Surface Decontamination and Shelf-Life Extension of Gilthead Sea Bream by Alternative Washing Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
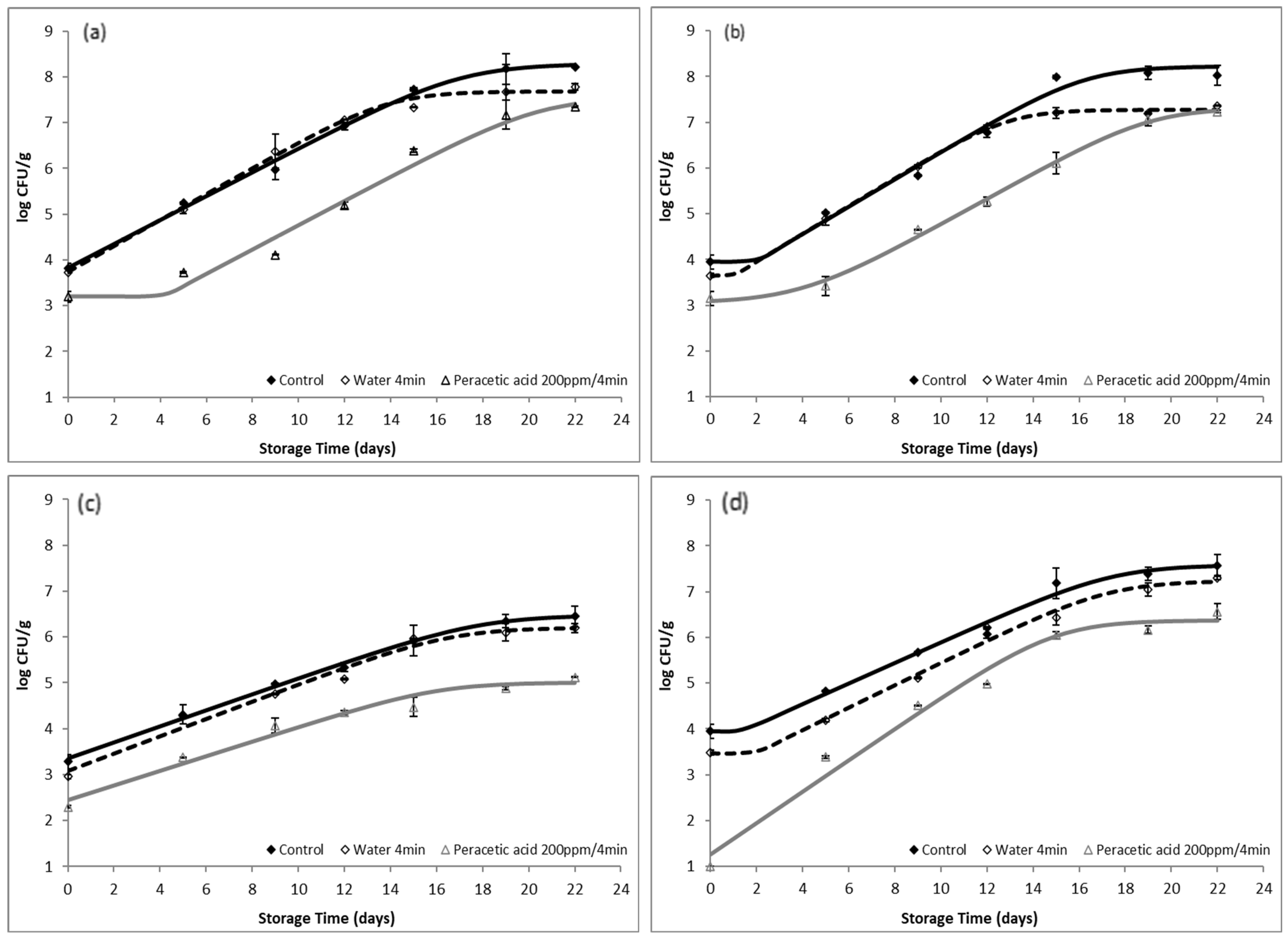
2.2. Microbiological Analysis
2.3. pH Measurement
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Efficacy of Organic Acids against Microbial Load of Fish
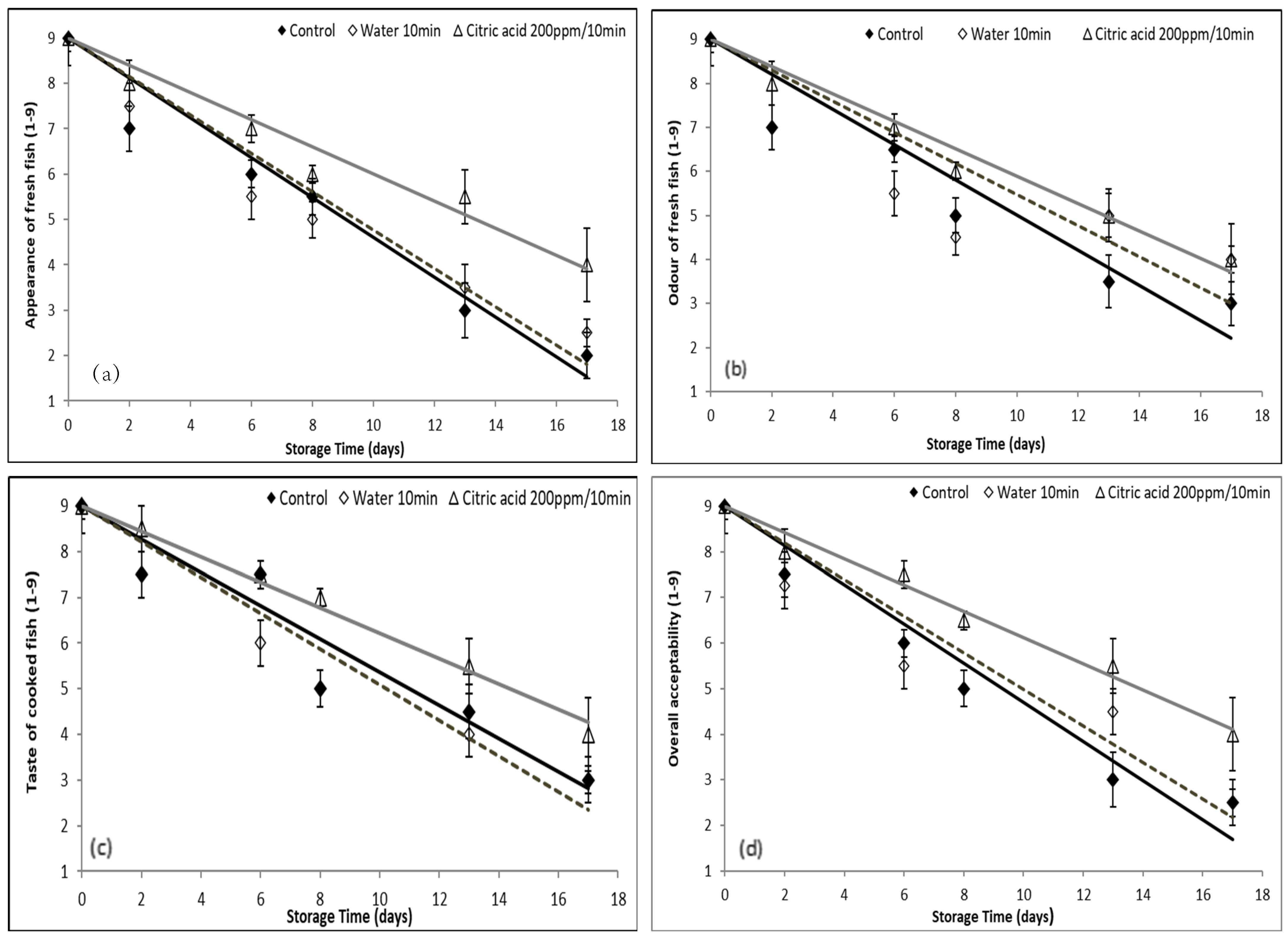
3.2. Evaluation of the Effect of Washing with Organic Acids on the Quality and Shelf Life of Gutted Fish
3.2.1. Application of Citric Acid as a Washing Medium for Gutted Fish
3.2.2. Application of PAA as a Washing Medium for Gutted Fish
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orban, E.; Nevigato, T.; Lena, G.D.; Masci, M.; Casini, I.; Gambelli, L.; Caproni, R. New trends in the seafood market. Sutchi catfish (Pangasius hypophthalmus) fillets from Vietnam: Nutritional quality and safety aspects. Food Chem. 2008, 110, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Thi, A.N.T.; Noseda, B.; Samapundo, S.; Nguyen, B.L.; Broekaert, K.; Rasschaert, G.; Heyndrickx, M.; Devlieghere, F. Microbial ecology of Vietnamese Tra fish (Pangasius hypophthalmus) fillets during processing. Int. J. Food Microbiol. 2013, 167, 144–152. [Google Scholar]
- Tsironi, T.; Taoukis, P. Advances in conventional and nonthermal processing of fish for quality improvement and shelf life extension. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–7. [Google Scholar]
- Ntzimani, A.; Angelakopoulos, R.; Semenoglou, I.; Dermesonlouoglou, E.; Tsironi, T.; Moutou, K.; Taoukis, P. Slurry ice as an alternative cooling medium for fish harvesting and transportation: Study of the effect on seabass flesh quality and shelf life. Aquac. Fish. 2021; in Press. [Google Scholar] [CrossRef]
- Allende, A.; Selma, M.V.; López-Gálvez, F.; Villaescusa, R.; Gil, M.I. Impact of wash water quality on sensory and microbial quality, including Escherichia coli cross-contamination, of fresh-cut escarole. J. Food Protect. 2008, 71, 2514–2518. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R.; Ryu, J.-H. Produce Handling and Processing Practices. Emerg. Infect. Dis. 1997, 3, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y. Fresh-cut produce wash water reuse affects water quality and packaged product quality and microbial growth in romaine lettuce. Hortic. Sci. 2007, 42, 1413–1419. [Google Scholar] [CrossRef]
- Sapers, G.M.; Miller, R.L.; Pilizota, V.; Mattrazzo, A.M. Antimicrobial treatments for minimally processed cantaloupe melon. J. Food Sci. 2001, 66, 345–349. [Google Scholar] [CrossRef]
- Pablos, C.; Romero, A.; De Diego, A.; Vargas, C.; Bascón, I.; Pérez-Rodríguez, F.; Marugán, J. Novel antimicrobial agents as alternative to chlorine with potential applications in the fruit and vegetable processing industry. Int. J. Food Microbiol. 2018, 285, 92–97. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT-Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Meireles, A.; Giaouris, E.; Simões, M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016, 82, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Thi, A.N.T.; Sampers, I.; Haute, S.V.; Samapundo, S.; Nguyen, B.L.; Heyndrickx, M.; Devlieghere, F. Decontamination of Pangasius fish (Pangasius hypophthalmus) with chlorine or peracetic acid in the laboratory and in a Vietnamese processing company. Int. J. Food Microbiol. 2015, 208, 93–101. [Google Scholar]
- Food and Drug Administration (FDA). Analysis and Evaluation of Preventive Control Measures for the Control and Reduction/Elimination of Microbial Hazards on Fresh and Fresh-Cut Produce. 2001. Available online: http://www.cfsan.fda.gov/~comm/ift3exec.html (accessed on 28 March 2022).
- Miller, F.A.; Ramos, B.; Gil, M.M.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Influence of pH, type of acid and recovery media on the thermal inactivation of Listeria innocua. Int. J. Food Microbiol. 2009, 133, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Loretz, M.; Stephan, R.; Zweifel, C. Antibacterial activity of decontamination treatments for cattle hides and beef carcasses. Food Control 2011, 22, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Loretz, M.; Stephan, R.; Zweifel, C. Antibacterial activity of decontamination treatments for pig carcasses. Food Control 2011, 22, 1121–1125. [Google Scholar] [CrossRef]
- Rajkovic, A.; Smigic, N.; Devlieghere, F. Contemporary strategies in combating microbial contamination in food chain. Int. J. Food Microbiol. 2010, 141, S29–S42. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Siragusa, G.R. The effectiveness of carcass decontamination systems for controlling the presence of pathogens on the surfaces of meat animal carcasses. J. Food Saf. 1995, 15, 229–238. [Google Scholar] [CrossRef]
- Stratford, M.; Eklund, T. Organic acids and esters. In Food Preservatives, 2nd ed.; Russell, N.J., Gould, G.W., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 48–84. [Google Scholar]
- Theron, M.M.; Lues, J.F.R. Organic Acids and Food Preservation; CRC Press: Boca Raton, FL, USA, 2011; p. 318. [Google Scholar]
- Brul, S.; Coote, P. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Masniyom, P.; Benjama, O. Effect of lactic, acetic and citric acids on quality changes of refrigerated green mussel, Perna viridis (Linnaeus, 1758). Songklanakarin J. Sci. Technol. 2007, 29, 1123–113424. [Google Scholar]
- Seo, Y.-S.; Lee, G.; Song, S.; Kim, K.; Cho, M. Combinatorial treatment using citric acid, malic acid, and phytic acid for synergistical inactivation of foodborne pathogenic bacteria. Korean J. Chem. Eng. 2021, 38, 826–832. [Google Scholar] [CrossRef]
- Luukkonen, T.; Pehkonen, S.O. Peracids in water treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Wang, D.; Yamaki, S.; Kawai, Y.; Yamazaki, K. Sanitizing efficacy and antimicrobial mechanism of peracetic acid against histamine-producing bacterium, Morganella psychrotolerans. LWT 2020, 126, 109263. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Oliveira, M.; Burgess, C.M.; Cropotova, J.; Rustad, T.; Sun, D.W.; Tiwari, B.K. Combined effects of ultrasound, plasma-activated water, and peracetic acid on decontamination of mackerel fillets. LWT 2021, 150, 111957. [Google Scholar] [CrossRef]
- Rasekh, J.G.; Waters, M.E.; Sidwell, V.D. The effect of washing on the quality characteristics of minced fresh croaker, Micropogon undulatus, held in frozen storage. Mar. Fish. Rev. 1980, 42, 26–30. [Google Scholar]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Geeraerd, A.; Valdramidis, V.P.; Van Impe, J.F.M. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. Mathematics of predictive food microbiology. Int. J. Food Microbiol. 1995, 26, 199–218. [Google Scholar] [CrossRef] [Green Version]
- Tsironi, T.; Maltezou, I.; Tsevdou, M.; Katsaros, G.; Taoukis, P.S. High pressure cold pasteurization of gilthead sea bream fillets: Selection of process conditions and validation of shelf-life extension. Food Bioprocess. Technol. 2015, 8, 681–690. [Google Scholar] [CrossRef]
- Vitro, R.; Sanz, D.; Alvarez, I.; Condon, S.; Raso, J. Application of the Weibull model to describe inactivation of Listeria monocytogenes and Escherichia coli by citric and lactic acid at different temperatures. J. Sci. Food Agric. 2006, 86, 865–870. [Google Scholar]
- Akbas, M.Y.; Olmez, H. Inactivation of Escherichia coli and Listeria monocytogenes on iceberg lettuce by dip wash treatments with organic acids. Lett. Appl. Microbiol. 2007, 44, 619–624. [Google Scholar] [CrossRef]
- Simeonidou, S.; Govaris, A.; Vareltzis, K. Quality assessment of seven Mediterranean fish during storage on ice. Food Res. Int. 1998, 30, 479–484. [Google Scholar] [CrossRef]
- Viji, P.; Panda, S.K.; Mohan, C.O.; Bindu, J.; Ravishankar, C.N.; Srinivasa Gopal, T.K. Combined effects of vacuum packaging and mint extract treatment on the biochemical, sensory and microbial changes of chill stored Indian mackerel. J. Food Sci. Technol. 2016, 53, 4289–4297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ünal Şengör, G.F.; Balaban, M.D.; Ceylan, Z.; Dogruyol, H. Determination of shelf life of gilthead seabream (Sparus aurata) with time temperature indicators. J. Food Process. Preserv. 2017, 42, e13426. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Boziaris, I.S. Monitoring of spoilage and determination of microbial communities based on 16S rRNA gene sequence analysis of whole sea bream stored at various temperatures. LWT-Food Sci. Technol. 2016, 66, 553–559. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Giannakourou, M.C.; Taoukis, P.S.; Nychas, G.-J.E. Application of shelf life decision system (SLDS) to marine cultured fish quality. Int. J. Food Microbiol. 2002, 73, 375–382. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.-J.E.; Boziaris, I.S. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 °C. Food Microbiol. 2015, 50, 44–53. [Google Scholar] [CrossRef]
- Outsoumanis, K.; Nychas, G.-J.E. Application of a systematic procedure to develop a microbial model for rapid fish shelf life predictions. Int. J. Food Microbiol. 2000, 60, 171–184. [Google Scholar] [CrossRef]
- Papaharisis, L.; Tsironi, T.; Dimitroglou, A.; Taoukis, P.; Pavlidis, M. Stress assessment, quality indicators and shelf life of three aquaculture important marine fish, in relation to harvest practices, water temperature and slaughter method. Aquac. Res. 2018, 50, 2608–2620. [Google Scholar] [CrossRef]
- Sallam, K.I. Chemical, sensory and shelf life evaluation of sliced salmon treated with salts of organic acids. Food Chem. 2007, 101, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Vandekinderen, I.; Devlieghere, F.; van Camp, J.; Denon, Q.; Alarcon, S.S.; Ragaert, P.; De Meulenaer, B. Impact of a decontamination step with peroxyacetic acid on the shelf-life, sensory quality and nutrient content of grated carrots packed under equilibrium modified atmosphere and stored at 7 °C. Postharvest Biol. Technol. 2009, 54, 141–152. [Google Scholar] [CrossRef]
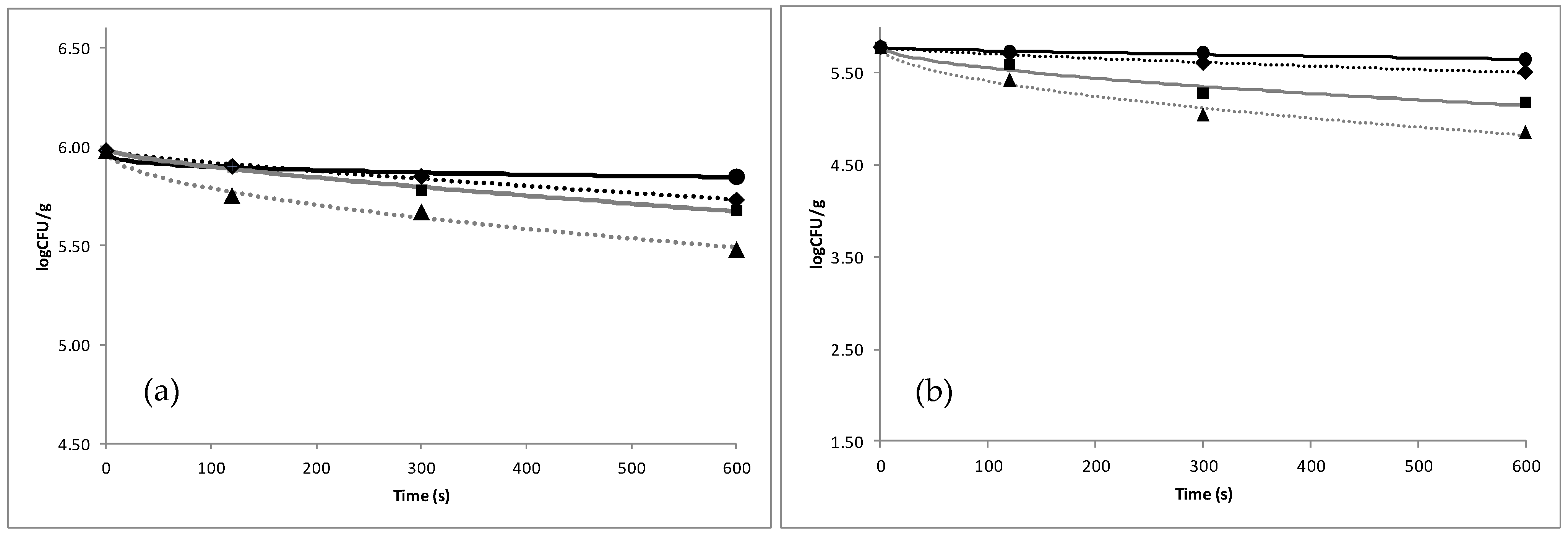

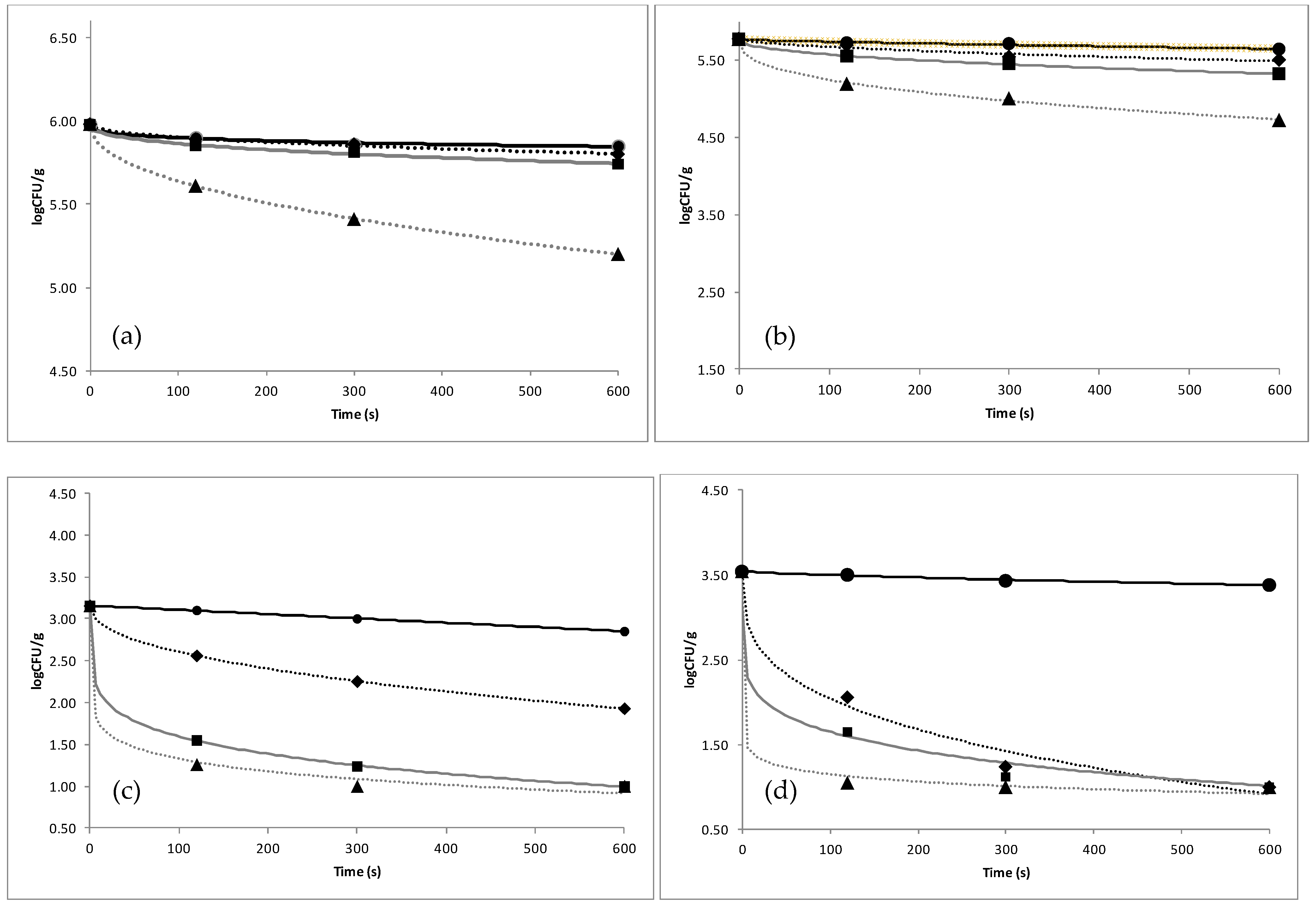
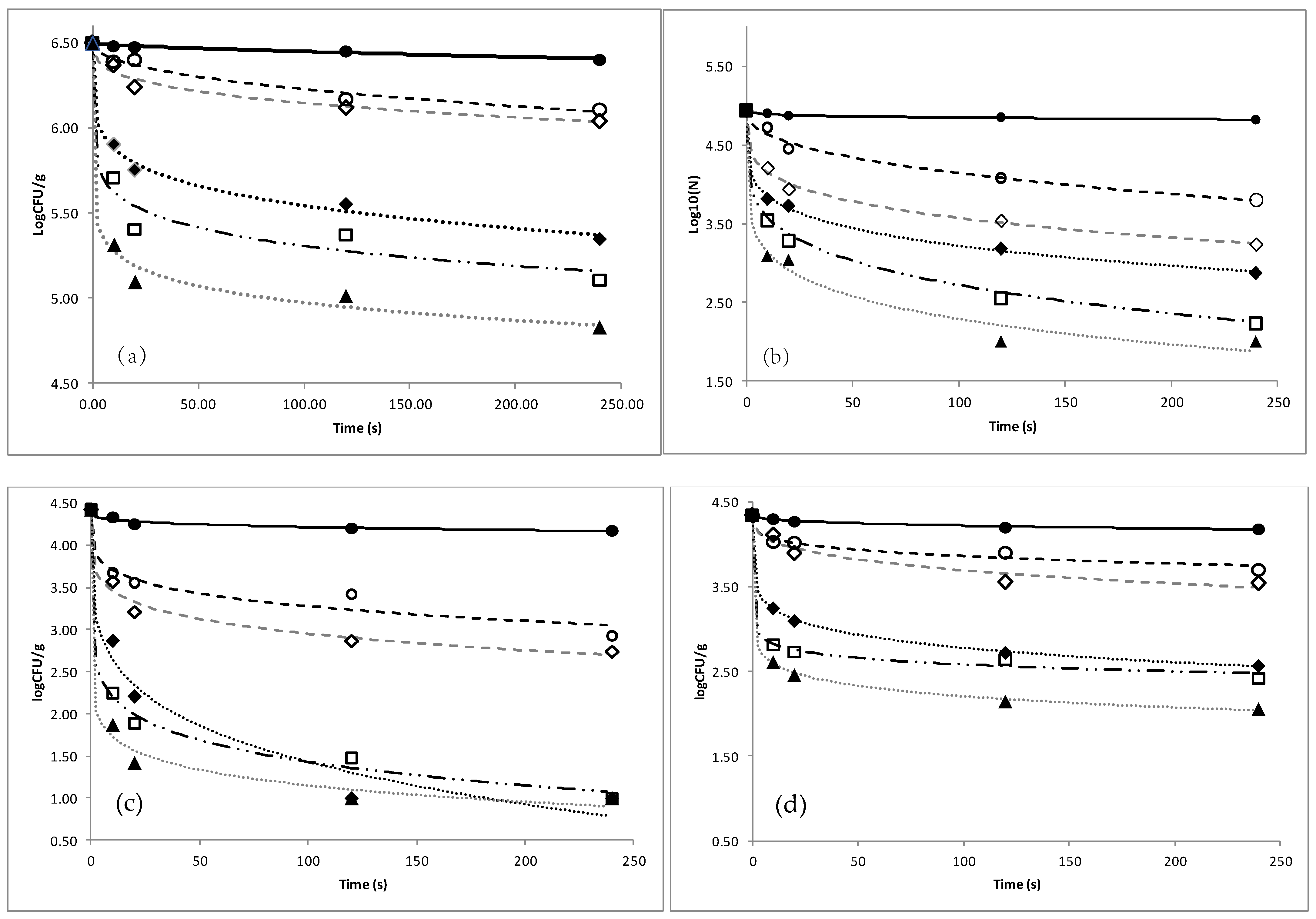


| Solution Concentration | Total Viable Count | Pseudomonas spp. | Enterobacteriaceae spp. | H2S Producing Bacteria | |
|---|---|---|---|---|---|
| Lactic acid | 0 ppm (water) | δ = 732,050 ± 2006 a p = 0.28 ± 0.10 R2 = 0.987 | δ = 8720 ± 1108 a p = 0.78 ± 0.17 R2 = 0.956 | δ = 1390 ± 128 a p = 1.01 ± 0.06 R2 = 0.999 | δ = 7155 ± 506 a p = 0.72 ± 0.10 R2 = 0.984 |
| 50 ppm | δ = 3464 ± 1261 b p = 0.81 ± 0.17 R2 = 0.992 | δ = 3248 ± 827 b p = 0.76 ± 0.11 R2 = 0.996 | δ = 182 ± 9 b p = 0.56 ± 0.02 R2 = 0.999 | δ = 29 ± 5 b p = 0.24 ± 0.09 R2 = 0.988 | |
| 100 ppm | δ = 3052 ± 1090 b p = 0.72 ± 0.15 R2 = 0.991 | δ = 1319 ± 71 c p = 0.57 ± 0.18 R2 = 0.955 | δ = 79 ± 3 c p = 0.39 ± 0.07 R2 = 995 | δ = 2.89 ± 0.35 c p = 0.17 ± 0.04 R2 = 998 | |
| 200 ppm | δ = 2340 ± 905 c p = 0.53 ± 0.13 R2 = 0.989 | δ = 653 ± 17 d p = 0.53 ± 0.17 R2 = 0.982 | δ = 75 ± 8 c p = 0.39 ± 0.12 R2 = 0.986 | δ = 2.44 ± 7.82 c p = 0.18 ± 0.11 R2 = 0.983 | |
| Citric acid | 0 ppm (water) | δ = 732,050 ± 2006 a p = 0.28 ± 0.10 R2 = 0.987 | δ = 8720 ± 1108 a p = 0.78 ± 0.17 R2 = 0.956 | δ = 1930 ± 128 a p = 1.01 ± 0.06 R2 = 0.999 | δ = 7155 ± 506 a p = 0.72 ± 0.10 R2 = 0.984 |
| 50 ppm | δ = 2492 ± 1907 b p = 0.47 ± 0.09 R2 = 0.993 | δ = 5088 ± 641 b p = 0.58 ± 0.13 R2 = 0.942 | δ = 380 ± 21 b p = 0.45 ± 0.01 R2 = 0.999 | δ = 26.99 ± 9 b p = 0.31 ± 0.06 R2 = 0.987 | |
| 100 ppm | δ = 2239 ± 1457 b p = 0.40 ± 0.07 R2 = 0.996 | δ = 3540 ± 45 c p = 0.45 ± 0.03 R2 = 0.999 | δ = 8.77 ± 1.40 c p = 0.18 ± 0.01 R2 = 0.999 | δ = 11.58 ± 3 b p = 0.20 ± 0.03 R2 = 0.961 | |
| 200 ppm | δ = 1024 ± 15 c p = 0.46 ± 0.01 R2 = 0.999 | δ = 545 ± 64 d p = 0.38 ± 0.05 R2 = 0.998 | δ = 0.61 ± 0.03 d p = 0.12 ± 0.06 R2 = 0.995 | δ = 0.000040 ± 0.000003 c p = 0.05 ± 0.01 R2 = 0.997 | |
| Peracetic acid | 0 ppm (water) | δ = 6517 ± 803 a p = 0.73 ± 0.28 R2 = 0.964 | δ = 417,470 ± 85,616 a p = 0.29 ± 0.08 R2 = 0.969 | δ = 73,894 ± 1463 a p = 0.13 ± 0.08 R2 = 0.959 | δ = 41,161 ± 3744 a p = 0.33 ± 0.06 R2 = 0.987 |
| 10 ppm | δ = 2859 ± 208 b p = 0.30 ± 0.08 R2 = 0.972 | δ = 169 ± 42 b p = 0.41 ± 0.08 R2 = 0.984 | δ = 52 ± 4 b p = 0.20 ± 0.08 R2 = 0.954 | δ = 2032 ± 171 b p = 0.30 ± 0.06 R2 = 0.966 | |
| 20 ppm | δ = 1777 ± 889 b p = 0.44 ± 0.11 R2 = 0.977 | d = 27 ± 9 c δ = 0.24 ± 0.03 R2 = 0.995 | δ = 11 ± 4 c p = 0.18 ± 0.04 R2 = 0.984 | δ = 371 ± 19 c p = 0.30 ± 0.10 R2 = 0.953 | |
| 50 ppm | δ = 124 ± 37 c p = 0.19 ± 0.03 R2 = 0.994 | δ = 6.67 ± 1.78 d p = 0.20 ± 0.01 R2 = 0.995 | δ = 0.70 ± 0.14 d p = 0.22 ± 0.03 R2 = 0.974 | δ = 4.76 ± 0.74 d p = 0.15 ± 0.01 R2 = 0.999 | |
| 150 ppm | δ = 26 ± 3 d p = 0.13 ± 0.05 R2 = 0.968 | δ = 5.08 ± 1,07 d p = 0.24 ± 0.04 R2 = 0.989 | δ = 0.020 ± 0.001 e p = 0.13 ± 0.02 R2 = 0.995 | δ = 0.010 ± 0.002 e p = 0.06 ± 0.01 R2 = 0.996 | |
| 200 ppm | δ = 1.75 ± 0.36 d p = 0.10 ± 0.03 R2 = 0.991 | δ = 0.28 ± 0.02 e p = 0.17 ± 0.04 R2 = 0.987 | δ = 0.00010 ± 0.00001 f p = 0.08 ± 0.02 R2 = 0.993 | δ = 0.012 ± 0.001 e p = 0.09 ± 0.01 R2 = 0.999 |
| Control | Water | Citric Acid (200 ppm/10 min) | Peracetic Acid (200 ppm/4 min) | |
|---|---|---|---|---|
| 0 °C | 11 | 12 | 16 | 18 |
| 5 °C | 6 | 7 | 11 | 9 |
| 10 °C | 4 | 5 | 6 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntzimani, A.; Semenoglou, I.; Dermesonlouoglou, E.; Tsironi, T.; Taoukis, P. Surface Decontamination and Shelf-Life Extension of Gilthead Sea Bream by Alternative Washing Treatments. Sustainability 2022, 14, 5887. https://doi.org/10.3390/su14105887
Ntzimani A, Semenoglou I, Dermesonlouoglou E, Tsironi T, Taoukis P. Surface Decontamination and Shelf-Life Extension of Gilthead Sea Bream by Alternative Washing Treatments. Sustainability. 2022; 14(10):5887. https://doi.org/10.3390/su14105887
Chicago/Turabian StyleNtzimani, Athina, Ioanna Semenoglou, Efimia Dermesonlouoglou, Theofania Tsironi, and Petros Taoukis. 2022. "Surface Decontamination and Shelf-Life Extension of Gilthead Sea Bream by Alternative Washing Treatments" Sustainability 14, no. 10: 5887. https://doi.org/10.3390/su14105887
APA StyleNtzimani, A., Semenoglou, I., Dermesonlouoglou, E., Tsironi, T., & Taoukis, P. (2022). Surface Decontamination and Shelf-Life Extension of Gilthead Sea Bream by Alternative Washing Treatments. Sustainability, 14(10), 5887. https://doi.org/10.3390/su14105887






