen, X.-H.; Mo, C.-H.; Huang, Y.-H.; He, M.-Y.; Li, Y.-W.; Feng, N.-X.; Katsoyiannis, A.; Cai, Q.-Y. Occurrence and dissipation mechanism of organic pollutants during the composting of sewage sludge: A critical review. Bioresour. Technol. 2021, 328, 124847. [Google Scholar] [CrossRef] [PubMed]

| Compound | R (%) | RSD (%) | MDL (µg kg−1 dm) | MQL (µg kg−1 dm) |
|---|---|---|---|---|
| LAS C10 | 79 | 4.4 | 0.18 | 0.61 |
| LAS C11 | 97 | 4.5 | 0.51 | 1.69 |
| LAS C12 | 97 | 4.2 | 0.50 | 1.66 |
| LAS C13 | 99 | 5.0 | 0.39 | 1.32 |
| NP1EO | 77 | 8.6 | 1.52 | 5.07 |
| NP2EO | 94 | 1.5 | 0.32 | 1.05 |
| NP | 73 | 4.8 | 0.31 | 1.04 |
| DEHP | 76 | 8.3 | 6.94 | 23.1 |
| Ecotoxicological Data | PNECwater | Log Kd | PNECsoil | |||
|---|---|---|---|---|---|---|
| Organism | Test | Toxicological Value | (µg L−1) | (μg kg−1) | ||
| (mg L−1) | ||||||
| C10 | Daphnia magna (invertebrate) | LC50 (48 h) | 13.9 1 | 13.9 | 2.72 1 | 7294.8 |
| C11 | Nannochloropsis gaditana (algae) | EC50 (72 h) | 1.38 2 | 1.38 | 2.60 1 | 549.4 |
| C12 | Pimephales promelas (fish) | EC50 (48 h) | 3.2 2 | 3.2 | 3.53 1 | 10,843.0 |
| C13 | Nannochloropsis gaditana (algae) | EC50 (72 h) | 0.18 2 | 0.18 | 2.09 5 | 22.1 |
| NP2EO | Mysidopsis bahia (invertebrate) | LC50 (48 h) | 0.11 3 | 0.11 | 3.76 6 | 633.0 |
| NP1EO | Mysidopsis bahia (invertebrate) | LC50 (48 h) | 0.11 3 | 0.11 | 3.64 7 | 480.2 |
| NP | Mysidopsis bahia (invertebrate) | LC50 (96 h) | 0.02 3 | 0.02 | 2.67 7 | 9.4 |
| DEHP | Selenastrum capricornutum (algae) | LC50 (48 h) | 0.1 4 | 0.1 | 3.90 8 | 794.3 |
| Parameter | Units | Pile A | Pile B | ||
|---|---|---|---|---|---|
| Day 0 | Day 127 | Day 0 | Day 127 | ||
| Kjeldahl nitrogen | % dm | 5.7 | 2.8 | 4.8 | 2.8 |
| Crude protein | % dm | 35.8 | 17.4 | 29.8 | 17.4 |
| Carbon | % dm | 41 | 17 | 40 | 21 |
| Organic matter | % dm | 71 | 30 | 69 | 37 |
| C/N | - | 7.2 | 6.1 | 8.4 | 7.6 |
| Ca (CaO) | % dm | 1.1 | 1.6 | 1.6 | 1.5 |
| Mg (MgO) | % dm | 0.65 | 0.17 | 0.41 | 0.34 |
| Extracted P (P2O5) | % dm | 2.9 | 0.972 | 1.96 | 0.589 |
| Total P (P2O5) | % dm | 6 | 4.7 | 5 | 4.5 |
| Extracted K (K2O) | % dm | 0.21 | 0.16 | 0.12 | 0.11 |
| Total Pb | mg kg−1dm | 135 | 99 | 89 | 81 |
| Total K (K2O) | % dm | 0.36 | 0.54 | 0.28 | 0.55 |
| Conductivity | mS cm−1 25 °C dm | 4.7 | 4.2 | 4.2 | 4.8 |
| Soluble salts | % | 0.30 | 0.27 | 0.27 | 0.31 |
| Total Fe | mg kg−1dm | 9054 | 16,099 | 14,054 | 18,345 |
| Total Mn | mg kg−1dm | 223 | 418 | 181 | 324 |
| Total Ni | mg kg−1dm | <20 | 23 | 28 | 30 |
| Total Cu | mg kg−1dm | 249 | 182 | 300 | 223 |
| Total Cr | mg kg−1dm | 49 | 56 | 55 | 63 |
| Total Cd | mg kg−1dm | 1.0 | 0.8 | 1.3 | 1.1 |
| Total Zn | mg kg−1dm | 771 | 643 | 1043 | 859 |
| Total Co | mg kg−1dm | <10 | <10 | <10 | 25 |
| Total Al | mg kg−1dm | 12,509 | 26,984 | 14,843 | 28,639 |
| Total Hg | mg kg−1dm | 1.20 | - | 1.30 | - |
| pH | Und pH dm | 8.2 | 8.3 | 8.1 | 8.0 |
| Moisture | % | 79 | 11 | 73 | 11 |
| Group | Compound | Pile A | Pile B | ||
|---|---|---|---|---|---|
| Mean mg kg−1dm | Range mg kg−1dm | Mean mg kg−1dm | Range mg kg−1dm | ||
| LAS | C10 | 127 | 67.9–214 | 242 | 128–371 |
| C11 | 889 | 502–1451 | 1690 | 888–2520 | |
| C12 | 1478 | 793–2462 | 2585 | 1395–3647 | |
| C13 | 1207 | 705–1926 | 2093 | 1159–2836 | |
| NPE | NP1EO | 1.39 | 0.93–2.28 | 1.93 | 1.23–2.79 |
| NP2EO | 13.6 | 11.2–16.8 | 14.8 | 8.68–20.1 | |
| NP | 6.45 | 4.89–9.26 | 5.26 | <MQL-10.6 | |
| Phthalate | DEHP | 7.61 | 4.91–12.8 | 12.4 | 6.56–15.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, J.; Mejías, C.; Arenas, M.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of Linear Alkylbenzene Sulfonates, Nonylphenol Ethoxylates and Di(2-ethylhexyl)phthalate in Composting Processes: Environmental Risks. Sustainability 2022, 14, 186. https://doi.org/10.3390/su14010186
Martín J, Mejías C, Arenas M, Santos JL, Aparicio I, Alonso E. Occurrence of Linear Alkylbenzene Sulfonates, Nonylphenol Ethoxylates and Di(2-ethylhexyl)phthalate in Composting Processes: Environmental Risks. Sustainability. 2022; 14(1):186. https://doi.org/10.3390/su14010186
Chicago/Turabian StyleMartín, Julia, Carmen Mejías, Marina Arenas, Juan Luis Santos, Irene Aparicio, and Esteban Alonso. 2022. "Occurrence of Linear Alkylbenzene Sulfonates, Nonylphenol Ethoxylates and Di(2-ethylhexyl)phthalate in Composting Processes: Environmental Risks" Sustainability 14, no. 1: 186. https://doi.org/10.3390/su14010186
APA StyleMartín, J., Mejías, C., Arenas, M., Santos, J. L., Aparicio, I., & Alonso, E. (2022). Occurrence of Linear Alkylbenzene Sulfonates, Nonylphenol Ethoxylates and Di(2-ethylhexyl)phthalate in Composting Processes: Environmental Risks. Sustainability, 14(1), 186. https://doi.org/10.3390/su14010186








