Treatment Technologies for Cooling Water Blowdown: A Critical Review
Abstract
1. Introduction
2. Overview of CWBD Pollutants and Impacts
2.1. CWBD Contaminants
2.2. Contaminants of Concerns in CWBD and Their Impacts
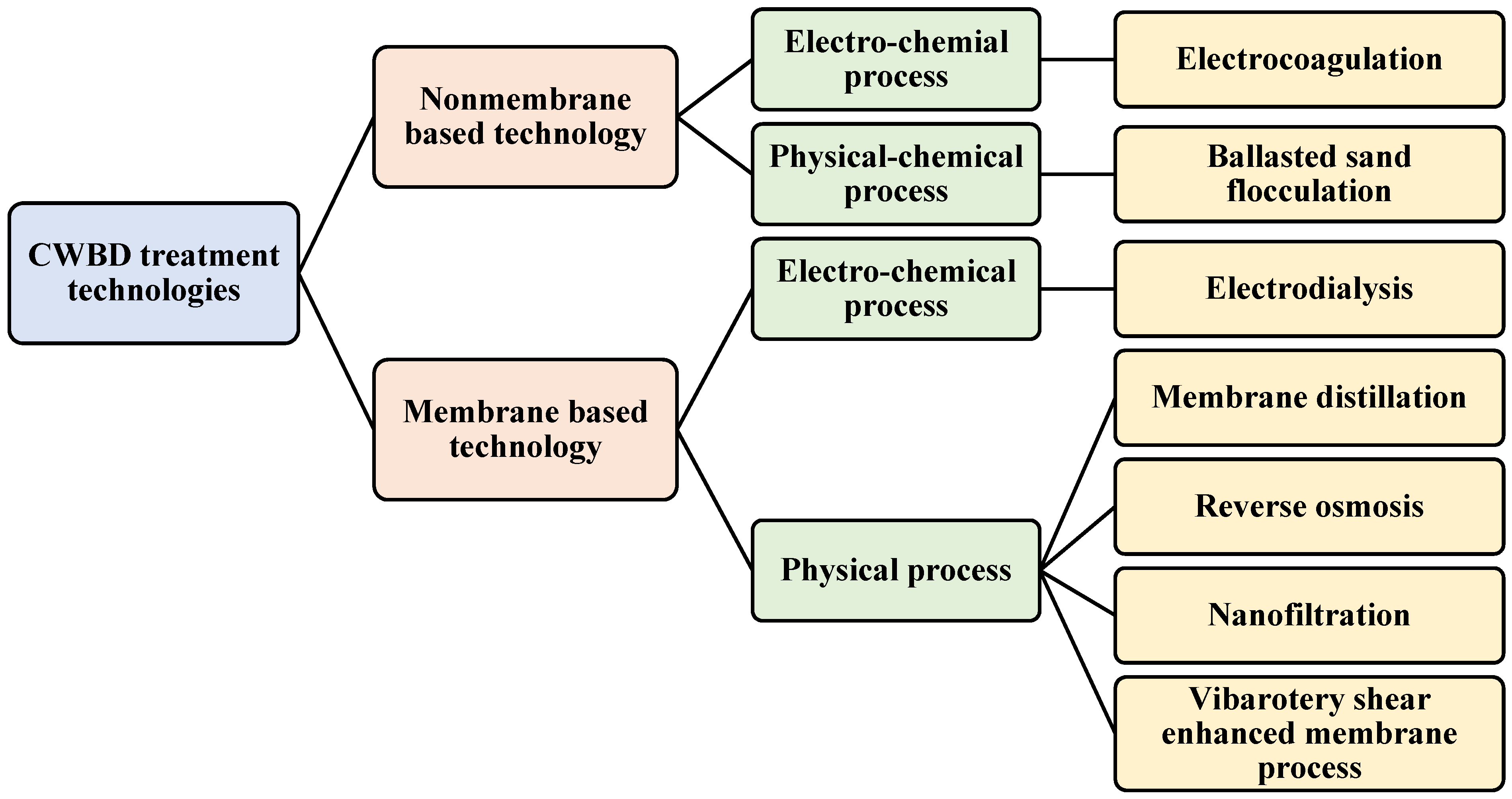
3. Overview on CWBD Water Treatment Technologies
3.1. Membrane-Based Technologies
3.1.1. Electrodialysis Process (ED)
3.1.2. Membrane or Thermal Membrane Distillation (MD or TMD)
3.1.3. Reverse Osmosis (RO)
3.1.4. Nanofiltration (NF)
3.1.5. Vibratory Sheared Enhanced Membrane Process (VSEP)
3.2. Non-Membrane Based-Technologies
3.2.1. Electrocoagulation Process (EC)
3.2.2. Ballasted Sand Flocculation Process (BSF)
4. Evaluation of CWBD Water Treatment Technologies
5. Regulations of Wastewater Effluent from Industries
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CWBD | Cooling water blowdown |
| RO | Reverse osmosis |
| ED | Electrodialysis |
| MD | Membrane distillation |
| EC | Electrocoagulation |
| NF | Nanofiltration |
| COD | Chemical oxygen demand |
| BOD | Biological oxygen demand |
| TDS | Total dissolved solids |
| TSS | Total suspended solids |
| BSF | Ballasted sand flocculation |
| VSEP | Vibratory shear enhanced membrane process |
References
- Dhadake, Y. Treatmnet of Cooling Tower Blowdown Water Using Electrodialysis; California State University: Long Beach, CA, USA, 2018. [Google Scholar]
- Afshari, F.; Dehghanpour, H. A Review Study on Cooling Towers; Types, Performance and Application. ALKU J. Sci. 2019, 1–10. Available online: https://dergipark.org.tr/en/pub/alku/issue/41490/489143 (accessed on 28 November 2021).
- Panjeshahi, M.; Ataei, A.; Gharaie, M.; Parand, R. Optimum design of cooling water systems for energy and water conservation. Chem. Eng. Res. Des. 2009, 87, 200–209. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Shoeib, M.A.; El-Khateeb, M.A.; Youssef, A.O.; Hafez, O.M. Electrochemical treatment of industrial cooling tower blowdown water using magnesium-rod electrode. Water Resour. Ind. 2020, 23, 100121. [Google Scholar] [CrossRef]
- Hafez, O.M.; Shoeib, M.; El-Khateeb, M.A.; Abdel-Shafy, H.; Youssef, A.O. Removal of scale forming species from cooling tower blowdown water by electrocoagulation using different electrodes. Chem. Eng. Res. Des. 2018, 136, 347–357. [Google Scholar] [CrossRef]
- Saha, P.; Bruning, H.; Wagner, T.V.; Rijnaarts, H.H. Removal of organic compounds from cooling tower blowdown by electrochemical oxidation: Role of electrodes and operational parameters. Chemosphere 2020, 259, 127491. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Wagner, T.V.; Ni, J.; Langenhoff, A.A.; Bruning, H.; Rijnaarts, H.H. Cooling tower water treatment using a combination of electrochemical oxidation and constructed wetlands. Process. Saf. Environ. Prot. 2020, 144, 42–51. [Google Scholar] [CrossRef]
- Wagner, V.T.; Parsons, J.R.; Rijnaarts, H.H.M.; de Voogt, P.; Langenhoff, A.A.M.; Wagner, T.V.; Parsons, J.R.; Rijnaarts, H.H.M.; de Voogt, P. Technology a Review on the Removal of Conditioning Chemicals from Cooling Tower Water in Constructed Wetlands. Crit. Rev. Environ. Sci. Technol. 2019, 48, 1094–1125. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Lu, S.; Yang, H.; Xie, W.; Zhao, H.; Zhang, Y.; Cao, X.; Tang, G.; Li, H.; et al. Treatment of cooling tower blowdown water by using adsorption-electrocatalytic oxidation: Technical performance, toxicity assessment and economic evaluation. Sep. Purif. Technol. 2020, 252, 117484. [Google Scholar] [CrossRef]
- Stratton, C.L.; Lee, G.F. Cooling Towers and Water Quality. J. Water Pollut. Control Fed. 1975, 47, 1901–1912. [Google Scholar]
- Ahmed, J.; Jamal, Y.; Shujaatullah, M. Recovery of cooling tower blowdown water through reverse osmosis (RO): Review of water parameters affecting membrane fouling and pretreatment schemes. Desalin. Water Treat. 2020, 189, 9–17. [Google Scholar] [CrossRef]
- Altman, S.J.; Jensen, R.P.; Cappelle, M.A.; Sanchez, A.L.; Everett, R.L.; Anderson, H.L.; McGrath, L.K. Membrane treatment of side-stream cooling tower water for reduction of water usage. Desalination 2012, 285, 177–183. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, C.; Doudrick, K.; Chan, C.K. Hexavalent chromium removal using metal oxide photocatalysts. Appl. Catal. B Environ. 2015, 176–177, 740–748. [Google Scholar] [CrossRef]
- Dickerson, B.R.; Vinyard, G.L. Effects of High Levels of Total Dissolved Solids in Walker Lake, Nevada, on Survival and Growth of Lahontan Cutthroat Trout. Trans. Am. Fish. Soc. 1999, 128, 507–515. [Google Scholar] [CrossRef]
- Maulbetsch, J.S.; DiFilippo, M.N. Performance, Cost, and Environmental Effects of Saltwater Cooling Towers. Environmental Effects of Mining; California Energy Commission: Sacramento, CA, USA, 2010; pp. 1–356. [Google Scholar]
- Weber-Scannell, P.K.; Duffy, L.K. Effects of Total Dissolved Solids on Aquatic Organisms: A Review of Literature and Recommendation for Salmonid Species. Am. J. Environ. Sci. 2007, 3, 1–6. [Google Scholar] [CrossRef]
- USGS. Phosphorus and Water; USGS: Reston, VA, USA, 2016.
- Papadopoulos, E.; Arsenos, G.; Ptochos, S.; Katsoulos, P.; Oikonomou, G.; Karatzia, M.A.; Karatzias, H. Pollutants in Wastewater Effluents Impacts. J. Hell. Vet. Med. Soc. 2014, 65, 115–120. [Google Scholar]
- Clarke, R. Groundwater: A Threatened Resource; UNEP Environmental Library, UN Environment Programme: Nairobi, Kenya, 2002; p. 36. [Google Scholar]
- Olariu, R. Treatment of Cooling Tower Blowdown Water. The Effect of Biodispersant on the Ultrafiltration Membrane. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2015. [Google Scholar]
- Fendorf, S.; Michael, H.A.; van Geen, A. Spatial and Temporal Variations of Groundwater Arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, K.M.; Hanh, H.T.; Kim, K.-W. Arsenic geochemistry and human health in South East Asia. Rev. Environ. Health 2011, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Abbas, F.; Ibrahim, M.; Rashid, U.; Khalid, S.; Ahmad, R.; Hakeem, K.R.; Majeed, T. Application of GIS for the identification and demarcation of selective heavy metal concentrations in the urban groundwater. J. Geogr. Sci. 2015, 25, 225–235. [Google Scholar] [CrossRef]
- Isa, N.M.; Aris, A.Z.; Lim, W.Y.; Sulaiman, W.N.A.W.; Praveena, S.M. Evaluation of heavy metal contamination in groundwater samples from Kapas Island, Terengganu, Malaysia. Arab. J. Geosci. 2014, 7, 1087–1100. [Google Scholar] [CrossRef]
- Galadima, A.; Garba, Z.N. Heavy Metals Pollution in Nigeria: Causes and Consequences. Elixir Pollut. 2012, 45, 7917–7922. [Google Scholar]
- Löwenberg, J.; Baum, J.A.; Zimmermann, Y.-S.; Groot, C.; Broek, W.V.D.; Wintgens, T. Comparison of pre-treatment technologies towards improving reverse osmosis desalination of cooling tower blow down. Desalination 2015, 357, 140–149. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Saji, V.S. Corrosion Inhibitors in Cooling Towers. Chem. Ind. Dig. 2002, 15, 74–80. [Google Scholar]
- ChemWorld. We Sell Direct to Everyone. 2016. Available online: https://www.chemworld.com/ (accessed on 28 November 2021).
- Pereira, L.; Duarte, E.; Fragoso, R. Water Use: Recycling and Desalination for Agriculture. Encycl. Agric. Food Syst. 2014, 5, 407–424. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sharma, R.; Handa, N.; Kaur, H.; Kaur, R.; Sirhindi, G.; Thukral, A. Prospects of Field Crops for Phytoremediation of Contaminants. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press: Cambridge, MA, USA, 2014; Volume 2, pp. 449–470. [Google Scholar]
- Sultana, M.S.; Jolly, Y.; Yeasmin, S.; Islam, A.; Satter, S.; Tareq, S.M. Transfer of Heavy Metals and Radionuclides from Soil to Vegetables and Plants in Bangladesh. In Soil Remediation and Plants: Prospects and Challenges; Elsevier: Amsterdam, The Netherlands, 2015; pp. 331–366. [Google Scholar]
- Fales, A.L. The Effects of Industrial Wastes on Sewage Treatment; New England Interstate Water Pollution Control Commission: Boston, MA, USA, 1970; pp. 970–985. [Google Scholar]
- Membrane Technology; Lenntech: Delft, The Netherlands, 2020.
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, L.; Doyen, A.; Roblet, C. Electrodialytic Phenomena, Associated Electromembrane Technologies and Applications in the Food, Beverage and Nutraceutical Industries; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 202–218. [Google Scholar]
- Wang, Y.; Jiang, C.; Bazinet, L.; Xu, T. Electrodialysis-Based Separation Technologies in the Food Industry. In Separation of Functional Molecules in Food by Membrane Technology; Academic Press: Cambridge, MA, USA, 2019; pp. 349–381. [Google Scholar]
- Koeman-Stein, N.; Creusen, R.; Zijlstra, M.; Groot, C.; Broek, W.V.D. Membrane distillation of industrial cooling tower blowdown water. Water Resour. Ind. 2016, 14, 11–17. [Google Scholar] [CrossRef]
- Ma, J.; Irfan, H.M.; Wang, Y.; Feng, X.; Xu, D. Recovering Wastewater in a Cooling Water System with Thermal Membrane Distillation. Ind. Eng. Chem. Res. 2018, 57, 10491–10499. [Google Scholar] [CrossRef]
- Kebria, M.R.S.; Rahimpour, A. Membrane Distillation: Basics, Advances, and Applications. Advances in Membrane Technologies; Abdelrasoul, A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Abraham, T.; Luthra, A. Socio-economic & technical assessment of photovoltaic powered membrane desalination processes for India. Desalination 2011, 268, 238–248. [Google Scholar] [CrossRef]
- Karanasiou, A.; Kostoglou, M.; Karabelas, A. An Experimental and Theoretical Study on Separations by Vacuum Membrane Distillation Employing Hollow-Fiber Modules. Water 2018, 10, 947. [Google Scholar] [CrossRef]
- Kiss, A.A.; Readi, O.M.K. An industrial perspective on membrane distillation processes. J. Chem. Technol. Biotechnol. 2018, 93, 2047–2055. [Google Scholar] [CrossRef]
- Pangarkar, B.; Deshmukh, S.; Sapkal, V. Review of membrane distillation process for water purification. Desalin. Water Treat. 2014, 57, 2959–2981. [Google Scholar] [CrossRef]
- Bappy, P.; Jabed, M.; Bahar, R.; Ariff, T.F. Low Energy and Low Cost Freshwater Production by Membrane Distillation. In Proceedings of the International Conference on Industrial Engineering and Operations Management, Kuala Lumpur, Malaysia, 8–10 March 2016; pp. 1799–1804. [Google Scholar]
- Shirazi, M.M.A.; Kargari, A. A Review on Applications of Membrane Distillation (MD) Process for Wastewater Treatment. J. Membr. Sci. Res. 2015, 1, 101–112. [Google Scholar]
- BioTech Water Researchers. Advantages and Disadvantages of Reverse Osmosis; BioTech Water Researchers: San Antonio, TX, USA, 2019. [Google Scholar]
- Farahani, M.H.D.A.; Borghei, S.M.; Vatanpour, V. Recovery of Cooling Tower Blowdown Water for Reuse: The Investigation of Different Types of Pretreatment Prior Nanofiltration and Reverse Osmosis. J. Water Process Eng. 2016, 10, 188–199. [Google Scholar] [CrossRef]
- Rabiee, H.; Khalilpour, K.R.; Betts, J.M.; Tapper, N. Energy-Water Nexus: Renewable-Integrated Hybridized Desalination Systems. In Polygeneration with Polystorage for Chemical and Energy Hubs; Academic Press: Cambridge, MA, USA, 2019; pp. 409–458. [Google Scholar]
- Elaine, H.; Doerge, T.A.; Baker, P.B. Water Facts: Number 6 Reverse Osmosis Units. no. 6.1994. Available online: https://files.knowyourh2o.com/Waterlibrary/privatewell/reverseosmosis.pdf (accessed on 28 November 2021).
- Macedo, A.T.Z.N.; Pulido, J.M.O.; Fragoso, R. The Use and Performance of Nanofiltration Membranes for Agro-Industrial Effluents Purification. In Nanofiltration; IntechOpen: London, UK, 2018; p. 65. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Shon, H.K.; Phuntsho, S.; Chaudhary, D.S.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment—A mini review. Drink. Water Eng. Sci. 2013, 6, 47–53. [Google Scholar] [CrossRef]
- Shahmansouri, A.; Bellona, C. Nanofiltration technology in water treatment and reuse: Applications and costs. Water Sci. Technol. 2015, 71, 309–319. [Google Scholar] [CrossRef]
- Leong, J.; Tan, J.; Heitz, A.; Ladewig, B.P. Use of vibratory shear enhanced processing to treat magnetic ion exchange concentrate: A techno-economic analysis. Desalination 2016, 383, 46–52. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Peleka, E.N.; Ntolia, A. Treatment of Tannery Wastewater with Vibratory Shear-Enhanced Processing Membrane Filtration. Separations 2019, 6, 20. [Google Scholar] [CrossRef]
- Balasubramanian, P. A Brief Review on Best Available Technologies for Reject Water (Brine) Management in Industries. Int. J. Environ. Sci. 2013, 3, 2010–2018. [Google Scholar]
- Zouboulis, A.; Petala, M. Performance of VSEP vibratory membrane filtration system during the treatment of landfill leachates. Desalination 2008, 222, 165–175. [Google Scholar] [CrossRef]
- Babu, D.S.; Singh, T.S.A.; Nidheesh, P.V.; Kumar, M.S. Industrial wastewater treatment by electrocoagulation process. Sep. Sci. Technol. 2020, 55, 3195–3227. [Google Scholar] [CrossRef]
- Hernández, I.L.; Díaz, C.B.; Cerecero, M.V.; Sánchez, P.T.A.; Juárez, M.C.; Lugo, V.L. Soft drink wastewater treatment by electrocoagulation—electrooxidation processes. Environ. Technol. 2016, 38, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.Y.; Morkovsky, P.; Gomes, J.A.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef]
- Akyol, A. Treatment of paint manufacturing wastewater by electrocoagulation. Desalination 2012, 285, 91–99. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.S.Z.; El-Taweel, Y.A.; Abdelwahab, O.; Nassef, E.M. Treatment of Petrochemical Wastewater Containing Phenolic Compounds by Electrocoagulation Using a Fixed Bed Electrochemical Reactor. Int. J. Electrochem. Sci. 2013, 8, 1534–1550. [Google Scholar]
- Zhao, S.; Huang, G.; Cheng, G.; Wang, Y.; Fu, H. Hardness, COD and turbidity removals from produced water by electrocoagulation pretreatment prior to Reverse Osmosis membranes. Desalination 2014, 344, 454–462. [Google Scholar] [CrossRef]
- Mansour, S.; Hasieb, I. Removal of Nickel from Drinking Water by Electrocoagulation Technique Using Alternating Current. Curr. Res. Chem. 2012, 4, 41–50. [Google Scholar] [CrossRef]
- Hashim, K.S.; Shaw, A.; Al Khaddar, R.; Pedrola, M.O.; Phipps, D. Iron removal, energy consumption and operating cost of electrocoagulation of drinking water using a new flow column reactor. J. Environ. Manag. 2017, 189, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Golder, A.; Samanta, A.; Ray, S. Removal of trivalent chromium by electrocoagulation. Sep. Purif. Technol. 2007, 53, 33–41. [Google Scholar] [CrossRef]
- Silva, J.F.; Graça, N.S.; Ribeiro, A.; Rodrigues, A.E. Electrocoagulation process for the removal of co-existent fluoride, arsenic and iron from contaminated drinking water. Sep. Purif. Technol. 2018, 197, 237–243. [Google Scholar] [CrossRef]
- Hashim, K.S.; Alkhaddar, R.; Jasim, N.; Shaw, A.; Phipps, D.; Kot, P.; Pedrola, M.O.; Alattabi, A.W.; Abdulredha, M.; Alawsh, R. Electrocoagulation as a green technology for phosphate removal from river water. Sep. Purif. Technol. 2019, 210, 135–144. [Google Scholar] [CrossRef]
- An, C.; Huang, G.; Yao, Y.; Zhao, S. Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci. Total Environ. 2017, 579, 537–556. [Google Scholar] [CrossRef]
- Islam, S.M.D.-U. Electrocoagulation (EC) technology for wastewater treatment and pollutants removal. Sustain. Water Resour. Manag. 2019, 5, 359–380. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Nariyan, E.; Sillanpää, M.; Wolkersdorfer, C. Electrocoagulation treatment of mine water from the deepest working European metal mine—Performance, isotherm and kinetic studies. Sep. Purif. Technol. 2017, 177, 363–373. [Google Scholar] [CrossRef]
- Anwer, E.A.; Majeed, B.A.A. Different Electrodes Connections in Electrocoagulation of Synthetic Blow down Water of Cooling Tower. Iraqi J. Chem. Pet. Eng. 2020, 21, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.; Ghosh, N.C.; Kazmi, A.A. Ballasted sand flocculation for water, wastewater and CSO treatment. Environ. Technol. Rev. 2016, 5, 57–67. [Google Scholar] [CrossRef]
- Kumar, S.; Kazmi, A.A.; Ghosh, N.C.; Singh, R. Optimization of Ballasted Sand Flocculation Process for Urban Runoff Treatment. J. Indian Water Resour. Soc. 2018, 37. Available online: http://117.252.14.250:8080/jspui/handle/123456789/4895 (accessed on 28 November 2021).
- Kazmi, A.A.; Kumar, S.; Ghosh, N.C. Ballasted Sand Flocculation Technology for Storm Water Run Off Treatmnet. In Proceedings of the 21st International Water Technology Conference, Port Said, Egypt, 28–30 June 2018; pp. 28–30. [Google Scholar]
- Veolia Water Technology. Actiflo® Sand-Ballasted Water Clarification and Softening Processes. Available online: https://www.oilandgasonline.com/doc/actiflo-ballasted-water-clarification-system-0001 (accessed on 28 November 2021).
- Sauvignet, P. Sand-Ballasted Flocculation Technology and Mobile Facilities. Quarry Manag. 2003, 30, 17–18. [Google Scholar]
- Treguer, R.; Blair, B.; Klaper, R.; Royer, S.; Magruder, C. Evaluation of Actiflo® Carb Process for the Combined Removal of Trace Organic Compounds and Phosphorous during Wastewater Tertiary Treatment. Proc. Water Environ. Fed. 2012, 2012, 7176–7196. [Google Scholar] [CrossRef][Green Version]
- Akhter, M.; Habib, G.; Qamar, S.U. Application of Electrodialysis in Waste Water Treatment and Impact of Fouling on Process Performance. J. Membr. Sci. Technol. 2018, 8, 1000182. [Google Scholar] [CrossRef]
- Mahmoudi, H. Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2. [Google Scholar]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Trishitman, D.; Cassano, A.; Basile, A.; Rastogi, N.K. Reverse osmosis for industrial wastewater treatment. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–228. [Google Scholar]
- Singh, R. Desalination and On-site Energy for Groundwater Treatment in Developing Countries Using Fuel Cells. In Emerging Membrane Technology for Sustainable Water Treatment; Elsevier: Amsterdam, The Netherlands, 2016; pp. 135–162. [Google Scholar]
- Culkin, B. Eccentric Drive Mechanism. U.S. Patent US5014564A, 14 May 1991. Available online: https://patents.google.com/patent/US5014564A/en (accessed on 28 November 2021).
- EMIS. Energie-En Milieu-Informatiesysteem Voor Het Vlaamse Gewest. 2020. Available online: https://emis.vito.be/nl (accessed on 28 November 2021).
- Al-Karaghouli, A.; Kazmerski, L.L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. [Google Scholar] [CrossRef]
- Chaoui, I.; Abderafi, S.; Vaudreuil, S.; Bounahmidi, T. Water desalination by forward osmosis: Draw solutes and recovery methods—Review. Environ. Technol. Rev. 2019, 8, 25–46. [Google Scholar] [CrossRef]
- Kim, I.S.; Hwang, M.; Choi, C. Membrane-Based Desalination Technology for Energy Efficiency and Cost Reduction. In Desalination Sustainability (A Technical, Socioeconomic, and Environmental Approach); Elsevier: Amsterdam, The Netherlands, 2017; pp. 31–74. [Google Scholar]
- Mulyanti, R.; Susanto, H. Wastewater Treatment by Nanofiltration Membranes. IOP Conf. Ser. Earth Environ. Sci. 2018, 142, 012017. [Google Scholar] [CrossRef]
- Strathmann, H. Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Binot, P.; Dahl, C.P.; Zuback, J.E. Methods of Treating Water Via Ballasted Flocculation and. Decantation. Patent CA2471034C, 24 April 2012. [Google Scholar]
- Sharma, G.; Choi, J.; Shon, H.K.; Phuntsho, S. Solar-powered electrocoagulation system for water and wastewater treatment. Desalin. Water Treat. 2011, 32, 381–388. [Google Scholar] [CrossRef]
- Hafez, O.M.; Shoeib, M.A.; El-khateeb, M.A.; Abdel-shafy, H.I.; Youssef, A.O. Pt Graphical Abstract Sc B; Surface Coating Department, Central Metallurgical Research and Development Institute: Cairo, Egypt, 2018. [Google Scholar]
- Bushart, S.; Tran, P.; Asay, R. Low-Level Liquid Waste Processing Pilot Studies Using a Vibratory Shear Enhanced Process (Vsep) for Fultration; WM Symposia, Inc.: Tucson, AZ, USA, 2002; pp. 1–8. [Google Scholar]
- Wateronline. End of Pipe Effluent Treatment for Pulp and Paper Mills 2021. Available online: https://www.wateronline.com/doc/end-of-pipe-effluent-treatment-for-pulp-and-p-0001 (accessed on 28 November 2021).
- Jin, X.; Li, E.; Lu, S.; Qiu, Z.; Sui, Q. Coking Wastewater Treatment for Industrial Reuse Purpose: Combining Biological Processes with Ultrafiltration, Nanofiltration and Reverse Osmosis. J. Environ. Sci. 2013, 25, 1565–1574. [Google Scholar] [CrossRef]
- VSEP. Applications—New Logic Research. Available online: https://www.vsep.com/technology/applications/ (accessed on 19 June 2021).
- New Logic Research. Membrane Filtration of Cooling Tower Blowdown Application. Available online: https://www.vsep.com/pdf/Membrane-Treatment-of-Cooling-Tower-Blowdown.pdf (accessed on 28 November 2021).
- Fayad, N. The Application of Electrocoagulation Process for Wastewater Treatment and for the Separation and Purification of Biological Media. Master’s Thesis, Université Clermont Auvergne, Clermont-Ferrand, France, 2018; pp. 5–226. [Google Scholar]
- Westerling, K. ED vs. RO the Benefits of Electrodialysis for Desalination. 2015. Available online: https://www.wateronline.com/doc/ed-vs-ro-the-benefits-of-electrodialysis-for-desalination-0001 (accessed on 28 November 2021).
- Mendez, E. Sustainable Desalination: Membrane Distillation Delivers Greener Clean Water—Filtration + Separation. 2012. Available online: https://www.filtsep.com/desalination/features/sustainable-desalination-membrane-distillation/ (accessed on 28 November 2021).
- Cui, H.; Huang, X.; Yu, Z.; Chen, P.; Cao, X. Application progress of enhanced coagulation in water treatment. RSC Adv. 2020, 10, 20231–20244. [Google Scholar] [CrossRef]
- USEPA. Wastewater Technology Fact Sheet Dechlorination; Environmental Protection Agency: Washington, DC, USA, 2000; pp. 1–7.
- Samcotech. Reverse Osmosis Vs Nanofiltration Membrane Process: What Is the Difference? Samcotech: New York, NY, USA, 2017. [Google Scholar]
- Singh, K.; Lataye, D.H.; Wasewar, K.L.; Yoo, C.K. Removal of fluoride from aqueous solution: Status and techniques. Desalin. Water Treat. 2013, 51, 3233–3247. [Google Scholar] [CrossRef]
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Madaeni, S.; Samieirad, S. Chemical cleaning of reverse osmosis membrane fouled by wastewater. Desalination 2010, 257, 80–86. [Google Scholar] [CrossRef]
- EMIS. Nanofiltration. 2010. Available online: https://emis.vito.be/en/bat/tools-overview/sheets/nanofiltration (accessed on 28 November 2021).
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2019, 380, 122231. [Google Scholar] [CrossRef]
- Subramani, A.; De Carolis, J.; Pearce, W.; Jacangelo, J.G. Vibratory shear enhanced process (VSEP) for treating brackish water reverse osmosis concentrate with high silica content. Desalination 2012, 291, 15–22. [Google Scholar] [CrossRef]
- Massot, A.; Mietton-Peuchot, M.; Peuchot, C.; Milisic, V. Nanofiltration and Reverse Osmosis in Winemaking. Desalination 2008, 231, 283–289. [Google Scholar] [CrossRef]
- Zeynali, R.; Akbari, M.; Ghasemzadeh, K.; Jalilnejad, E.; Basile, A. Chapter 4—Achievements in High Pressure Membrane Processes Nf and Ro for Wastewater and Water Treatment; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Water, Produced, and Bilge Water. Using Vsep to Treat Produced Water. 1993. Available online: https://www.vsep.com/pdf/ProducedWater.pdf (accessed on 28 November 2021).
- Biniaz, P.; Ardekani, N.T.; Makarem, M.A.; Rahimpour, M.R. Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD). ChemEngineering 2019, 3, 8. [Google Scholar] [CrossRef]
- Luo, W.; Phan, H.V.; Li, G.; Hai, F.; Price, W.E.; Elimelech, M.; Nghiem, L.D. An Osmotic Membrane Bioreactor–Membrane Distillation System for Simultaneous Wastewater Reuse and Seawater Desalination: Performance and Implications. Environ. Sci. Technol. 2017, 51, 14311–14320. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent Advances in Membrane Distillation Processes: Membrane Development, Configuration Design and Application Exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Ghyselbrecht, K.; Van Houtte, E.; Pinoy, L.; Verbauwhede, J.; Van der Bruggen, B.; Meesschaert, B. Treatment of RO concentrate by means of a combination of a willow field and electrodialysis. Resour. Conserv. Recycl. 2012, 65, 116–123. [Google Scholar] [CrossRef]
- Bartels, C.; Wilf, M.; Andes, K.; Iong, J. Design considerations for wastewater treatment by reverse osmosis. Water Sci. Technol. 2005, 51, 473–482. [Google Scholar] [CrossRef]
- Luminor. Reverse Osmosis Water Filtration Overview. 2021. Available online: https://www.luminoruv.com/education/reverse-osmosis (accessed on 28 November 2021).
- Van Der Bruggen, B.; Vandecasteele, C. Removal of Pollutants from Surface Water and Groundwater by Nanofiltration: Overview of Possible Applications in the Drinking Water Industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Tibebe, D.; Kassa, Y.; Bhaskarwar, A.N. Treatment and characterization of phosphorus from synthetic wastewater using aluminum plate electrodes in the electrocoagulation process. BMC Chem. 2019, 13, 107. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Olson, N.; Ives, R.; Nshimyimana, J.P.; Rusinek, C.A.; Rose, J.B.; Liao, W. Techno-Economic Analysis of Electrocoagulation on Water Reclamation and Bacterial/Viral Indicator Reductions of a High-Strength Organic Wastewater—Anaerobic Digestion Effluent. Sustainability 2020, 12, 2697. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Khosravi, T.; Sharafi, K.; Mouradi, M. Comparing operational cost and performance evaluation of electrodialysis and reverse osmosis systems in nitrate removal from drinking water in Golshahr, Mashhad. Desalin. Water Treat. 2015, 57, 5391–5397. [Google Scholar] [CrossRef]
- Kullab, A.; Martin, A. Membrane distillation and applications for water purification in thermal cogeneration plants. Sep. Purif. Technol. 2011, 76, 231–237. [Google Scholar] [CrossRef]
- Young, J.C.; Edwards, F.G. Factors affecting ballasted flocculation reactions. Water Environ. Res. 2003, 75, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Birnhack, L.; Nir, O.; Lahav, O. Establishment of the Underlying Rationale and Description of a Cheap Nanofiltration-Based Method for Supplementing Desalinated Water with Magnesium Ions. Water 2014, 6, 1172–1186. [Google Scholar] [CrossRef]
- Elazhar, F.; Touir, J.; Elazhar, M.; Belhamidi, S.; El Harrak, N.; Zdeg, A.; Hafsi, M.; Amor, Z.; Taky, M.; Elmidaoui, A. Techno-economic comparison of reverse osmosis and nanofiltration in desalination of a Moroccan brackish groundwater. Desalin. Water Treat. 2014, 55, 2471–2477. [Google Scholar] [CrossRef]
- US Department of the Interior Bureau of Reclamation. Brine-Concentrate Treatment and Disposal Options Report—Part 1; US Department of the Interior Bureau of Reclamation: Washington, DC, USA, 2009; pp. 1–46.
- Qureshi, A.S. Challenges and Prospects of Using Treated Wastewater to Manage Water Scarcity Crises in the Gulf Cooperation Council (GCC) Countries. Water 2020, 12, 1971. [Google Scholar] [CrossRef]
- Chin, D.A. Water-Quality Engineering in Natural Systems: Fate and Transport Processes in the Water Environment; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 50. [Google Scholar]
- EPA. Guidelines for Water Reuse; EPA: Washington, DC, USA, 2012.
- IAEA. Executive by-Law for the Environment Protection Law. Supreme Council for the Environment & Natural Reserves Decree Law No. 30; Supreme Council for the Environment & Natural Reserves: Doha City, Qatar, 2002. [Google Scholar]
- Al-Muzaini, M.; Mendonca, V.; Al-Sariri, T.; Al-Jabri, M. Waste Water Discharge into the Marine Environment in the Sultanate of Oman: Sources and Standards—An Overview of the Current Situation. In Proceedings of the 3rd Marine Waste Water Discharges (MWWD), Catania, Italy, 27 September–2 October 2004. [Google Scholar]
- Abu Dhabi Specification. Environmental Specifications for Land-Based Liquid Discharges to the Marine Environment; Abu Dhabi Quality & Conformity Council: Abu Dhabi, United Arab Emirates, 2017.
| Parameter | Unit | [4] | [11,26] (J. Löwenberg, 2015) | [7] | [1] **** |
|---|---|---|---|---|---|
| pH | 8.2 | 7.9 | 6.8 ± 0.2 | ||
| Calcium (Ca2+) | mg/L | 392 ** | 1204 ** | 338 ± 7.6 | 125.9 |
| Magnesium (Mg2+) | mg/L | 280 ** | 259 ** | 58 ± 2.4 | 12.5 |
| Silica (SiO2) | mg/L | 27 *** | 0.9 | ||
| Chloride (Cl−) | mg/L | 162 | 500 | 458 ± 10 | 205.32 |
| Zinc (Zn2+) | mg/L | 1.2 | |||
| Phosphate (PO43−) | mg/L | 6.61 * | 5.9 | ||
| Iron (Fe2+) | mg/L | 0.1 | 0.6343 | ||
| Sulphate (SO42−) | mg/L | 711 | 1043 ± 52 | 469.05 | |
| Aluminium (Al3+) | mg/L | 0.0596 | |||
| Barium (Ba2+) | mg/L | 0.145 | 0.1142 | ||
| Potassium (K+) | mg/L | 75 ± 1.3 | 8.1 | ||
| Sodium (Na+) | mg/L | 334 ± 2.9 | 262.8 | ||
| Copper (Cu) | mg/L | 0.224 | |||
| Strontium (Sr2+) | mg/L | 1.500 | 1.0853 | ||
| Bromide (Br−) | mg/L | 43.35 | |||
| Fluorine (F) | mg/L | 8.1 | |||
| Total suspended solids (TSS) | mg/L | 12 | |||
| Total dissolved solids (TDS) | mg/L | 1297 | 1329 | ||
| Total organic carbon (TOC) | mg/L | 41 ± 1.3 | |||
| Chemical oxygen demand (COD) | mg/L | 107 ± 6.4 | |||
| Nitrate (NO3−) | mg/L | 86.7 | 57 ± 1.8 | ||
| Turbidity | NTU | 7.93 | 7.3 | ||
| CWBD water source | Effluent of a urea fertilizer plant | From a cooling tower (CT) next to the Dow premises in Terneuzen (The Netherlands) | From (CT) of Dow Benelux BV (Terneuzen, The Netherlands) | From CSULB cooling towers | |
| Criteria | Electrocoagulation (EC) | Electrodialysis (ED) | Membrane Distillation (MD) | Ballasted Sand Flocculation (BSF) | Reverse Osmosis (RO) | Nanofiltration (NF) | Vibratory Sheared Enhanced Membrane Process (VSEP) |
|---|---|---|---|---|---|---|---|
| Process scale | All levels [70] | Large-scale [80] | Pilot scale, large scale are implemented mostly in desalination process [42] | Pilot and large-scale [74] | Large-scale [48] | Implemented in large scale for coking wastewater treatment [97]; Pilot scale implementation for rubber wastewater | Large-scale VSEP as a recovery system for CWBD water in several facilities such as gas production, coal gas-fired power plant, and biomass plants [98,99] |
| Maintenance | Low [100] | Maintenance and the cost of maintenance associated only with the membrane [1]. Low maintenance [101] | Low [102] | High maintenance Required for example for the hydro-cyclone [103,104] | High maintenance requirement due to fouling [26]; Low maintenance if pre-treatment process is used [105] | Low maintenance if pre-treatment process is used [105] | Low maintenance because system has few moving parts [85] |
| Chemicals additives to the process | None [94] | None [34,106] | None [42] | Require high amount of chemicals, e.g., Alum and FeCl3 compared to traditional processes [74] | None [107]; Requires chemicals for membrane cleaning to prevent fouling [108] | None [109] | None [85] |
| Utility and energy requirements | 0.18–3.05 kWh/m3 with magnesium electrodes for CWBD treatment [4]; Requires electricity [5]; Al electrode and monopolar-parallel connection, the energy consumption is less compared to bipolar-series [73] | From 1.1 to 2.9 kWh/m3 for the treatment of almond industry wastewater; Requires a source of electricity; Less energy intensive compared to RO and thermal processes [1]; A lot of electricity is consumed in case of high level of salt in the influent stream [91]; | Required energy is mainly in form of heat, and with a small amount of electricity for pumps [37]; The process requires high energy [39,110] | High energy for hydro-cyclone [92] | Relative: energy demand increases as a result of the fouling issue [26] | Less energy compared to RO processs [90] | With same conditions, the energy consumption of VSEP process is three times higher than RO process [111] |
| The quality of permeate (effluent water) | Low content of TDS, odorless, and colorless effluent [73] | Higher water recovery rate than effluent from RO process [110]; Requires post-treatment to remove the remaining sludge [73] | Low water recovery [110]; Low permeate flux compared to RO process [43]; Low (salt concentration near zero) [38] | Comparable quality to conventional treatment technologies [74] | High quality [112] | Produce clean, high-quality water, and the permeate has a stable flowrate [51,113] | High quality [34,114]; Solid-free permeate [85] |
| The sludge quality (rejection) | Low sludge discharge, stable, and non-toxic [71,94]; Sludge contains mainly metallic oxides/hydroxides [73] | High sludge discharge [73] | Non-volatile compounds, macromolecules, and inorganic ions are all highly rejected from the water stream to the sludge with (99–100%) and the separation theoretically can reach 100% [115,116,117] | Flocs easily eliminated by settling [74]; Sludge layer is distinct, clear, and supernatant [78] | A concentrated stream with high salinity [118] | Low discharge sludge [109]; Retentate concentrations lower than RO for low value salts in the influent stream [109]; Rejection efficacy altered when fouling occurs [50] | Concentrated waste stream with 30 to 35% total solids (higher than the feed) [95,96] |
| Contaminants | CWBD Treatment Technologies | ||
|---|---|---|---|
| EC [4] | ED [1] | MD [37] | |
| Total dissolved Solids (TDS) | √ | √ | |
| Calcium (Ca2+) | √ | √ | √ |
| Magnesium (Mg2+) | √ | √ | √ |
| Silica (SiO2) | √ | ||
| Chloride (Cl−) | √ | √ | √ |
| Zinc (Zn2+) | √ | ||
| Phosphate (PO43−) | √ | ||
| Iron (Fe2+) | √ | √ | |
| Sulphate (SO42−) | √ | √ | √ |
| Aluminium (Al3+) | |||
| Barium (Ba2+) | |||
| Potassium (K+) | √ | √ | |
| Sodium (Na+) | √ | √ | |
| Copper (Cu) | √ | ||
| Strontium (Sr2+) | |||
| Bromide (Br−) | √ | ||
| Fluorine (F) | √ | ||
| Manganese (Mn2+) | √ | ||
| Nitrate (NO3−) | |||
| EC | ED | MD | BSF | RO | NF | VSEP | |
|---|---|---|---|---|---|---|---|
| Operational cost | |||||||
| Capital cost | |||||||
| Total cost | |||||||
| References | [5,122,123] | [122,124] | [44,125] | [74,126] | [48,122] | [127,128] | [129] |
| Color coding | Meaning | ||||||
| Low | |||||||
| Moderate | |||||||
| High |
| Standards of Water and Wastewater for Irrigation | Standards of Discharging into Marine | Standards of Discharging Liquid Waste to Public Foul Sewage Networks | |||||
|---|---|---|---|---|---|---|---|
| Parameter | EPA Irrigation ppm [132] | Qatar Irrigation ppm [133] | Qatar Discharges into Water Environment or Marine ppm [133] | UAE Discharges to Marine at Point of Discharge ppm [134] | Oman Discharges to Marine ppm [135] | Qatar Discharged Liquid Waste for Treatment by Public Sewage Work ppm [133] | Qatar Industrial Effluent Discharged to Sewers ppm [133] |
| Aluminium | 5.0 | 15 | 3 | 20 | 30 | - | |
| Arsenic | 0.10 | 0.1 | - | 0.05 | 0.05 | 5 | 5 |
| Beryllium | 0.10 | - | - | 0.05 | 5 | - | |
| Boron | 0.75 | 1.5 | 1.5 | 1 | - | - | |
| Cadmium | 0.01 | 0.05 | 0.05 | 0.05 | 0.50 | 2 | 10 |
| Chromium | 0.1 | 0.01 | 0.2 | 0.2 | 5 | 2 | |
| Cobalt | 0.05 | 0.2 | 2 | 0.2 | - | - | |
| Copper | 0.2 | 0.2 | 0.5 | 0.5 | 0.50 | 5 | 4 |
| Fluoride | 1 | 15 | 1 | 10 | - | - | |
| Iron | 5 | 1 | 1 | 2 | 2.0 | 25 | - |
| Lead | 5 | 0.1 | 0.1 | 0.1 | 0.10 | 5 | 5 |
| Lithium | 2.5 | - | - | - | - | - | |
| Manganese | 0.2 | 0.05 | 0.2 | 0.2 | - | - | |
| Molybdenum | 0.01 | - | - | - | - | - | |
| Nickel | 0.2 | 0.2 | 0.5 | 0.1 | 0.10 | 5 | - |
| Selenium | 0.02 | - | 0.02 | 0.02 | 0.02 | - | - |
| Vanadium | 0.1 | - | - | - | - | - | |
| Zinc | 2 | 0.5 | 2 | 0.5 | 0.10 | 10 | 4 |
| TDS | - | 2000 | 1500 | - | 4000 | - | |
| Sulphate | - | 400 | 0.1 | - | 1000 | 1000 | |
| Phosphate as P (PO4 −3) | - | 30 | 2 | 2 | 0.10 | - | - |
| Ammonia as N | - | - | 3 | 2 | - | - | |
| Chlorine residual | - | 0.1 | 0.05 | - | - | - | |
| Cyanide (total) | - | - | 0.1 | 0.05 | 0.10 | 1 | 1 |
| Nitrates | - | - | - | 30 | - | - | |
| COD | - | 150 | 100 | 100 | 3000 | 3000 | |
| BOD5 | - | 10 | 50 | 30 | 30 | 1000 | - |
| Total organic carbon (TOC) | - | - | - | 75 | 1000 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, M.; Eljack, F.; Kazi, M.-K.; Almomani, F.; Ahmed, E.; El Jack, Z. Treatment Technologies for Cooling Water Blowdown: A Critical Review. Sustainability 2022, 14, 376. https://doi.org/10.3390/su14010376
Soliman M, Eljack F, Kazi M-K, Almomani F, Ahmed E, El Jack Z. Treatment Technologies for Cooling Water Blowdown: A Critical Review. Sustainability. 2022; 14(1):376. https://doi.org/10.3390/su14010376
Chicago/Turabian StyleSoliman, Mariam, Fadwa Eljack, Monzure-Khoda Kazi, Fares Almomani, Elalim Ahmed, and Ziad El Jack. 2022. "Treatment Technologies for Cooling Water Blowdown: A Critical Review" Sustainability 14, no. 1: 376. https://doi.org/10.3390/su14010376
APA StyleSoliman, M., Eljack, F., Kazi, M.-K., Almomani, F., Ahmed, E., & El Jack, Z. (2022). Treatment Technologies for Cooling Water Blowdown: A Critical Review. Sustainability, 14(1), 376. https://doi.org/10.3390/su14010376







