Fish Food Production Using Agro-Industrial Waste Enhanced with Spirulina sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials for the Processing of Fish Food
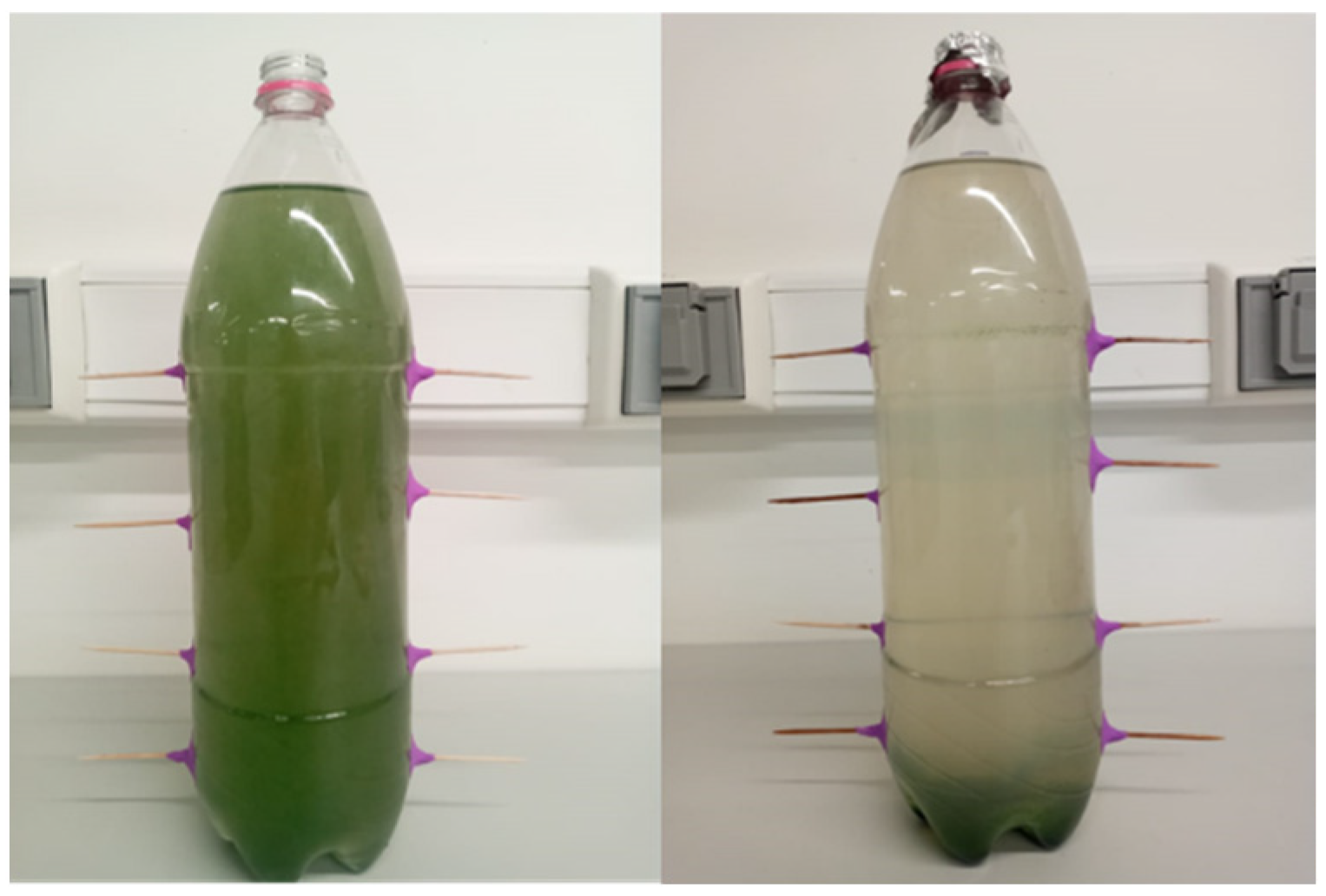
2.2. Spirulina sp. Production
2.3. Manufacture of Flours from Agro-Industrial Waste
2.4. Processing of Food for Fish
3. Results and Discussion
3.1. Raw Materials for the Processing of Fish Food
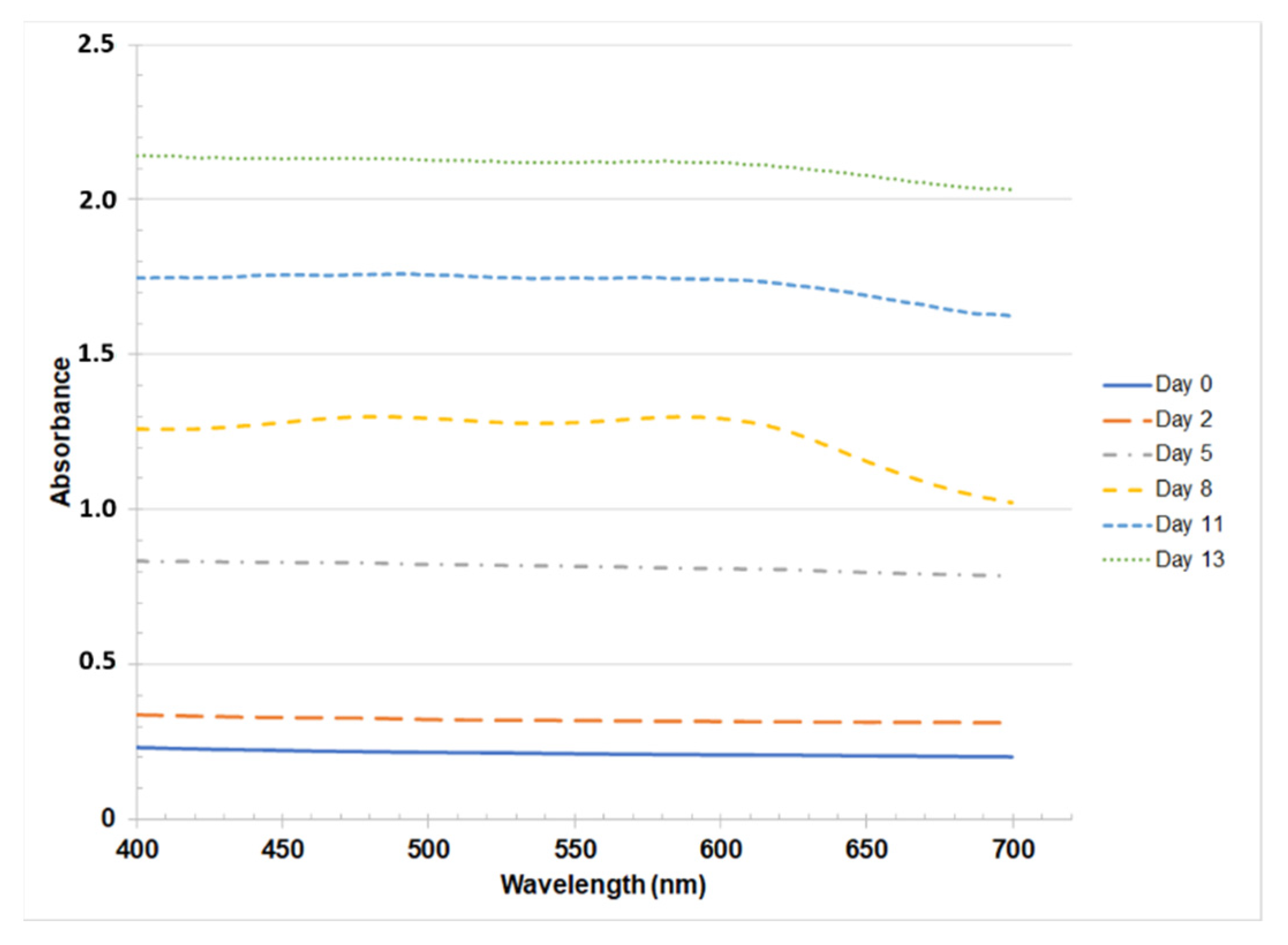
3.1.1. Spirulina sp.
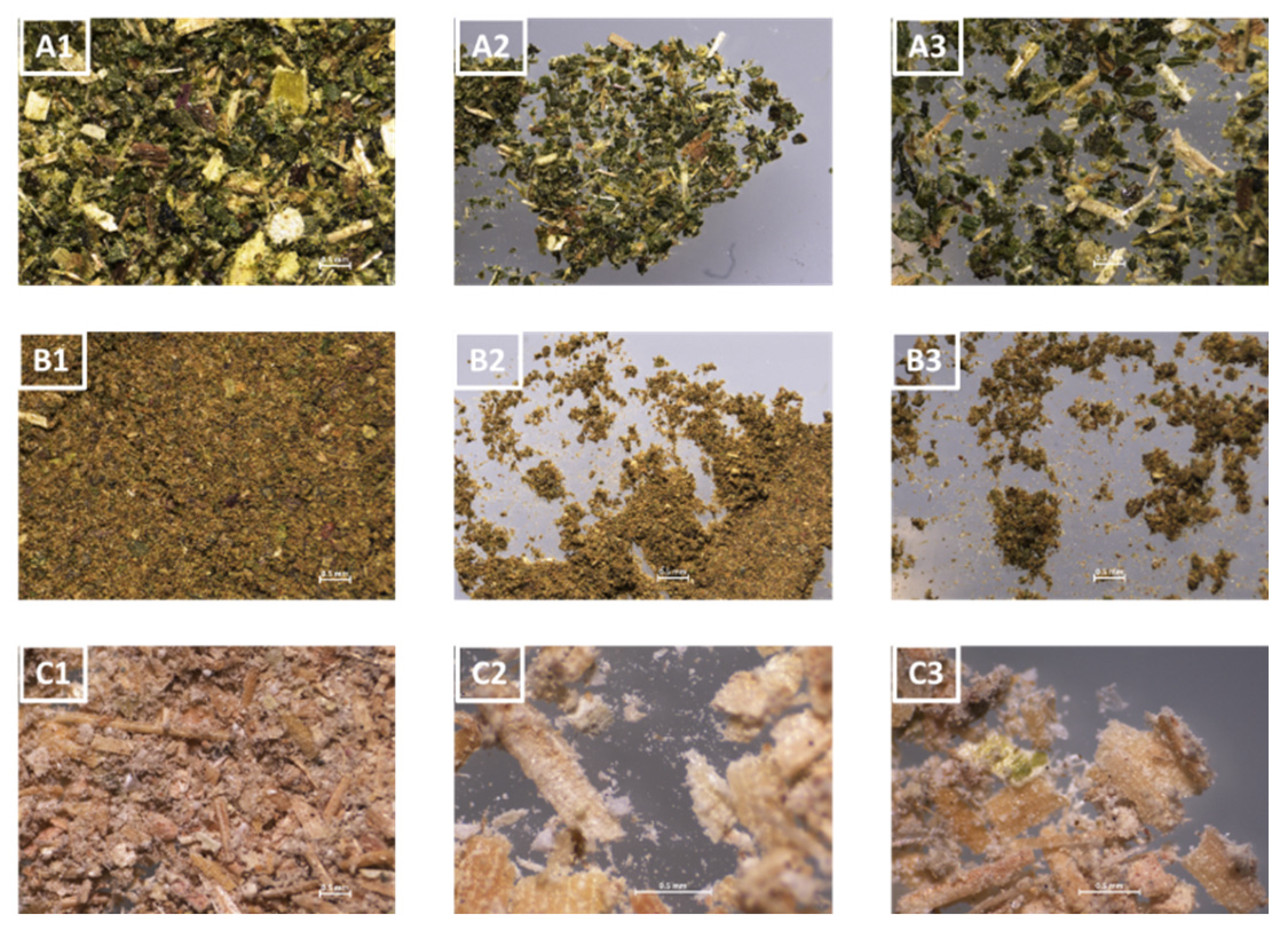
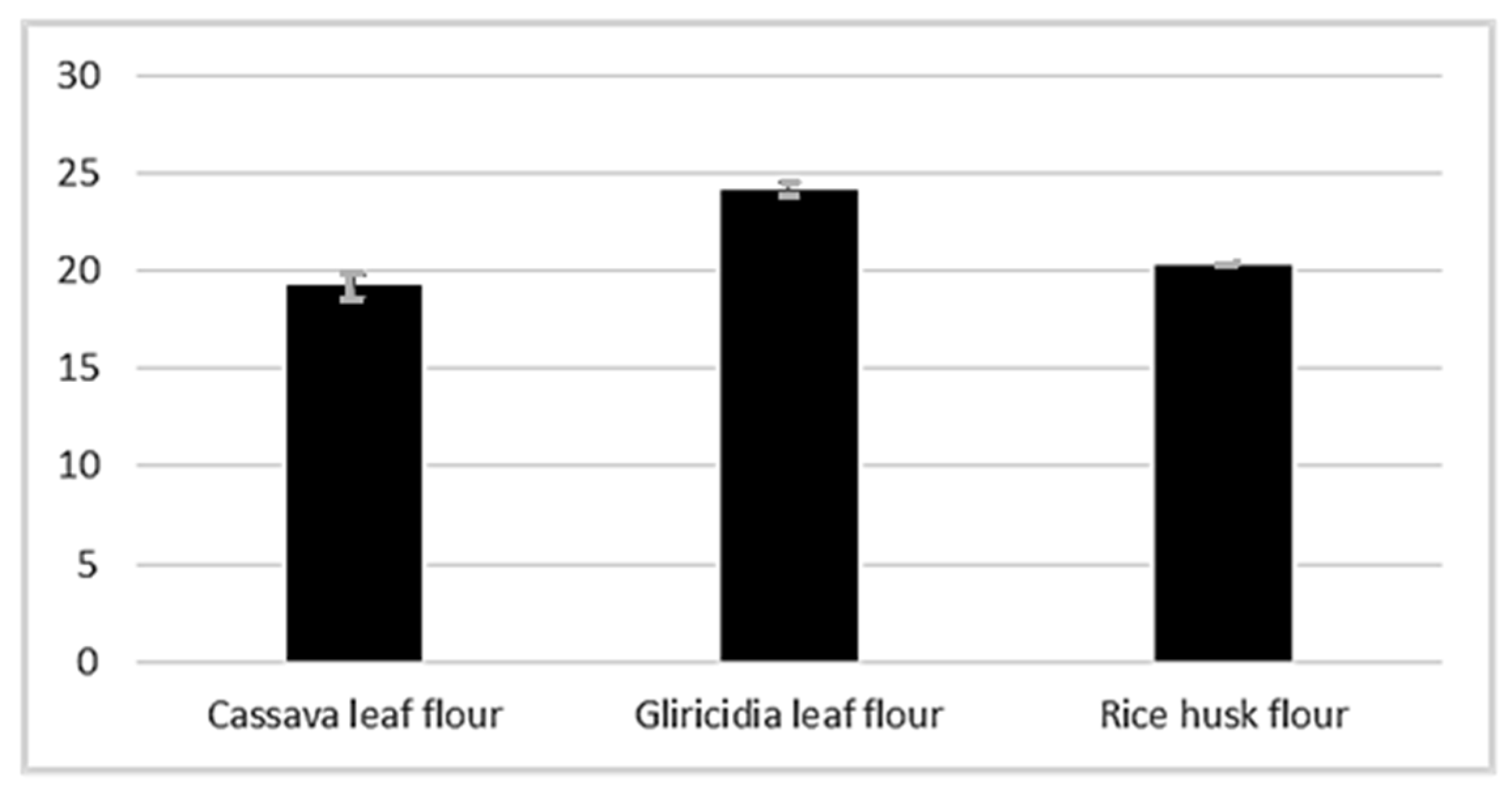
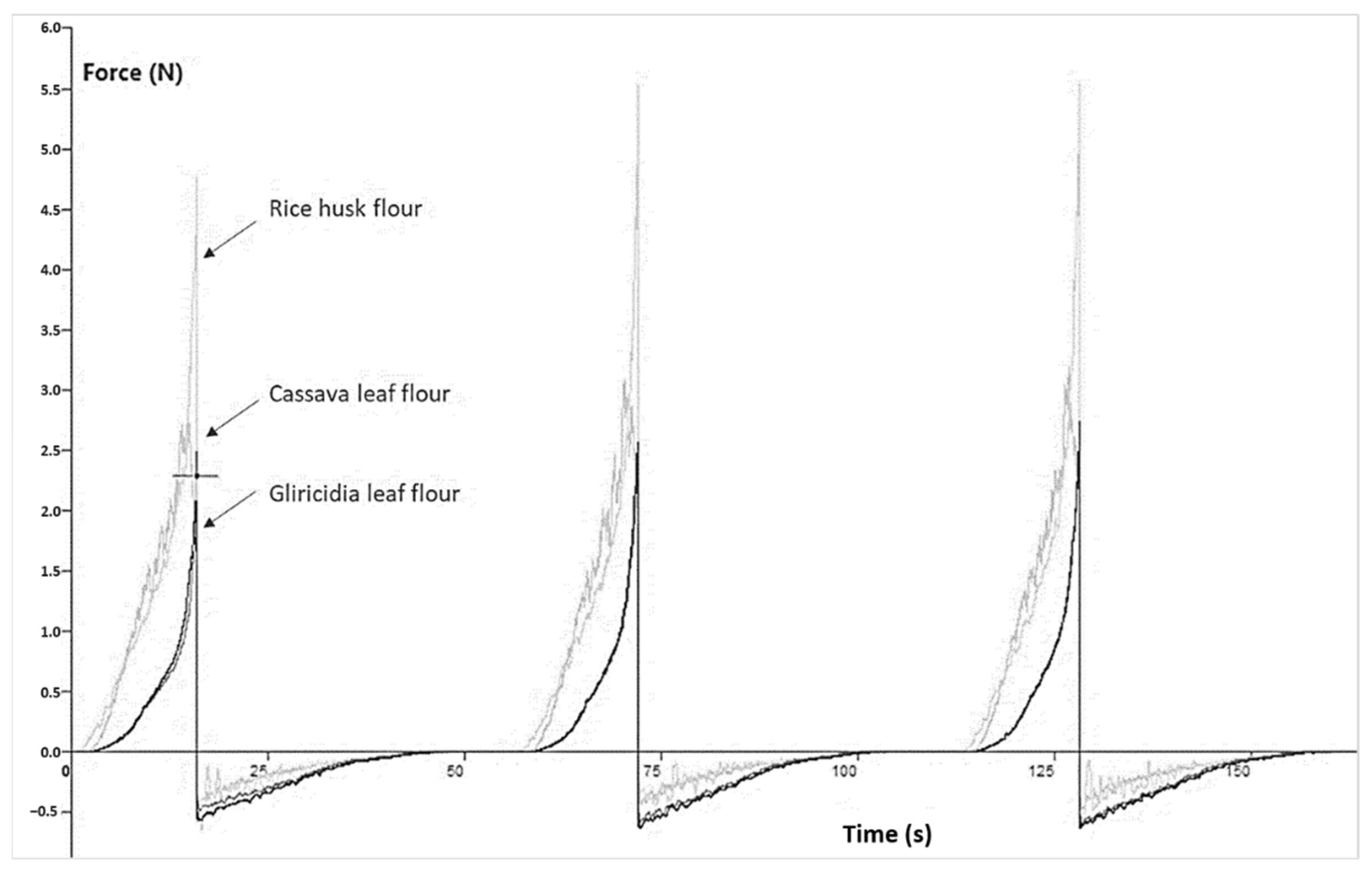
3.1.2. Flours Produced from Agro-Industrial Waste
3.2. Development of Fish Food
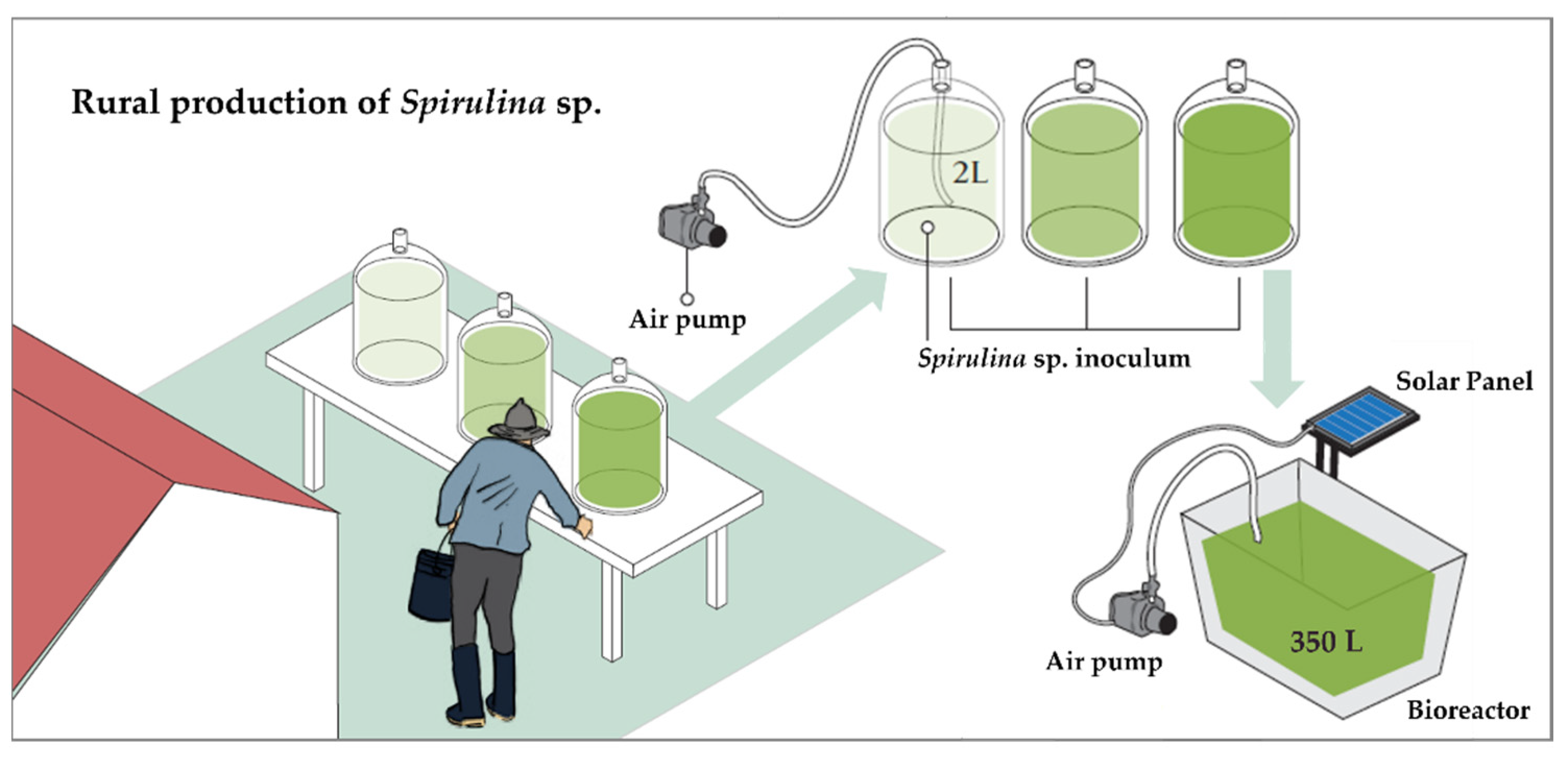
3.3. Pilot Production of Spirulina sp. to Guarantee the Sustainability of the Aquaculture Sector
- Production of Spirulina sp. as raw material by the farmers
- Production of fish food incorporating the Spirulina produced.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Obi, F.O.; Ugwuishiwu, B.O.; Nwakaire, J.N. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- Bedekar, P.A.; Bhalkar, B.N.; Patil, S.M.; Govindwar, S.P. Moringa oleifera-mediated coagulation of textile wastewater and its biodegradation using novel consortium-BBA grown on agricultural waste substratum. Environ. Sci. Pollut. Res. 2016, 23, 20963–20976. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Da Silva Batista, N.; Latawiec, A.; Rodrigues, A.; Strassburg, B.; Silva, D.; Araujo, E.; De Moraes, L.; Guerra, J.; Galvão, G.; et al. The Effects of Gliricidia-Derived Biochar on Sequential Maize and Bean Farming. Sustainability 2018, 10, 578. [Google Scholar] [CrossRef] [Green Version]
- Mak, T.M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef]
- Youenou, B.; Hien, E.; Deredjian, A.; Brothier, E.; Favre-Bonté, S.; Nazaret, S. Impact of untreated urban waste on the prevalence and antibiotic resistance profiles of human opportunistic pathogens in agricultural soils from Burkina Faso. Environ. Sci. Pollut. Res. 2016, 23, 25299–25311. [Google Scholar] [CrossRef] [Green Version]
- Miskat, M.I.; Ahmed, A.; Chowdhury, H.; Chowdhury, T.; Chowdhury, P.; Sait, S.; Park, Y.-K. Assessing the Theoretical Prospects of Bioethanol Production as a Biofuel from Agricultural Residues in Bangladesh: A Review. Sustainability 2020, 12, 8583. [Google Scholar] [CrossRef]
- Lansche, J.; Awiszus, S.; Latif, S.; Müller, J. Potential of Biogas Production from Processing Residues to Reduce Environmental Impacts from Cassava Starch and Crisp Production—A Case Study from Malaysia. Appl. Sci. 2020, 10, 2975. [Google Scholar] [CrossRef]
- Patsios, S.I.; Dedousi, A.; Sossidou, E.N.; Zdragas, A. Sustainable Animal Feed Protein through the Cultivation of YARROWIA Lipolytica on Agro-Industrial Wastes and by-Products. Sustainability 2020, 12, 1398. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garcia, G.; Stone, J.; Rahimifard, S. Opportunities for waste valorisation in the food industry—A case study with four UK food manufacturers. J. Clean. Prod. 2019, 211, 1339–1356. [Google Scholar] [CrossRef]
- Choi, W.M.; Lam, C.L.; Mo, W.Y.; Wong, M.H. The use of food wastes as feed ingredients for culturing grass carp (Ctenopharyngodon idellus) in Hong Kong. Environ. Sci. Pollut. Res. 2016, 23, 7178–7185. [Google Scholar] [CrossRef] [PubMed]
- Cadillo-Benalcazar, J.J.; Giampietro, M.; Bukkens, S.G.F.; Strand, R. Multi-scale integrated evaluation of the sustainability of large-scale use of alternative feeds in salmon aquaculture. J. Clean. Prod. 2020, 248, 119210. [Google Scholar] [CrossRef]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Meyfroidt, P. Trade-offs between environment and livelihoods: Bridging the global land use and food security discussions. Glob. Food Sec. 2018, 16, 9–16. [Google Scholar] [CrossRef]
- Adéyèmi, A.D.; Kayodé, A.P.P.; Chabi, I.B.; Odouaro, O.B.O.; Nout, M.J.; Linnemann, A.R. Screening local feed ingredients of Benin, West Africa, for fish feed formulation. Aquac. Rep. 2020, 17, 100386. [Google Scholar] [CrossRef]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as feed: Using economic allocation to quantify the Fish In: Fish Out ratio of major fed aquaculture species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Rosas, V.T.; Poersch, L.H.; Romano, L.A.; Tesser, M.B. Feasibility of the use of Spirulina in aquaculture diets. Rev. Aquac. 2019, 11, 1367–1378. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–nutritious, sustainable aqua- and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Dewi, E.N.; Amalia, U.; Mel, M. The Effect of Different Treatments to the Amino Acid Contents of Micro Algae Spirulina sp. Aquat. Procedia 2016, 7, 59–65. [Google Scholar] [CrossRef]
- Abdelkhalek, N.K.M.; Eissa, I.A.M.; Ahmed, E.; Kilany, O.E.; El-Adl, M.; Dawood, M.; Hassan, A.M.; Abdel-Daim, M.M. Protective role of dietary Spirulina platensis against diazinon-induced Oxidative damage in Nile tilapia; Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2017, 54, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sankarapandian, V.; Nitharsan, K.; Parangusadoss, K.; Gangadaran, P.; Ramani, P.; Maran, B.A.V.; Jogalekar, M.P. Prebiotic Potential and Value-Added Products Derived from Spirulina laxissima SV001—A Step towards Healthy Living. BioTech 2022, 11, 13. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhattacharya, S. Characterization of agglomeration process as a function of moisture content using a model food powder. J. Texture Stud. 2006, 37, 35–48. [Google Scholar] [CrossRef]
- Abdullah, E.; Salam, A.; Aziz, A. Cohesiveness and Flowability Properties of Silica Gel Powder. Phys. Int. 2010, 1, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Seth, D.; Rajamanickam, G. Development of extruded snacks using soy, sorghum, millet and rice blend—A response surface methodology approach. Int. J. Food Sci. Technol. 2012, 47, 1526–1531. [Google Scholar] [CrossRef]
- Arroyo, M. Aprovechamiento de La Harina de Plecostomus Spp Como Ingrediente En Alimento Para El Crecimiento de Tilapia (Oreochromis Niloticus); Instituto Politécnico Nacional de México: Mexico City, Mexico, 2008. [Google Scholar]
- Nicoletti, M. Microalgae Nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef]
- Becker, W. Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 380–391. [Google Scholar]
- Altmann, B.A.; Rosenau, S. Spirulina as Animal Feed: Opportunities and Challenges. Foods 2022, 11, 965. [Google Scholar] [CrossRef]
- Toughan, H.; Khalil, S.R.; El-Ghoneimy, A.A.; Awad, A.; Seddek, A.S. Effect of dietary supplementation with Spirulina platensis on Atrazine-induced oxidative stress- mediated hepatic damage and inflammation in the common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2018, 149, 135–142. [Google Scholar] [CrossRef]
- Rojas, E.; Ávila, M.; Parada, G. Application of nutritional strategies and their effect in continuous culture of Spirulina (Arthrospira platensis). Lat. Am. J. Aquat. Res. 2012, 40, 763–771. [Google Scholar] [CrossRef]
- Delrue, F.; Alaux, E.; Moudjaoui, L.; Gaignard, C.; Fleury, G.; Perilhou, A.; Richaud, P.; Petitjean, M.; Sassi, J.-F. Optimization of Arthrospira platensis (Spirulina) Growth: From Laboratory Scale to Pilot Scale. Fermentation 2017, 3, 59. [Google Scholar] [CrossRef] [Green Version]
- Colla, L.M.; Oliveira Reinehr, C.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Çelekli, A.; Yavuzatmaca, M.; Bozkurt, H. Modeling of biomass production by Spirulina platensis as function of phosphate concentrations and pH regimes. Bioresour. Technol. 2009, 100, 3625–3629. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.G.; Braune, S.; Waldeck, P.; Küpper, J.-H.; Petrick, I.; Jung, F. Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor. Life 2021, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Benemann, J. Microalgae for Biofuels and Animal Feeds. Energies 2013, 6, 5869–5886. [Google Scholar] [CrossRef] [Green Version]
- Pantoja, J.O.; Sánchez, S.; Hoyos, J.L. Obtención de un alimento extruido para tilapia roja (oreochromis spp) utilizando ensilaje biológico de pescado. Biotecnol. En El Sect. Agropecu. Y Agroind. 2011, 9, 178–186. [Google Scholar]
- Lara, R.; Castro, T.; Castro, J.; Castro, G.; Malpica Sánchez, A.; García Castillo, V. La importancia de Spirulina en la alimentación acuícola. Contactos 2005, 57, 13–14. [Google Scholar]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Rosenau, S.; Oertel, E.; Dietz, C.; Wessels, S.; Tetens, J.; Mörlein, D.; Ciulu, M. Total Replacement of Fishmeal by Spirulina (Arthrospira platensis) and Its Effect on Growth Performance and Product Quality of African Catfish (Clarias gariepinus). Sustainability 2021, 13, 8726. [Google Scholar] [CrossRef]
- Ramírez-Rodrigues, M.M.; Estrada-Beristain, C.; Metri-Ojeda, J.; Pérez-Alva, A.; Baigts-Allende, D.K. Spirulina platensis protein as sustainable ingredient for nutritional food products development. Sustainability 2021, 13, 6849. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravi Kumar, R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Ramírez-Carmona, M.; Casas, E.; Orrego, E.; Acevedo, A.; Giraldo, S. Biomasa de Residuos Agrícolas en el Departamento de Antioquia; Universidad Pontificia Bolivariana: Medellin, Colombia, 2010. [Google Scholar]
- Burns, A.; Gleadow, R.; Cliff, J.; Zacarias, A.; Cavagnaro, T. Cassava: The Drought, War and Famine Crop in a Changing World. Sustainability 2010, 2, 3572–3607. [Google Scholar] [CrossRef] [Green Version]
- FAO. Norme Générale Codex Pour les Additifs Alimentaires; FAO: Rome, Italy, 2015.
- Romero, N.; Areche, C.; Cubides-Cárdenas, J.; Escobar, N.; García-Beltrán, O.; Simirgiotis, M.J.; Céspedes, Á. In Vitro Anthelmintic Evaluation of Gliricidia sepium, Leucaena leucocephala, and Pithecellobium dulce: Fingerprint Analysis of Extracts by UHPLC-Orbitrap Mass Spectrometry. Molecules 2020, 25, 3002. [Google Scholar] [CrossRef]
- Compean-Martínez, J.; Salazar-Ulloa, M.; Chávez-Soriano, L.; Muñoz-Córdoba, G.; Son-de Fernex, E. Anthelmintic-like Activity of Leucaena leucocephala Aqueous Extract Against Gyrodactylus spp. in Naturally Infected Tilapia Fingerlings. N. Am. J. Aquac. 2021, 83, 354–362. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.; Juliano, P. Encapsulated and Powdered Foods; CRC Press: Boca Raton, FL, USA, 2005; pp. 41–70. [Google Scholar]
- Dhanalakshmi, K.; Ghosal, S.; Bhattacharya, S. Agglomeration of Food Powder and Applications. Crit. Rev. Food Sci. Nutr. 2011, 51, 432–441. [Google Scholar] [CrossRef]
- Feng, J.Q.; Hays, D.A. Relative importance of electrostatic forces on powder particles. Powder Technol. 2003, 135–136, 65–75. [Google Scholar] [CrossRef]
- Bhandari, B.; Ho, T. Handbook of Food Preservation; CRC Press: New York, NY, USA, 2020; pp. 387–402. [Google Scholar]
- Bika, D.; Tardos, G.I.; Panmai, S.; Farber, L.; Michaels, J. Strength and morphology of solid bridges in dry granules of pharmaceutical powders. Powder Technol. 2005, 150, 104–116. [Google Scholar] [CrossRef]
- Martin, A.; Osen, R.; Greiling, A.; Karbstein, H.P.; Emin, A. Effect of rapeseed press cake and peel on the extruder response and physical pellet quality in extruded fish feed. Aquaculture 2019, 512, 734316. [Google Scholar] [CrossRef]
- Berman, A. Optimización Del Proceso de Extrusión Para La Elaboración de Pelets Para Alimentación de Tilapia (Oreochromis Niloticus) En Zamorano; Escuela Agrícola Panamericana: Tegucigalpa, Honduras, 2007. [Google Scholar]
- Hoyos-Concha, J.L.; Villada-Castillo, H.S.; Fernández-Quintero, A.; Ortega-Toro, R. Use of trout by-products (Oncorhynchus mykiss) for the production of an extruded fish feed. Rev. Mex. Ing. Química 2022, 21, 1–13. [Google Scholar] [CrossRef]
- Kabir, K.A.; Verdegem, M.C.J.; Verreth, J.A.J.; Phillips, M.J.; Schrama, J.W. Dietary non-starch polysaccharides influenced natural food web and fish production in semi-intensive pond culture of Nile tilapia. Aquaculture 2020, 528, 735506. [Google Scholar] [CrossRef]
- Fagbenro, O.A.; Jauncey, K. Physical and nutritional properties of moist fermented fish silage pellets as a protein supplement for tilapia (Oreochromis niloticus). Anim. Feed Sci. Technol. 1998, 71, 11–18. [Google Scholar] [CrossRef]
- Mo, W.Y.; Lun, C.H.I.; Choi, W.M.; Man, Y.B.; Wong, M.H. Enhancing growth and non-specific immunity of grass carp and Nile tilapia by incorporating Chinese herbs (Astragalus membranaceus and Lycium barbarum) into food waste based pellets. Environ. Pollut. 2016, 219, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; El-Sayed, G.O.; Shady, S.H. Growth, biochemical variables, and zinc bioaccumulation in Nile tilapia, Oreochromis niloticus (L.) as affected by water-born zinc toxicity and exposure period. Int. Aquat. Res. 2016, 8, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Fagbenro, O.; Jauncey, K. Chemical and nutritional quality of stored fermented fish (tilapia) silage. Bioresour. Technol. 1993, 46, 207–211. [Google Scholar] [CrossRef]
- Kanmani, N.; Romano, N.; Ebrahimi, M.; Nurul Amin, S.M.; Kamarudin, M.S.; Karami, A.; Kumar, V. Improvement of feed pellet characteristics by dietary pre-gelatinized starch and their subsequent effects on growth and physiology in tilapia. Food Chem. 2018, 239, 1037–1046. [Google Scholar] [CrossRef]
- Makwinja, R.; Geremew, A. Roles and requirements of trace elements in tilapia nutrition: Review. Egypt. J. Aquat. Res. 2020, 46, 281–287. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Thoresen, L.; Albrektsen, S.; Grimaldo, E.; Grimsmo, L.; Whitaker, R.D.; Sele, V.; Wiech, M. Processing Mixed Mesopelagic Biomass from the North-East Atlantic into Aquafeed Resources; Implication for Food Safety. Foods 2021, 10, 1265. [Google Scholar] [CrossRef]
- Manomaitis, L.; Cremer, M. Growth Performance of Tilapia Fed Soy-Based Feed in Low Volume, High Density Cages on Phu Long Reservoir, Dalai, Ninh Binh, Yen Khanh District, Vietnam; Results of ASA-IM/Soy-in-Aquaculture; American Soybean Association International Marketing (ASA-IM): St. Louis, MO, USA, 2006. [Google Scholar]
- Jagtap, S.; Garcia-Garcia, G.; Duong, L.; Swainson, M.; Martindale, W. Codesign of Food System and Circular Economy Approaches for the Development of Livestock Feeds from Insect Larvae. Foods 2021, 10, 1701. [Google Scholar] [CrossRef]




| Compound | Concentration (mol∙m−3) |
|---|---|
| NaHCO3 | 200 |
| K2HPO4 | 3.67 |
| NaNO3 | 29.41 |
| K2SO4 | 5.73 |
| NaCl | 17.11 |
| MgSO4 7H2O | 2.62 |
| CaCl2 2H2O | 0.27 |
| FeSO4 7H2O | 0.04 |
| EDTA | 200 |
| Flours (%) | ||||
|---|---|---|---|---|
| Formulation | Spirulina sp. | Cassava Leaves | Gliricidia Leaves | Rice Husk |
| 1 | 20 | 40 | 20 | 20 |
| 2 | 35 | 35 | 20 | 10 |
| 3 | 20 | 50 | 20 | 10 |
| Raw Materials | % Moisture | % Ashes | % Protein | % Fat |
|---|---|---|---|---|
| Cassava leaf flour | 10.23 ± 0.15 | 8.91 ± 0.0 | 28.33 ± 0.15 | 4.42 ± 0.19 |
| Gliricidia leaf flour | 9.15 ± 0.14 | 5.94 ± 0.0 | 25.63 ± 0.09 | 3.43 ± 0.52 |
| Rice husk flour | 7.32 ± 0.18 | 16.50 ± 0.01 | 7.17 ± 0.02 | 0.61 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C.; Sánchez-Osorno, D. Fish Food Production Using Agro-Industrial Waste Enhanced with Spirulina sp. Sustainability 2022, 14, 6059. https://doi.org/10.3390/su14106059
Ramírez-Carmona M, Rendón-Castrillón L, Ocampo-López C, Sánchez-Osorno D. Fish Food Production Using Agro-Industrial Waste Enhanced with Spirulina sp. Sustainability. 2022; 14(10):6059. https://doi.org/10.3390/su14106059
Chicago/Turabian StyleRamírez-Carmona, Margarita, Leidy Rendón-Castrillón, Carlos Ocampo-López, and Diego Sánchez-Osorno. 2022. "Fish Food Production Using Agro-Industrial Waste Enhanced with Spirulina sp." Sustainability 14, no. 10: 6059. https://doi.org/10.3390/su14106059
APA StyleRamírez-Carmona, M., Rendón-Castrillón, L., Ocampo-López, C., & Sánchez-Osorno, D. (2022). Fish Food Production Using Agro-Industrial Waste Enhanced with Spirulina sp. Sustainability, 14(10), 6059. https://doi.org/10.3390/su14106059









