Applications of Starch Biopolymers for a Sustainable Modern Agriculture
Abstract
:1. Introduction
2. The State of Plastics Uses in Agriculture
2.1. Plastic Films in Agricultural Applications
2.1.1. Greenhouse Cover
2.1.2. Mulch
2.1.3. Low Tunnels
2.1.4. Silage
2.1.5. Nets
2.2. Piping, Irrigation and Drainage, and Packaging
3. Biodegradable Polymers and Research Gaps
3.1. Classification of Biodegradable Polymers, Polysaccharides, and Starches
3.2. Limitations, Research Gaps in Starch, and Surface Modifications
3.3. Starch Blending
3.3.1. Starch/PVA
3.3.2. Starch/PLA
3.3.3. Starch/PCL
3.3.4. Starch/PHB-HV
3.3.5. Starch/PBS and Starch/PBSA
3.3.6. Ternary Blends
3.3.7. Nanocomposites: Fillers in the Starch Matrix
3.3.8. Starch-Based Nanocrystals
3.3.9. Essential Oils Impregnated Starch Blends
3.4. Biodegradable Starch Polymers for Agriculture
3.4.1. Mulching
3.4.2. Silage
3.4.3. Packaging and Containers
4. Biodegradability of Starch and Starch Blends
4.1. Biodegradation of Starch/PVA
4.2. Biodegradation of Starch/PLA
4.3. Biodegradation of Starch/PCL Blends
4.4. Biodegradation of Starch/PHB-V
4.5. Biodegradation of Starch/PBS and Starch/PBSA
4.6. Biodegradation of Ternary Blends
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, E.S. Green Plastics: An Introduction to the New Science of Biodegradable Plastics; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- FAO. Assessment of Agricultural Plastics and Their Sustainability—A Call for Action; FAO: Rome, Italy, 2021. [Google Scholar]
- Ray, S.S.; Bousmina, M. Biodegradable Polymers and Their Layered Silicate Nanocomposites: In Greening the 21st Century Materials World. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Jiang, W.; Qu, D.; Mu, D.; Wang, L.R. China’s energy-saving greenhouses. Chron. Hort. 2004, 44, 15–17. [Google Scholar]
- Jouët, J.P. Plastics in the world. Plasticulture 2001, 2, 106–127. [Google Scholar]
- Jouet, J.P. The situation of plasticulture in the world. Plasticulture 2004, 761, 46–57. [Google Scholar]
- Reynolds, A. Market Overview. Agricultural film markets, trends, and business development. Proc. Agric. Film. 2009, 2009, 24–26. [Google Scholar]
- Reynolds, A. Updated views on the development opportunities in agricultural film markets. In Proceedings of the Agricultural Film 2010—International Conference on Greenhouse, Tunnel, Mulch and Agricultural Films and Covers, Barcelona, Spain, 22–24 November 2010; Applied Market Information Ltd.: Bristol, UK, 2010; pp. 22–24. [Google Scholar]
- Garnaud, J.C. Agricultural and Horticultural Applications of Polymers; Rapra Technology Ltd.: Oxford, UK; Pergamon Press: Shawbury, UK, 1988. [Google Scholar]
- Brown, R.P. Polymers in Agriculture and Horticulture; Rapra Technology Ltd.: Shawbury, UK, 2004. [Google Scholar]
- Dwivedi, P.; Mishra, P.K.; Mondal, M.K.; Srivastava, N. Non-biodegradable polymeric waste pyrolysis for energy recovery. Heliyon 2019, 5, e02198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobling, S. Improving starch for food and industrial applications. Curr. Opin. Plant Biol. 2004, 7, 210–218. [Google Scholar] [CrossRef]
- Eliasson, A.-C. Starch in Food: Structure, Function and Applications; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Elvira, C.; Mano, J.; San Roman, J.; Reis, R. Starch-based biodegradable hydrogels with potential biomedical applications as drug delivery systems. Biomaterials 2002, 23, 1955–1966. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, J.; Liu, Q. Starch—A potential biomaterial for biomedical applications. In Nanomaterials and Nanosystems for Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2007; pp. 83–98. [Google Scholar]
- USDA. Agricultural yearbook. In United States: Department of Agriculture, Economic Research Service; USDA: Washington, DC, USA, 2010. [Google Scholar]
- Alobi, N.O.; Sunday, E.A.; Magu, T.O.; Oloko, G.O.; Nyong, B.E. Analysis of Starch from Non- Edible Root and Tubers as Sources of Raw Materials for the Synthesis of Biodegradable Starch Plastics. J. Basic Appl. Res. 2017, 3, 27–32. [Google Scholar]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Vliegenthart, J.F.G. Crystallinity in starch plastics: Consequences for material properties. Trends Biotechnol. 1997, 15, 208–213. [Google Scholar] [CrossRef]
- Rindlav-Westling, Å.; Stading, M.; Gatenholm, P. Crystallinity and morphology in films of starch, amylose and amylopectin blends. Biomacromolecules 2002, 3, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics—A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Crystallinity in starch bioplastics. Ind. Crops Prod. 1996, 5, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Hulleman, S.; Kalisvaart, M.; Janssen, F.; Feil, H.; Vliegenthart, J. Origins of B-type crystallinity in glycerol-plasticised, compression-moulded potato starches. Carbohydr. Polym. 1999, 39, 351–360. [Google Scholar] [CrossRef]
- Müller, C.M.; Laurindo, J.B.; Yamashita, F. Effect of nanoclay incorporation method on mechanical and water vapor barrier properties of starch-based films. Ind. Crops Prod. 2011, 33, 605–610. [Google Scholar] [CrossRef]
- Castano, J.; Rodríguez-Llamazares, S.; Sepúlveda, E.; Giraldo, D.; Bouza, R.; Pozo, C. Morphological and structural changes of starch during processing by melt blending. Starch Stärke 2017, 69, 1600247. [Google Scholar] [CrossRef]
- Atwell, W.A.; Hood, L.F.; Lineback, D.R.; Varriano-Marston, E.; Zohel, H.F. The terminology and methodology associated with basic starch phenomena. Cereal Foods World 1998, 33, 306–311. [Google Scholar]
- Chen, P.; Yu, L.; Chen, L.; Li, X. Morphology and microstructure of maize starches with different amylose/amylopectin content. Starch Stärke 2006, 58, 611–615. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Chen, P.; Yu, L.; Chen, L.; Tong, Z. Morphologies and thermal properties of hydroxypropylated high-amylose corn starch. Cereal Chem. 2010, 87, 144. [Google Scholar] [CrossRef]
- Qiao, D.; Yu, L.; Liu, H.; Zou, W.; Xie, F.; Simon, G.; Petinakis, E.; Shen, Z.; Chen, L. Insights into the hierarchical structure and digestion rate of alkali-modulated starches with different amylose contents. Carbohydr. Polym. 2016, 144, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Moret-Ferguson, S.; Maximenko, N.A.; Proskurowski, G.; Peacock, E.E.; Hafner, J.; Reddy, C.M. Plastic accumulation in the North Atlantic subtropical gyre. Science 2010, 329, 1185–1188. [Google Scholar] [CrossRef] [Green Version]
- Young, A.H. Fractionation of starch. In Starch: Chemistry and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 249–283. [Google Scholar]
- Singh, N.; Singh, J.; Kaur, L.; Singh Sodhi, N.; Singh Gill, B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Nawaz, H.; Arêas, E.P.G. Chemistry and Applications of Polysaccharide Solutions in Strong Electrolytes/Dipolar Aprotic Solvents: An Overview. Molecules 2013, 18, 1270–1313. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Biodegradable Polymers. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Brunswick, NJ, USA, 2014; pp. 303–335. [Google Scholar]
- Garc, N.L.; Fam, L.; Accorso, N.B.D.; Goyanes, S. Biodegradable Starch Nanocomposites. In Eco-Friendly Polymer Nanocomposites; Thakur, V.K., Thakur, M.K., Eds.; Springer: New Delhi, India, 2015; pp. 17–77. [Google Scholar]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, C.; Rost, F.; Onyango, C.; Jaros, D.; Rohm, H. Crystallinity, thermal and morphological characteristics of resistant starch type III produced by hydrothermal treatment of debranched cassava starch. Starch Stärke 2009, 61, 634–645. [Google Scholar] [CrossRef]
- Rolland-Sabaté, A.; Sánchez, T.; Buléon, A.; Colonna, P.; Jaillais, B.; Ceballos, H.; Dufour, D. Structural characterization of novel cassava starches with low and highamylose contents in comparison with other commercial sources. Food Hydrocoll. 2012, 27, 161–174. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of cellulose fibers addition on the mechanical properties and water vapor barrier of starch-based films. Food Hydrocoll. 2009, 23, 1328–1333. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Gandini, A.A. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Oxford, UK, 2008. [Google Scholar]
- Zhoua, Y.; Hoovera, R.; Liub, Q. Relationship between a-amylase degradation and the structure and physicochemical properties of legume starches. Carbohydr. Polym. 2004, 57, 299–317. [Google Scholar] [CrossRef]
- Waduge, R.N.; Hoover, R.; Vasanthan, T.; Gao, J.; Li, J. Effect of annealing on the structure and physicochemical properties of barley starches of varying amylose content. Food Res. Int. 2006, 39, 59–77. [Google Scholar] [CrossRef]
- Boudries, N.; Belhaneche, N.; Nadjemi, B.; Deroanne, C.; Mathlouthi, M.; Roger, B.; Sindic, M. Physicochemical and functional properties of starches from sorghum cultivated in the Sahara of Algeria. Carbohydr. Polym. 2009, 78, 475–480. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing, and Applications, 1st ed.; William Andrew: Norwich, NY, USA, 2012; ISBN 9781455730032. [Google Scholar]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of poly (lactic) acid and starch for biodegradable food packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef]
- Silva, G.C.; Galleguillos Madrid, F.M.; Hernández, D.; Pincheira, G.; Peralta, A.K.; Urrestarazu Gavilán, M.; Vergara Carmona, V.; Fuentes-Peñailillo, F. Microplastics and Their Effect in Horticultural Crops: Food Safety and Plant Stress. Agronomy 2021, 11, 1528. [Google Scholar] [CrossRef]
- Castilla, N. lnvernaderos de Pla´stico. Tecnologı´a y Manejo; Mundi-Prensa: Madrid, Spain, 2004. [Google Scholar]
- PlasticsEurope. Plastics—The Facts. PlasticsEurope: Brussels, Belgium. 2018. Available online: https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf (accessed on 20 February 2022).
- Scarascia-Mugnozza, G.; Sica, C.; Russo, G. Plastic materials in European agriculture: Actual use and perspectives. J. Agric. Eng. 2011, 42, 15–28. [Google Scholar] [CrossRef]
- Espí, E.; Salmerón, A.; Fontecha, A.; García, Y.; Real, A.I. PLastic Films for Agricultural Applications. J. Plast. Film Sheeting 2006, 22, 85. [Google Scholar] [CrossRef]
- Papadakis, G.; Briassoulis, D.; Scarascia Mugnozza, G.; Vox, G.; Feuilloley, P.; Stoffers, J.A. Review Paper (SE-Structures and Environmental): Radiometric and thermal properties of, and testing methods for, greenhouse covering materials. J. Agric. Eng. Res. 2000, 77, 7–38. [Google Scholar] [CrossRef]
- Adam, A.; Kouider, S.A.; Hamou, A.; Saiter, J.A. Studies of polyethylene multi-layer films used as greenhouse covers under Saharan climatic conditions. Polym. Test. 2005, 24, 834–838. [Google Scholar] [CrossRef]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; Krumbein, A. UV-B induced secondary plant metabolites—potential benefits for plant and human health. Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crops Res. 2006, 95, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Bisaglia, C.; Tabacco, E.; Borreani, G. The use of plastic film instead of netting when tying round bales for wrapped baled silage. Biosyst. Eng. 2011, 108, 1–8. [Google Scholar] [CrossRef]
- Castellano, S.; Mugnozza, G.S.; Russo, G.; Briassoulis, D.; Mistriotis, A.; Hemming, S.; Waaijenberg, D. Plastic nets in agriculture: A general review of types and applications. Appl. Eng. Agric. 2008, 24, 799–808. [Google Scholar] [CrossRef]
- Beckman, E. The World of Plastics, in Numbers. 2018. Available online: http://theconversation.com/the-world-of-plastics-in-numbers-100291 (accessed on 20 February 2022).
- De Van, V.K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Schwach, E.; Avérous, L. Starch-based biodegradable blends: Morphology and interface properties. Polym. Int. Soc. Chem. Ind. 2004, 53, 2115–2124. [Google Scholar] [CrossRef]
- Moad, G. Chemical modification of starch by reactive extrusion. Prog. Polym. Sci. 2011, 36, 218–237. [Google Scholar] [CrossRef]
- Apopei, D.F.; Dinu, M.V.; Trochimczuk, A.W.; Dragan, E.S. Sorption isotherms of heavy metal ions onto semi-interpenetrating polymer network cryogels based on polyacrylamide and anionically modified potato starch. Ind. Eng. Chem. Res. 2012, 51, 10462–10471. [Google Scholar] [CrossRef]
- Abdul-Raheim, A.R.M.; El-Saeed Shimaa, M.; Farag Reem, K.; Abdel-Raouf Manar, E. Low cost biosorbents based on modified starch iron oxide nanocomposites for selective removal of some heavy metals from aqueous solutions. Adv. Mater. Lett. 2016, 7, 402–409. [Google Scholar] [CrossRef]
- Liu, T.; Han, X.; Wang, Y.; Yan, L.; Du, B.; Wei, Q.; Wei, D. Magnetic chitosan/anaerobic granular sludge composite: Synthesis, characterization, and application in heavy metal ions removal. J. Colloid Interface Sci. 2017, 508, 405–414. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ko, Y.G.; Deri, F. Biodegradable polymer blends and composites: An overview. Polym. Sci. Ser. A 2014, 56, 812–829. [Google Scholar] [CrossRef]
- Šárka, E.; Dvořáček, V. Waxy starch as a perspective raw material (a review). Food Hydrocoll. 2017, 69, 402–409. [Google Scholar] [CrossRef]
- Kong, X.; Kasapis, S.; Bao, J. Viscoelastic properties of starches and flours from two novel rice mutants induced by gamma irradiation. LWT-Food Sci. Technol. 2015, 60, 578–582. [Google Scholar] [CrossRef]
- Chakraborty, M.; Matkovic, K.; Grier, D.G.; Jarabek, E.L.; Berzonsky, W.A.; McMullen, M.S.; Doehlert, D.C. Physicochemical and functional properties of tetraploid and hexaploid waxy wheat starch. Starch Stärke 2004, 56, 339–347. [Google Scholar] [CrossRef]
- Haaj, S.B.; Thielemans, W.; Magnin, A.; Boufi, S. Starch nanocrystals and starch nanoparticles from waxy maize as nanoreinforcement: A comparative study. Carbohydr. Polym. 2016, 143, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-M.; Gao, Q.-Y. Physicochemical properties, structure and in vitro digestion of resistant starch from waxy rice starch. Carbohydr. Polym. 2011, 84, 1151–1157. [Google Scholar] [CrossRef]
- Sun, Q.; Li, G.; Dai, L.; Ji, N.; Xiong, L. Green preparation and characterisation of waxy maize starch nanoparticles through enzymolysis and recrystallisation. Food Chem. 2014, 162, 223–228. [Google Scholar] [CrossRef]
- Hoover, R. Starch retrogradation. Food Rev. Int. 1995, 11, 331–346. [Google Scholar] [CrossRef]
- Jane, J.-L.; Ao, Z.; Duvick, S.A.; Wiklund, M.; Yoo, S.-H.; Wong, K.-S.; Gardner, C. Structures of amylopectin and starch granules: How are they synthesized? J. Appl. Glycosci. 2003, 50, 167–172. [Google Scholar] [CrossRef]
- Hermansson, A.-M.; Svegmark, K. Developments in the understanding of starch functionality. Trends Food Sci. Technol. 1996, 7, 345–353. [Google Scholar] [CrossRef]
- Svegmark, K.; Helmersson, K.; Nilsson, G.; Nilsson, P.-O.; Andersson, R.; Svensson, E. Comparison of potato amylopectin starches and potato starches—Influence of year and variety. Carbohydr. Polym. 2002, 47, 331–340. [Google Scholar] [CrossRef]
- Suzuki, A.; Hizukuri, S.; Takeda, Y. Physicochemical studies of kuzu starch. Cereal Chem. 1981, 58, 286–290. [Google Scholar]
- Richardson, S.; Nilsson, G.S.; Bergquist, K.-E.; Gorton, L.; Mischnick, P. Characterisation of the substituent distribution in hydroxypropylated potato amylopectin starch. Carbohydr. Res. 2000, 328, 365–373. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Nony, F.; Khelladi, S.; Fitoussi, J.; Farzaneh, S. 13-Epoxy/amine reactive systems for composites materials and their thermomechanical properties. In Advances in Composites Manufacturing and Process Design; Elsevier: Amsterdam, The Netherlands, 2015; pp. 269–296. [Google Scholar]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7–8, 100028. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; El Halal, S.L.D.M.; Lim, L.-T.; Dias, Á.R.G.; da Rosa Zavareze, E. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, I.; Hoover, R. Effect of annealing on themolecular structure and physicochemical properties ofstarches from different botanical sources: A review. Carbohydr. Polym. 2008, 74, 691–703. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch Stärke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Prompiputtanapon, K.; Sorndech, W.; Tongta, S. Surface modification of tapioca starch by using the chemical and enzymatic method. Starch Stärke 2020, 72, 1900133. [Google Scholar] [CrossRef]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-Based Hydrogels: Present Status and Applications. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.; Ibrahim, H. An overview on starch-based sustainable hydrogels: Potential applications and aspects. J. Polym. Environ. 2021, 30, 19–52. [Google Scholar] [CrossRef]
- Xiao, C. Current advances of chemical and physical starch-based hydrogels. Starch Stärke 2013, 65, 82–88. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.; Majumdar, D. Effect of heat treatment of starch on the properties of the starch hydrogels. Mater. Lett. 2008, 62, 215–218. [Google Scholar] [CrossRef]
- Zia-ud, D.; Xiong, H.; Fei, P. Physical and chemical modification of starches: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Chapter 5—Physical Modification of Starch. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 223–253. [Google Scholar]
- Pashkuleva, I.; Marques, A.P.; Vaz, F.; Reis, R.L. Surface modification of starch based biomaterials by oxygen plasma or UV-irradiation. J. Mater. Sci. Mater. Med. 2010, 21, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, L.; McCarthy, O. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Jyothi, A.N. Starch Graft Copolymers: Novel Applications in Industry. Compos. Interfaces 2010, 17, 165–174. [Google Scholar] [CrossRef]
- Cazotti, J.C.; Fritz, A.T.; Garcia-Valdez, O.; Smeets, N.M.; Dubé, M.A.; Cunningham, M.F. Graft Modification of Starch Nanoparticles Using Nitroxide-Mediated Polymerization and the “Grafting to” Approach. Biomacromolecules 2020, 21, 4492–4501. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W.; Dufresne, A. Polymer grafting onto starch nanocrystals. Biomacromolecules 2007, 8, 2916–2927. [Google Scholar] [CrossRef]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch Stärke 2018, 70, 1600351. [Google Scholar] [CrossRef]
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Zain-ul-Abdin; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical modification of starch and its application as an adsorbent material. RSC Adv. 2016, 6, 78264–78285. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Production, structure, physicochemical and functional properties of maize, cassava, wheat, potato and rice starches. Starch Stärke 2015, 67, 14–29. [Google Scholar] [CrossRef]
- BeMiller, J.; Whistler, R. Starch: Chemistry and Technology, 3rd ed.; Food Science and Technology International Series; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Kaseem, M.; Hamad, K.; Deri, F. Thermoplastic starch blends: A review of recent works. Polym. Sci. Ser. A 2012, 54, 165–176. [Google Scholar] [CrossRef]
- Mani, R.; Bhattacharya, M. Properties of injection moulded blends of starch and modified biodegradable polyesters. Eur. Polym. J. 2001, 37, 515–526. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of citric acid/glycerol co-plasticized thermoplastic starch prepared by melt blending. Carbohydr. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Fitzsimons, S.M.; Mulvihill, D.M.; Morris, E.R. Co-gels of whey protein isolate with crosslinked waxy maize starch: Analysis of solvent partition and phase structure by polymer blending laws. Food Hydrocoll. 2008, 22, 468–484. [Google Scholar] [CrossRef]
- Rahmat, A.R.; Rahman, W.A.W.A.; Sin, L.T.; Yussuf, A. Approaches to improve compatibility of starch filled polymer system: A review. Mater. Sci. Eng. C 2009, 29, 2370–2377. [Google Scholar] [CrossRef]
- Abbott, A.P.; Abolibda, T.Z.; Qu, W.; Wise, W.R.; Wright, L.A. Thermoplastic starch–polyethylene blends homogenised using deep eutectic solvents. RSC Adv. 2017, 7, 7268–7273. [Google Scholar] [CrossRef] [Green Version]
- Nazarzadeh, Z.E.; Najafi, M.P.; Azariyan, E.; Sharifian, I. Conductive and biodegradable polyaniline/starch blends and their composites with polystyrene. Iran. Polym. J. 2011, 20, 319–328. [Google Scholar]
- Chen, L.; Qiu, X.; Deng, M.; Hong, Z.; Luo, R.; Chen, X.; Jing, X. The starch grafted poly (l-lactide) and the physical properties of its blending composites. Polymer 2005, 46, 5723–5729. [Google Scholar] [CrossRef]
- Raquez, J.M.; Nabar, Y.; Narayan, R.; Dubois, P. In situ compatibilization of maleated thermoplastic starch/polyester melt-blends by reactive extrusion. Polym. Eng. Sci. 2008, 48, 1747–1754. [Google Scholar] [CrossRef]
- Kalambur, S.; Rizvi, S.S. An overview of starch-based plastic blends from reactive extrusion. J. Plast. Film Sheeting 2006, 22, 39–58. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Dean, K.; Yuan, Q.; Chen, L.; Zhang, X. Effect of compatibilizer distribution on the blends of starch/biodegradable polyesters. J. Appl. Polym. Sci. 2007, 103, 812–818. [Google Scholar] [CrossRef]
- Xie, F.; Halley, P.J.; Avérous, L. Rheology to understand and optimize processibility, structures and properties of starch polymeric materials. Prog. Polym. Sci. 2012, 37, 595–623. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Teixeira, E.; Curvelo, A.; Corrêa, A.; Marconcini, J.; Glenn, G.; Mattoso, L. Properties of thermoplastic starch from cassava bagasse and cassava starch and their blends with poly (lactic acid). Ind. Crops Prod. 2012, 37, 61–68. [Google Scholar] [CrossRef]
- Priya, B.; Gupta, V.; Pathania, D.; Singha, A. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.; Yamashita, F.; Müller, C.; Pires, A. Effect of cooling and coating on thermoplastic starch/poly(lactic acid) blend sheets. Polym. Test. 2014, 33, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Fabunmi, O.; Tabil, L., Jr.; Panigrahi, S.; Chang, P. Developing Biodegradable Plastics from starch. In Proceedings of the ASABE/CSBE North Central Intersectional Conference, Saskatoon, SK, Canada, 5–7 October 2006. [Google Scholar]
- Bendaoud, A.; Yvan, C. Effects of relative humidity and ionic liquids on the water content and glass transition of plasticized starch. Carbohydr. Polym. 2013, 97, 665–675. [Google Scholar] [CrossRef]
- Lu, D.; Xiao, C.; Xu, S. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Encalada, K.; Aldás, M.B.; Proaño, E.; Valle, V. An overview of starch-based biopolymers and their biodegradability. Cienc. Ing. 2018, 39, 245–258. [Google Scholar]
- Wang, J.; Cheng, F.; Zhu, P. Structure and properties of urea-plasticized starch films with different urea contents. Carbohydr. Polym. 2014, 101, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Yao, F.C.; De Min, J.; Dong, X. Effect of a Complex Plasticizer on the Structure and Properties of the Thermoplastic PVA/Starch Blends. Polym. Plast. Technol. Eng. 2009, 48, 489–495. [Google Scholar] [CrossRef]
- Azahari, N.; Othman, N.; Ismail, H. Biodegradation Studies of Polyvinyl Alcohol/Corn Starch Blend Films in Solid and Solution Media. J. Phys. Sci. 2011, 22, 15–31. [Google Scholar]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Brito, L.; Vaca, F.; Bruno, M.I.; Sebastião, P. Molecular Dynamic Evaluation of starch-PLA blends nanocomposite with organoclay by proton NMR relaxometry. Polym. Test. 2013, 32, 1181–1185. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.; Ma, X. Preparation and characterization of thermoplastic starch/PLA blends by onestep reactive extrusion. Polym. Int. 2007, 56, 1440–1447. [Google Scholar] [CrossRef]
- Singh, R.; Pandey, J.; Rutot, D.; Degée, P.; Dubois, P. Biodegradation of poly(ε-caprolactone)/starch blends and composites in composting and culture environments: The effect of compatibilization on the inherent biodegradability of the host polymer. Carbohydr. Res. 2003, 33, 1759–1769. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.; Ugwu, C.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Muñoz, A.; Talens, P.; Chiralt, A. Improvement of properties of glycerol plasticized starch films by blending with a low ratio of polycaprolactone and/or polyethylene glycol. Food Hydrocoll. 2016, 56, 9–19. [Google Scholar] [CrossRef]
- Vikman, M.; Hulleman, S.H.D.; Van, D.Z.M.; Myllarinen, P.; Feil, H. Morphology and Enzymatic Degradation of Thermoplastic Starch–Polycaprolactone Blends. J. Appl. Polym. Sci. 1999, 74, 2594–2604. [Google Scholar] [CrossRef]
- Sugih, A.K.; Drijfhout, J.P.; Picchioni, F.; Janssen, L.P.B.M.; Heeres, H.J. Synthesis and Properties of Reactive Interfacial Agents for Polycaprolactone-Starch Blends. J. Appl. Polym. Sci. 2009, 114, 2315–2326. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards Controlled Degradation of Poly(lactic) Acid in Technical Applications. J. Carbon Res. 2021, 7, 42. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, K.; Pereira, J.; Smith, A.; Carvalho, C.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Wang, X.-L.; Yang, K.-K.; Wang, Y.-Z. Properties of Starch Blends with Biodegradable Polymers. J. Macromol. Sci. 2003, 43, 385–409. [Google Scholar] [CrossRef]
- Kanitporn, S.; Koombhongse, P.; Chirachanchai, S. Starch grafted poly(butylene succinate) via conjugating reaction and its role on enhancing the compatibility. Carbohydr. Polym. 2013, 102, 95–102. [Google Scholar]
- Babu, R.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Jbilou, F.; Joly, C.; Galland, S.; Belard, L.; Desjardin, V.; Bayard, R.; Dole, P.; Degraeve, P. Biodegradation study of plasticised corn flour/poly(butylene succinate-co-butylene adipate) blends. Polym. Test. 2013, 32, 1565–1575. [Google Scholar] [CrossRef]
- Maubane, L.; Suprakas, S.; Kalala, J. The effect of starch amylose content on the morphology and properties of melt-processed butyl-etherified starch/poly[(butylene succinate)-co-adipate] blends. Carbohydr. Polym. 2017, 155, 89–100. [Google Scholar] [CrossRef]
- Liao, H.-T.; Wu, C.-S. Preparation and characterization of ternary blends composed of polylactide, poly(E-caprolactone) and starch. Mater. Sci. Eng. 2009, 515, 207–214. [Google Scholar] [CrossRef]
- Sarazin, P.; Li, G.; Orts, W.; Favis, B. Binary and ternary blends of polylactide, polycaprolactone and thermoplastic starch. Polymer 2008, 49, 599–609. [Google Scholar] [CrossRef]
- Carmona, V.; Corrêa, A.; Marconcini, J.; Mattoso, L. Properties of a Biodegradable Ternary Blend of Thermoplastic Starch (TPS), Poly(ε-Caprolactone) (PCL) and Poly(Lactic Acid) (PLA). J. Polym. Environ. 2014, 23, 83–89. [Google Scholar] [CrossRef]
- Ren, J.; Hongye, F.; Tianbin, R.; Weizhong, Y. Preparation, characterization and properties of binary and ternary blends with thermoplastic starch, poly(lactic acid) and poly(butylene adipate-co-terephthalate). Carbohydr. Polym. 2009, 77, 576–582. [Google Scholar] [CrossRef]
- Tachaphiboonsap, S.; Jarukumjorn, K. Toughness and Compatibility Improvement of Thermoplastic Starch/Poly(lactic Acid) Blends. Adv. Mater. Res. 2013, 747, 67–71. [Google Scholar] [CrossRef]
- Shirai, M.; Olivato, J.; Garcia, P.; Müller, C.; Grossmann, M.; Yamashita, F. Thermoplastic starch/polyester films: Effects of extrusion process and poly (lactic acid) addition. Mater. Sci. Eng. C 2013, 33, 4112–4117. [Google Scholar] [CrossRef]
- Ma, P.; Xu, P.; Chen, M.; Dong, W.; Cai, X.; Schmit, P.; Spoelstra, A.; Lemstra, P. Structure–property relationships of reactively compatibilized PHB/EVA/starch blends. Carbohydr. Polym. 2014, 108, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Syafri, E.; Kasim, A.; Abral, H.; Asben, A. Effect of Precipitated Calcium Carbonate on Physical, Mechanical and Thermal Properties of Cassava Starch Bioplastic Composites. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 1950. [Google Scholar] [CrossRef] [Green Version]
- Belibi, P.; Daou, J.; Ndjaka, J.-M.; Michelin, L.; Brendle, J.; Nsom, B.; Durand, B. Tensile and water barrier properties of cassava starch composite films reinforced by synthetic zeolite and beidellite. J. Food Eng. 2013, 115, 339–346. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A. Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Ahmed, J.; Brijesh Tiwari, B.K.; Imam, S.H.; Rao, M.A. Starch-Based Polymeric Materials and Nanocomposites: Chemistry, Processing, and Applications; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L. Starch-based nano-biocomposites. Prog. Polym. Sci. 2013, 38, 1590–1628. [Google Scholar] [CrossRef] [Green Version]
- Oleyaei, S.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Salaberria, A.; Labidi, J.; Fernandes, S. Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Orue, A.; Corcuera, M.A.; Pena, C.; Eceiza, A.; Arbelaiz, A. Bionanocomposites based on thermoplastic starch and cellulose nanofibers. J. Thermoplast. Compos. Mater. 2014, 29, 817–832. [Google Scholar] [CrossRef]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Preparation and characterization of thermoplastic starch and cellulose nanofibers as green nanocomposites: Extrusion processing. Int. J. Biol. Macromol. 2018, 112, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Harunsyah; Sariadi; Raudah. The effect of clay nanoparticles as reinforcement on mechanical properties of bioplastic base on cassava starch. J. Phys. Conf. Ser. 2018, 953, 7. [Google Scholar] [CrossRef]
- Wahyuningtiyas, N.; Suryanto, H. Properties of Cassava Starch based Bioplastic Reinforced by Nanoclay. J. Mech. Eng. Sci. Technol. 2018, 2, 20–26. [Google Scholar] [CrossRef]
- Wittaya, T. Rice Starch-Based Biodegradable Films: Properties Enhancement. In Structure and Function of Food Engineering; Ayman, A.E., Ed.; IntechOpen: London, UK, 2012; Chapter 5. [Google Scholar]
- Dufresne, A.; Sabu, T.; Laly, P. Biopolymer Nanocomposites: Processing, Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Souza, A.; Goto, G.; Mainardi, J.; Coelho, A.; Tadini, C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci. Technol. 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J.K. Biocontrol Potential of Essential Oils in Organic Horticulture Systems: From Farm to Fork. Front. Nutr. 2022, 8, 1275. [Google Scholar] [CrossRef]
- Varona, S.; Kareth, S.; Martín, Á.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluids 2010, 54, 369–377. [Google Scholar] [CrossRef]
- De Barros Fernandes, R.V.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Caetano, K.D.S.; Hessel, C.T.; Tondo, E.C.; Flores, S.H.; Cladera-Olivera, F. Application of active cassava starch films incorporated with oregano essential oil and pumpkin residue extract on ground beef. J. Food Saf. 2017, 37, e12355. [Google Scholar] [CrossRef]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Glenn, G.M.; Klamczynski, A.P.; Woods, D.F.; Chiou, B.; Orts, W.J.; Imam, S.H. Encapsulation of plant oils in porous starch microspheres. J. Agric. Food Chem. 2010, 58, 4180–4184. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The synergistic effects of cinnamon essential oil and nano TiO2 on antimicrobial and functional properties of sago starch films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch–chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Rohani, S.M.R.; Oromiehie, A.R. Antibacterial, antioxidant and optical properties of edible starch-chitosan composite film containing Thymus kotschyanus essential oil. In Veterinary Research Forum; Urmia University: Urmia, Iran, 2012; p. 167. [Google Scholar]
- De Prisco, N.; Immirzi, B.; Malinconico, M.; Mormile, P.; Petti, L.; Gatta, G. Sustainable greenhouse systems. J. Appl Polym. Sci. 2002, 86, 622–632. [Google Scholar] [CrossRef]
- Immirzi, B.; Malinconico, M.; Romano, G.; Russo, R.; Santagata, G. Biodegradable films of natural polysaccharides blends. J. Mater. Sci. Lett. 2003, 22, 1389–1392. [Google Scholar] [CrossRef]
- Briassoulis, D. An overview on the mechanical behaviour of biodegradable agricultural films. J. Polym. Environ. 2004, 12, 65–81. [Google Scholar] [CrossRef]
- Russo, R.; Giuliani, A.; Immirzi, B.; Malinconico, M.; Romano, G. Alginate/Polyvinylalcohol Blends for Agricultural Applications: Structure-Properties Correlation, Mechanical Properties and Greenhouse Effect Evaluation. Macromol. Symp. 2004, 218, 241–250. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Petti, L.; Romano, G. Physical behavior of biodegradable alginate-poly(vinyl alcohol) blend films. J. Polym. Sci. Pol. Phys. 2005, 43, 1205–1213. [Google Scholar] [CrossRef]
- Vox, G.; Schettini, E. Evaluation of the radiometric properties of starch-based biodegradable films for crop protection. Polym. Test. 2007, 26, 639–651. [Google Scholar] [CrossRef]
- Malinconico, M.; Immirzi, B.; Massenti, S.; La Mantia, F.P.; Mormile, P.; Petti, L. Blends of polyvinylalcohol and functionalised polycaprolactone. A study on the melt extrusion and post-cure of films suitable for protected cultivation. J. Mater. Sci. 2002, 37, 4973–4978. [Google Scholar] [CrossRef]
- Kaplan, D.L.; Mayer, J.M.; Greenberger, M.; Gross, R.A.; McCarthy, S.P. Degradation methods and degradation kinetics of polymer films. Polym. Degrad. Stab. 1994, 45, 165–172. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable Polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Narayan, R. Drivers for biodegradable/compostable plastics and role of composting in waste management and sustainable agriculture. Bioprocess. Solid Waste Sludge 2001, 1, 1. Available online: http://www.orbit-online.net/journal/archiv/index.html (accessed on 1 March 2022).
- Doran, J.W. Soil health and global sustainability: Translating science into practice. Agric. Ecosyst. Environ. 2002, 88, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Bastioli, C. Properties and application of Mater-Bi starch-based materials. Polym. Degrad. Stab. 1998, 59, 263–272. [Google Scholar] [CrossRef]
- Souza, M.A.d.; Vilas-Boas, I.T.; Leite-da-Silva, J.M.; Abrahão, P.D.N.; Teixeira-Costa, B.E.; Veiga-Junior, V.F. Polysaccharides in Agro-Industrial Biomass Residues. Polysaccharides 2022, 3, 95–120. [Google Scholar] [CrossRef]
- Gáspár, M.; Benkő, Z.; Dogossy, G.; Réczey, K.; Czigány, T. Reducing water absorption in compostable starch-based plastics. Polym. Degrad. Stab. 2005, 90, 563–569. [Google Scholar] [CrossRef]
- Marques, P.T.; Lima, A.M.F.; Bianco, G.; Laurindo, J.B.; Borsali, R.; Meins, J.F.; Soldi, V. Thermal properties and stability of cassava starch films cross-linked with tetraethylene glycol diacrylate. Polym. Degrad. Stab. 2006, 91, 726–732. [Google Scholar] [CrossRef]
- Shogren, R.L. Biodegradable Mulches from Renewable Resources. J. Sustain. Agric. 2000, 16, 33–47. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Copinet, A.; Tighzert, L.; Coma, V. Mechanical and barrier properties of biodegradable films made form chitosan and poly(lactic acid) blends. J. Polym. Environ. 2004, 12, 1–6. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan-Starch Composite Film Preparation and Characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Rhim, J.W. Physical and mechanical properties of water-resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Schettini, E.; Vox, G.; Malinconico, M.; Immirzi, B.; Santagata, G. Physical properties of innovative biodegradable spray coating for soil mulching in greenhouse cultivation. Acta Hortic. 2005, 691, 725–732. [Google Scholar] [CrossRef]
- Brandelero, R.P.H.; Alfaro, A.T.; Marques, P.T.; Brandelero, E.M. New Approach of Starch and Chitosan Films as Biodegradable Mulching. Rev. Virtual Quim. 2019, 11, 3. Available online: http://rvq.sbq.org.br (accessed on 20 February 2022).
- Oatley, C.W. The Scanning Electron Microscope; Cambridge University Press: Cambridge, UK, 1972. [Google Scholar]
- Finkenstadt, V.L.; Tisserat, B. Poly (lactic acid) and Osage orange wood fiber composites for agricultural mulch films. Ind. Crops Prod. 2010, 31, 316–320. [Google Scholar] [CrossRef]
- Holmes, B.J.; Springman, R. Recycling Silo-Bags and Other Agricultural Plastic Films (A 3875). Cooperative Extension of the University of Wisconsin-Extension. 2009. Available online: http://www.uwex.edu/ces/crops/uwforage/A3875_Recycling_silo_bags_and_other_ag_plastics.pdf (accessed on 14 February 2022).
- Bhatti, J.A. Current State and Potential for Increasing Plastics Recycling in the U.S. Master’s Thesis, Columbia University, New York, NY, USA, 2010. Available online: http://www.seas.columbia.edu/earth/wtert/sofos/bhatti_thesis.pdf (accessed on 14 February 2022).
- Borreani, G.; Tabacco, E.; Guerrini, S.; Ponti, R. Opportunities in developing novel biodegradable films to cover silages. In Proceedings of the Agricultural Film 2013, International Industry Conference on Silage, Mulch and Tunnel Films Used in Agriculture, Madrid, Spain, 16–18 September 2013; Applied Market Information Ltd.: Madrid, Spain, 2013; pp. 4.1–4.13. [Google Scholar]
- Momani, B. Assessment of the Impacts of Bioplastics: Energy Usage, Fossil Fuel Usage, Pollution, Health Effects, Effects on the Food Supply, and Economic Effects Compared to Petroleum Based Plastics; Worcester Polytechnic Institute: Worcester, MA, USA, 2009; Available online: http://www.wpi.edu/Pubs/E-project/Available/E-project-031609-205515/unrestricted/bioplastics.pdf (accessed on 14 February 2022).
- Keller, A. Biodegradable stretch films for silage bales: Basically possible. Agrarforschung Schweiz 2000, 7, 64–169. [Google Scholar]
- Ivonkovic, A.; Zeljko, K.; Talic, S.; Lasic, M. Biodegradable packaging in the food industry. J. Food Saf. Food Qual. 2017, 68, 23–52. [Google Scholar]
- Raheem, D. Application of plastics and paper as food packaging materials—An overview. Emir. J. Food Agric. 2012, 25, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Averous, L.; Baqquillon, N. Biocomposite based on plasticizes starch: Thermal and mechanical behaviours. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Adeodato Vieira, M.G.; Altenhofen da Silva, M.; Oliveira dos Santos, L.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Shogren, R.; Doane, W.; Garlotta, D.; Lawton, J.; Willett, J. Biodegradation of starch/polylactic acid/poly (hydroxyesterether) composite bars in soil. Polym. Degrad. Stab. 2003, 79, 405–411. [Google Scholar] [CrossRef]
- Lawton, J.W.; Shogren, R.L.; Tiefenbacher, K.F. Aspen fiber addition improves the mechanical properties of baked cornstarch foams. Ind. Crops Prod. 2004, 19, 41–48. [Google Scholar] [CrossRef]
- Parvin, F.; Rahman, M.A.; Islam, J.M.M.; Mubarak, A.; Ahmad Khan, M.A.; Saadat, A.H.M. Preparation and Characterization of Starch/PVA Blend for Biodegradable Packaging Material. Adv. Mater. Res. 2010, 123–125, 351–354. [Google Scholar] [CrossRef]
- Li, T.; Turng, L.-S.; Gong, S.; Erlacher, K. Polylactide, nanoclay, and core–shell rubber composites. Polym. Eng. Sci. 2006, 46, 1419. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, C.; Ma, S.; Feng, J.; Yang, Y.; Zhang, R.; Zhu, J. The properties of poly(lactic acid)/starch blends with a functionalized plant oil: Tung oil anhydride. Carbohydr. Polym. 2013, 95, 77–84. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, L.; Ma, S.; Yang, Y.; Zhang, C.; Tang, Z.; Zhu, J. Effect of castor oil enrichment layer produced by reaction on the properties of PLA/HDI-g-starch blends. Carbohydr. Polym. 2013, 94, 235–243. [Google Scholar] [CrossRef]
- Khalil, F.; Galland, S.; Cottaz, A.; Joly, C.; Degraeve, P. Polybutylene succinate adipate/starch blends: A morphological study for the design of controlled release films. Carbohydr. Polym. 2014, 108, 272–280. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Development and characterization of sugar palm starch and poly(lactic acid) bilayer films. Carbohydr. Polym. 2016, 146, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Zhang, X.; He, C. Review Fully biodegradable Poly(lactic acid)/Starch blends: A review of toughening strategies. Int. J. Biol. Macromol. 2018, 109, 99–113. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Influence of Nanocellulose Additive on the Film Properties of Native Rice Starch-based Edible Films for Food Packaging. Recent Pat. Nanotechnol. 2019, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Johari, A.; Jusoh, M. Isolation, Characterization, and Application of Nanocellulose from Oil Palm Empty Fruit Bunch Fiber as Nanocomposites. J. Nanomater. 2014, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Godbole, S.; Gote, S.; Latkar, M.; Chakrabarti, T. Preparation and characterization of biodegradable poly-3-hydroxybutyrate–starch blend films. Bioresour. Technol. 2003, 86, 33–37. [Google Scholar] [CrossRef]
- Nasseri, R.; Mohammadi, N. Starch-based nanocomposites: A comparative performance study of cellulose whiskers and starch nanoparticles. Carbohydr. Polym. 2014, 106, 432–439. [Google Scholar] [CrossRef]
- Schyrr, B.; Pasche, S.; Voirin, G.; Weder, C.; Simon, Y.C.; Foster, E.J. Biosensors Based on Porous Cellulose Nanocrystal–Poly(vinyl Alcohol) Scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 12674–12683. [Google Scholar] [CrossRef]
- Montes, S.; Carrasco, P.M.; Ruiz, V.; Cabañero, G.; Grande, H.J.; Labidi, J.; Odriozola, I. Synergistic reinforcement of poly(vinyl alcohol) nanocomposites with cellulose nanocrystal-stabilized graphene. Compos. Sci. Technol. 2015, 117, 26–31. [Google Scholar] [CrossRef]
- Wang, B.; Walther, A. Self-Assembled, Iridescent, Crustacean-Mimetic Nanocomposites with Tailored Periodicity and Layered Cuticular Structure. ACS Nano 2015, 9, 10637–10646. [Google Scholar] [CrossRef] [PubMed]
- Shey, J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Properties of baked starch foam with natural rubber latex. Ind. Crops Prod. 2006, 24, 34–40. [Google Scholar] [CrossRef]
- Fang, Q.; Hanna, M.A. Characteristics of biodegradable Mater-Bi®-starch based foams as affected by ingredient formulations. Ind. Crops Prod. 2001, 13, 219–227. [Google Scholar] [CrossRef]
- Ganjyal, G.; Reddy, N.; Yang, Y.; Hanna, M.A. Biodegradable packaging foams of starch acetate blended with corn stalk fibers. Mater. Sci. 2004, 93, 2627–2633. [Google Scholar] [CrossRef]
- Glenn, G.M.; Imam, S.H.; Orts, W.J. Starch-based foam composite materials: Processing and bioproducts. MRS Bull. 2011, 36, 696–702. [Google Scholar] [CrossRef]
- Glenn, G.M.; Klamczynski, A.P.; Ludvik, C.; Shey, J.; Imam, S.H.; Chiou, B.; McHugh, T.; De Grandi-Hoffman, G.; Orts, W.; Wood, D.; et al. Permeability of starch gel matrics and select films to solvent vapors. J. Agric. Food Chem. 2006, 54, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.J.; Francois, N.J. Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr. Polym. 2016, 148, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Basnayake, B.F.A. Patent of Discovery of a Process to Retard the Release of Nitrogen Fertilizer by Using Charcoal and Manioc; University of Peradeniya: Peradeniya, Sri Lanka, 1994; No: 10665. [Google Scholar]
- Gamage, D.A.S.; Basnayake, B.F.A.; Costa, J.; Vidanagamage, K. Evaluation of total N, P, K and organic matter contents of soil amended with paddy husk charcoal coated urea and comparison of the yield of paddy. In Proceedings of the International Conference on Sustainable Built Environment, Kandy, Sri Lanka, 16–18 December 2012. [Google Scholar]
- Gamage, D.A.S. Development of Nutrient Management Technologies for Sustainable Rice Farming for Mitigating Water and Atmospheric Pollution. Ph.D. Thesis, Postgraduate Institute of Agriculture, University of Peradeniya, Peradeniya, Sri Lanka, 2015. [Google Scholar]
- Villa, M.; Capill, V.; Gardé, J.; Rivera, J. Degradación biológica de polímeros mediante la selección y producción de potenciales cultivos iniciadores. Retema Rev. Técnica Medio Ambiente 2009, 136, 80–83. [Google Scholar]
- Wagner, M.; Oehlman, J. Endocrine disruptors in bottled mineral water: Total estrogenic burden and migration from plastic bottles. Environ. Sci. Pollut. Res. 2009, 16, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Katami, T.; Yasuhara, A.; Okuda, T.; Shibamoto, T. Formation of PCDDs, PCDFs, and coplanar PCBs from polyvinyl chloride during combustion in an incinerator. Environ. Sci. Technol. 2002, 36, 1320–1324. [Google Scholar] [CrossRef]
- Gironès, J.; López, J.; Mutjé, P.; Carvalho, A.; Curvelo, A.; Vilaseca, F. Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos. Sci. Technol. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- European Bioplastics, European Bioplastics. December 2021. Available online: https://www.european-bioplastics.org/global-bioplastics-production-will-more-than-triple-within-the-next-five-years/ (accessed on 25 February 2022).
- Pagga, U. Compostable packaging materials–test methods and limit values for biodegradation. Appl. Microbiol. Biotechnol. 1999, 51, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Jayasekara, R.; Harding, I.H.; Bowater, I.; Christie, G.B.Y. Biodegradation by Composting of Surface Modified Starch and PVA Blended Films. J. Polym. Environ. 2003, 11, 49–56. [Google Scholar] [CrossRef]
- Chai, W.L.; Chow, J.D.; Chen, C.C. Effects of modified starch and different molecular weight polyvinyl alcohols on biodegradable characteristics of polyvinyl alcohol/starch blends. J. Polym. Environ. 2012, 20, 550–564. [Google Scholar] [CrossRef]
- Pšeja, J.; Charvátová, H.; Hruzík, P.; Hrnčiřík, J.; Kupec, J. Anaerobic biodegradation of blends based on polyvinyl alcohol. J. Polym. Environ. 2006, 14, 185–190. [Google Scholar] [CrossRef]
- Hrnčiřík, J.; Pšeja, J.; Kupec, J.; Bernkopfová, S. Anaerobic biodegradation of polyvinyl alcohol modified by extracellular polysaccharides. J. Polym. Environ. 2010, 18, 98–103. [Google Scholar] [CrossRef]
- Yun, Y.; Yoon, S. Effect of amylose contents of starches on physical properties and biodegradability of starch/PVAblended films. Polym. Bull. 2010, 64, 553–568. [Google Scholar] [CrossRef]
- Negim, E.; Rakhmetullayeva, R.; Yeligbayeva, G.; Urkimbaeva, P.; Primzharova, S.; Kaldybekov, D.; Craig, W. Improving biodegradability of polyvinyl alcohol/starch blend films for packaging applications. Int. J. Basic Appl. Sci. 2014, 3, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Râpă, M.; Grosu, E.; Stoica, P.; Andreica, M.; Hetvary, M. Polyvinyl alcohol and starch blends: Properties and biodegradation behavior. J. Environ. Res. Prot. 2014, 11, 34–42. [Google Scholar]
- Tanase, E.; Popa, E.; Rapa, M.; Popa, O.; Popa, I. Biodegradation study of some food packaging biopolymers based on PVA. Bull. UASVM Anim. Sci. Biotechnol. 2016, 73, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Ray, D.; Mitra, D.; Mukhopadhyay, A. Isolation and characterisation of starch/polyvinyl alcohol degrading fungi from aerobic compost environment. Int. Biodeterior. Biodegrad. 2013, 82, 9–12. [Google Scholar] [CrossRef]
- Imam, S.; Cinelli, P.; Gordon, S.; Chiellini, E. Characterization of biodegradable composite films prepared from blends of poly (vinyl alcohol), cornstarch, and lignocellulosic fiber. J. Polym. Environ. 2005, 13, 47–55. [Google Scholar] [CrossRef]
- Raj, B.; Somashekar, R. Structure–property relation in polyvinyl alcohol/starch composites. J. Appl. Polym. Sci. 2004, 91, 630–635. [Google Scholar]
- Gattin, R.; Copinet, A.; Bertrand, C.; Couturier, Y. Biodegradation study of a starch and poly (lactic acid) coextruded material in liquid, composting and inert mineral media. Int. Biodeterior. Biodegrad. 2002, 50, 25–31. [Google Scholar] [CrossRef]
- Copinet, A.; Bertrand, C.; Longieras, A.; Coma, V.; Couturier, Y. Photodegradation and biodegradation study of a starch and poly (lactic acid) coextruded material. J. Polym. Environ. 2003, 11, 169–179. [Google Scholar] [CrossRef]
- Petinakis, E.; Liu, X.; Yu, L.; Way, C.; Sangwan, P.; Dean, K.; Edward, G. Biodegradation and thermal decomposition of poly (lactic acid)-based materials reinforced by hydrophilic fillers. Polym. Degrad. Stab. 2010, 95, 1704–1707. [Google Scholar] [CrossRef]
- Rudeekit, Y.; Siriyota, P.; Intaraksa, P.; Chaiwutthinan, P.; Tajan, M.; Leejarkpai, T. Compostability and Ecotoxicity of Poly (lactic acid) and Starch Blends. Adv. Mater. Res. 2012, 506, 323–326. [Google Scholar] [CrossRef]
- Shin, B.; Jang, S.; Kim, B. Thermal, morphological, and mechanical properties of biobased and biodegradable blends of poly (lactic acid) and chemically modified thermoplastic starch. Polym. Eng. Sci. 2011, 51, 826–834. [Google Scholar] [CrossRef]
- Rodrigues, C.; Tofanello, A.; Nantes, I.; Rosa, D. Biological Oxidative Mechanisms for Degradation of Poly (lactic acid) Blended with Thermoplastic Starch. ACS Sustain. Chem. Eng. 2015, 3, 2756–2766. [Google Scholar] [CrossRef]
- Wu, C. Improving polylactide/starch biocomposites by grafting polylactide with acrylic acid–characterization and biodegradability assessment. Macromol. Biosci. 2005, 5, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Shin, B.; Lee, T.; Narayan, R. Thermal properties, and morphology of biodegradable PLA/starch compatibilized blends. J. Ind. Eng. Chem. 2007, 13, 457–464. [Google Scholar]
- Ohkita, T.; Lee, S. Thermal degradation and biodegradability of poly (lactic acid)/corn starch biocomposites. J. Appl. Polym. Sci. 2006, 100, 3009–3017. [Google Scholar] [CrossRef]
- Ishiaku, U.; Pang, K.; Lee, W.; Ishak, Z. Mechanical properties and enzymic degradation of thermoplastic and granular sago starch filled poly (ε-caprolactone). Eur. Polym. J. 2002, 38, 393–401. [Google Scholar] [CrossRef]
- Yavuz, H.; Babaç, C. Preparation and biodegradation of starch/polycaprolactone films. J. Polym. Environ. 2003, 11, 107–113. [Google Scholar] [CrossRef]
- Hubackova, J.; Dvorackova, M.; Svoboda, P.; Mokrejs, P.; Kupec, J.; Pozarova, I.; Koutny, M. Influence of various starch types on PCL/starch blends anaerobic biodegradation. Polym. Test. 2013, 32, 1011–1019. [Google Scholar] [CrossRef]
- Kim, C.; Jung, K.; Kim, J.; Park, J. Modification of aliphatic polyesters and their reactive blends with starch. J. Polym. Environ. 2004, 12, 179–187. [Google Scholar] [CrossRef]
- Rosa, D.; Rodrigues, T.; Graças, F.G.C.; Calil, M. Effect of thermal aging on the biodegradation of PCL, PHB-s with starch in soil compost, V, and their blend. J. Appl. Polym. Sci. 2003, 89, 3539–3546. [Google Scholar] [CrossRef]
- Ali, S.F.A. Biodegradation properties of poly-εcaprolactone, starch and cellulose acetate butyrate composites. J. Polym. Environ. 2014, 22, 359–364. [Google Scholar] [CrossRef]
- Praznik, W.; Huber, A.; Watzinger, S.; Beck, R. Molecular Characteristics of High Amylose Starches. Starch Starke 1994, 46, 88–94. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J. A new biopolymer-based polycaprolactone/starch modified clay nanocomposite. Cellul. Chem. Technol. 2014, 48, 515–520. [Google Scholar]
- Guarás, M.; Alvarez, V.; Ludueña, L. Processing and characterization of thermoplastic starch/polycaprolactone/compatibilizer ternary blends for packaging applications. J. Polym. Res. 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Di Franco, C.; Cyras, V.P.; Busalmen, J.P.; Ruseckaite, R.A.; Vázquez, A. Degradation of polycaprolactone/starch blends and composites with sisal fibre. Polym. Degrad. Stab. 2004, 86, 95–103. [Google Scholar] [CrossRef]
- Campos, A.; Teodoro, K.B.R.; Teixeira, E.M.; Corrêa, A.C.; Marconcini, J.M.; Wood, D.F.; Mattoso, L.H. Properties of thermoplastic starch and TPS/polycaprolactone blend reinforced with sisal whiskers using extrusion processing. Polym. Eng. Sci. 2013, 53, 800–808. [Google Scholar] [CrossRef]
- Lai, S.-M.; Don, R.-M.; Huang, Y.C. Preparation and properties of biodegradable thermoplastic starch/poly(hydroxy butyrate) blends. J. Appl. Polym. Sci. 2006, 100, 2371–2379. [Google Scholar] [CrossRef]
- Magalhães, N.; Andrade, C. Properties of melt-processed poly (hydroxybutyrate-co-hydroxyvalerate)/starch 1:1 blend nanocomposites. Polímeros 2013, 23, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, D.; Mukai, M.; Kubota, K.; Kai, T.; Kaneko, N.; Araki, K.; Kubo, M. Degradation of Bioplastics in Soil and Their Degradation Effects on Environmental Microorganisms. J. Agric. Chem. Environ. 2016, 5, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Chen, C.; Gao, L.; Jiao, L.; Xu, H.; Guo, W. Physical and degradation properties of binary or ternary blends composed of poly (lactic acid), thermoplastic starch and GMA grafted POE. Polym. Degrad. Stab. 2011, 96, 175–182. [Google Scholar] [CrossRef]

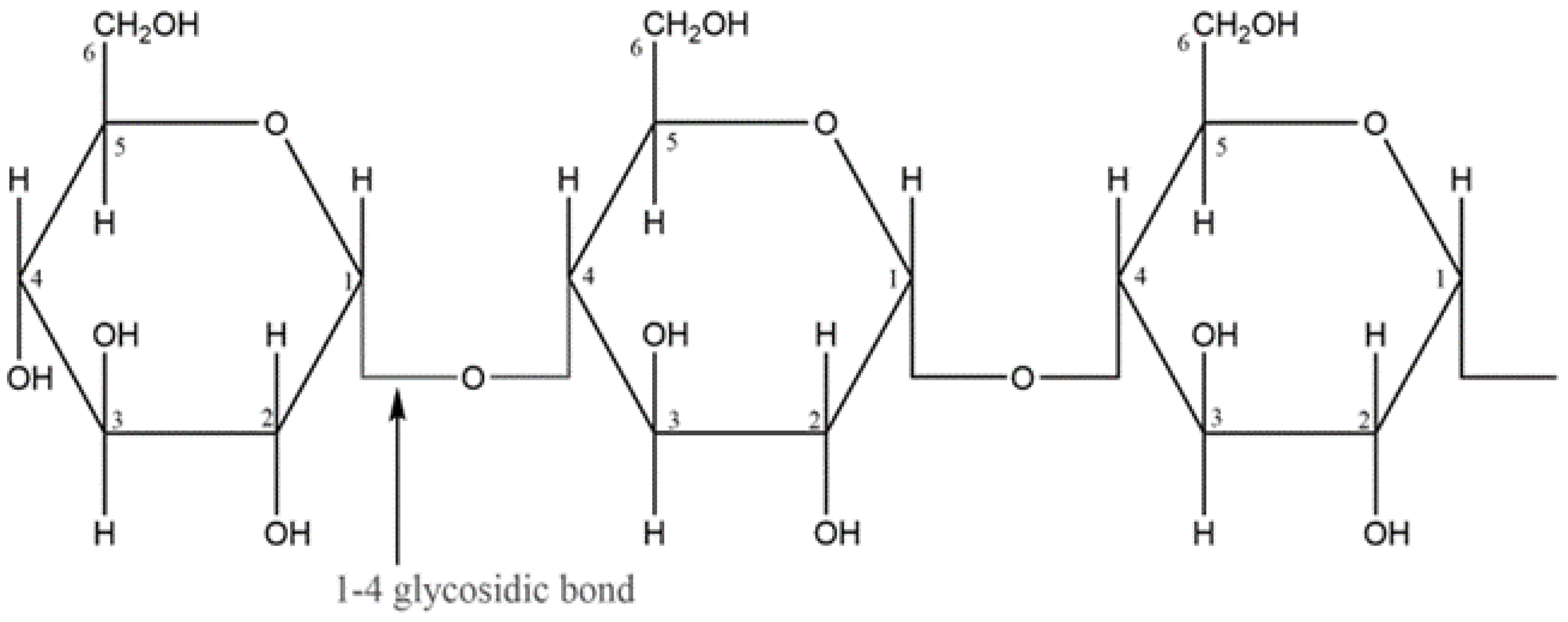
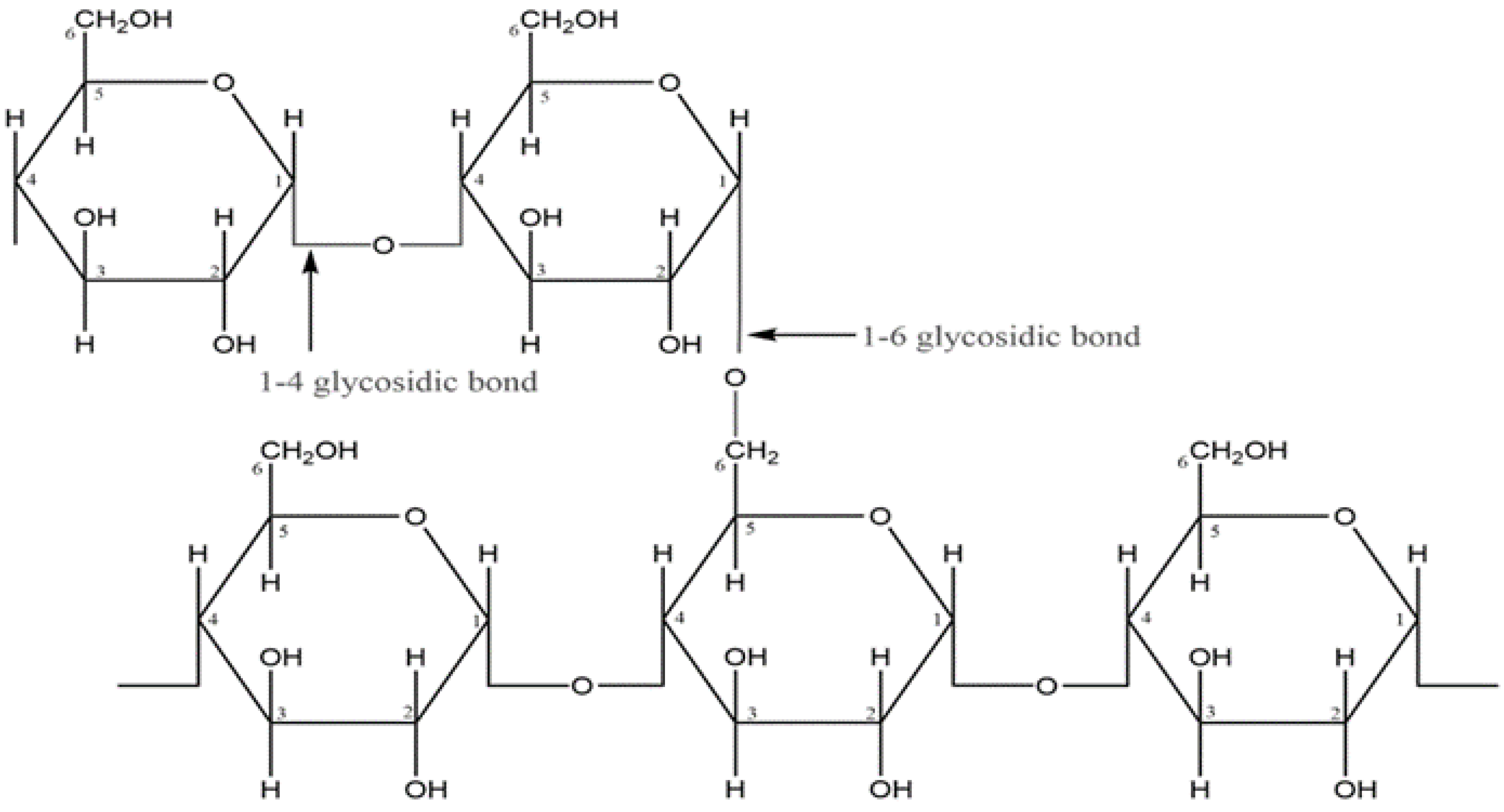




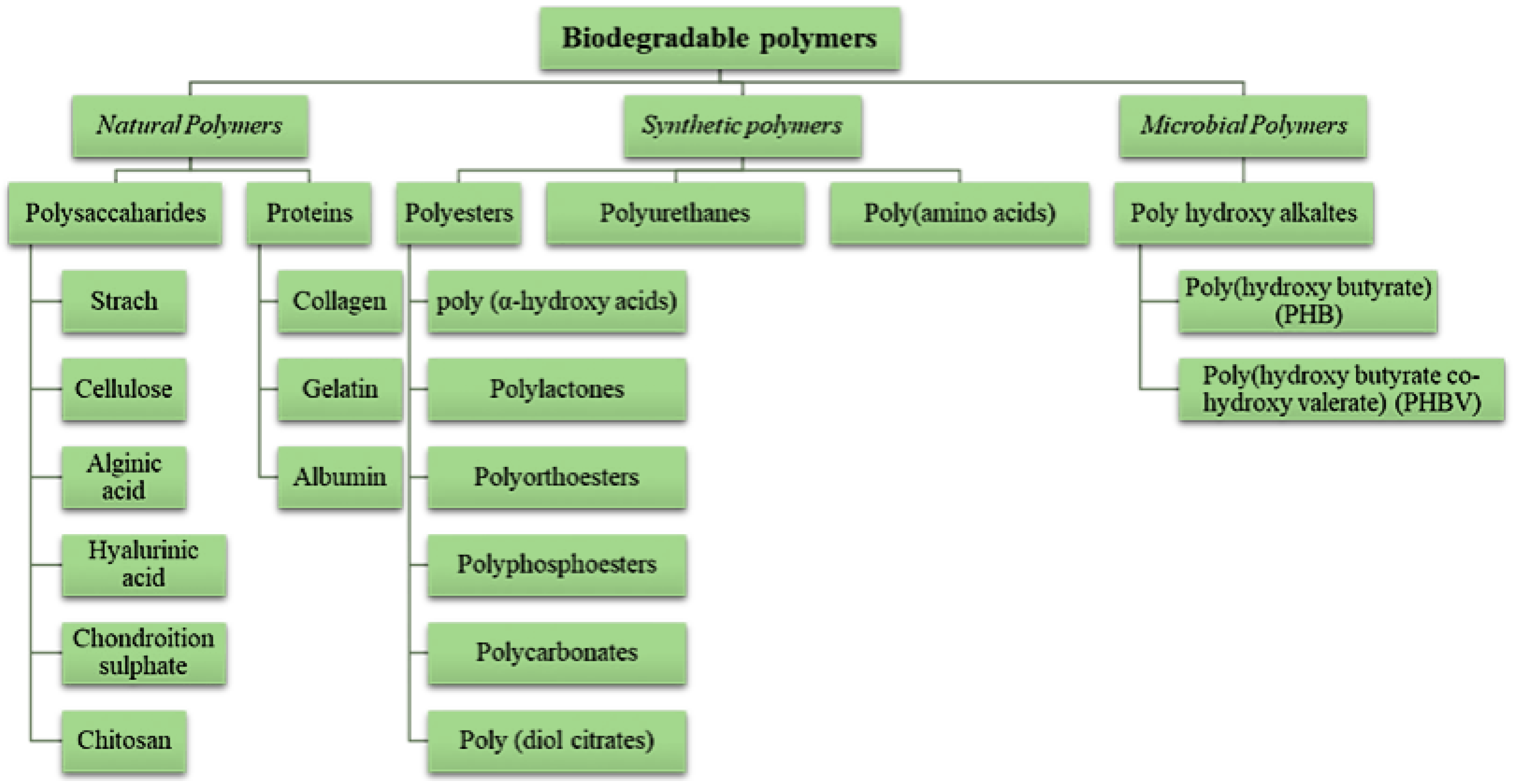
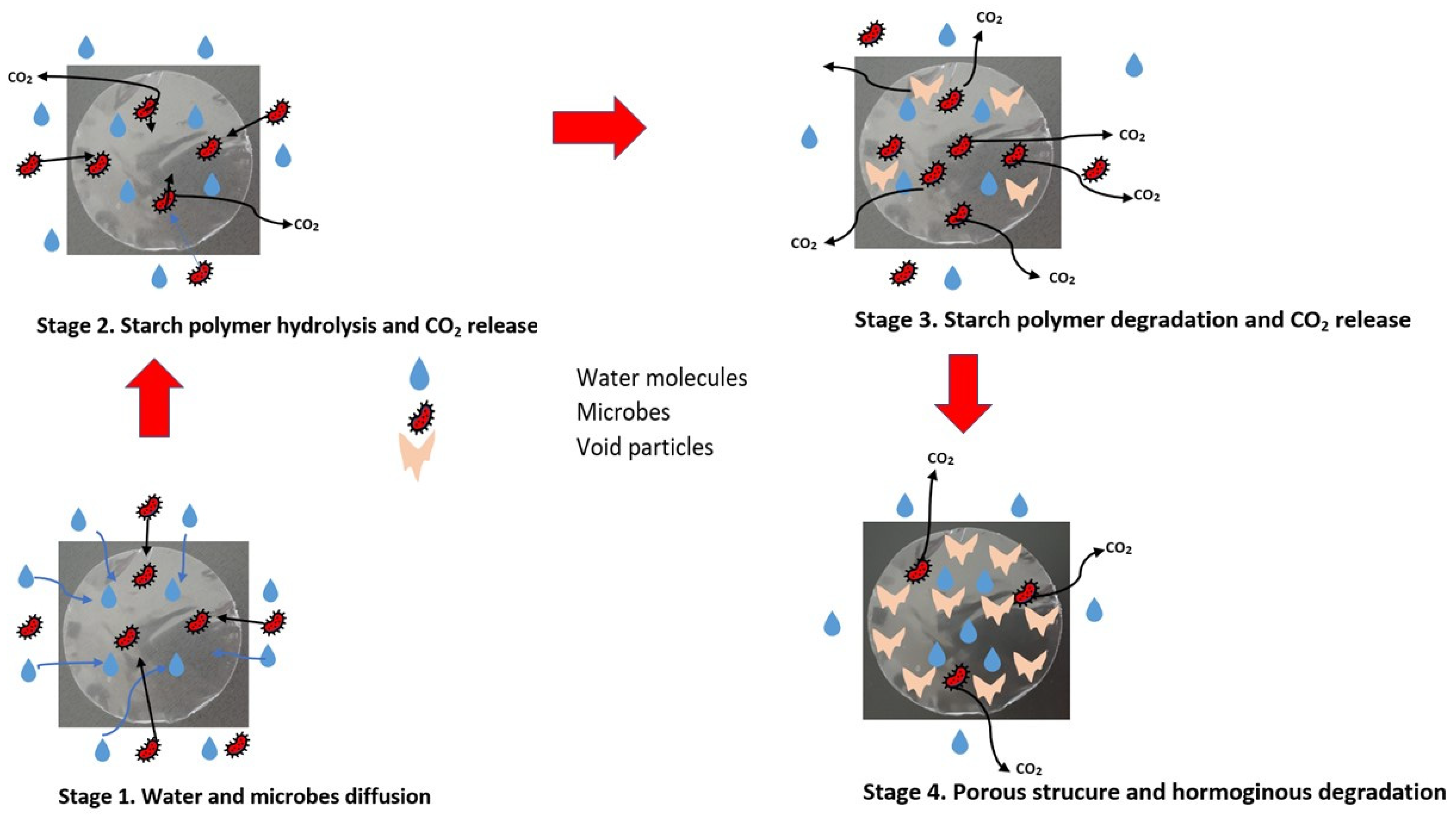
| Starch Source | Amylose (%) | Amylopectin (%) | Crystallinity (%) | References |
|---|---|---|---|---|
| Roots and tubers | ||||
| Potato | 17–24 | 76–83 | 23–53 | [32,33,34,35,36,37] |
| Cassava | 16–22 | 81–83 | 31–59 | [32,34,36,37,38,39] |
| Sweet potato | 18 | 81 | [34] | |
| Yam | 15–22 | 78–91 | [37,38] | |
| Cereals and pulses | ||||
| Corn | 17–28 | 72–83 | 43–48 | [32,34,35,36,37,40] |
| Rice | 15–35 | 65–85 | 38 | [32,34,35,36,37,41] |
| Wheat | 20–25 | 75–80 | 36–39 | [32,34,35,36,37,41] |
| Smooth pea | 33–50 | 50–67 | 30 | [34,42] |
| Wrinkled pea | 61–88 | 12–39 | 17 | [34] |
| Barely | 27.5 | 72.5 | 37–44 | [34,43] |
| Lentil | 29–45 | 71–54 | 32 | [34] |
| Sorghum | 25 | 75 | 22–28 | [36,44,45] |
| Blend | Properties | Applications | References |
|---|---|---|---|
| Starch/PVA |
|
| [113,205,206,207] |
| Starch/PLA |
|
| [124,138,208,209,210,211,212,213,214] |
| Starch/nanocellulose/PLA/PBS |
|
| [215,216] |
| Starch/PVA/Nanocellulose |
|
| [217] |
| Starch/PBSA |
|
| [211] |
| Starch/PHB |
|
| [218] |
| Starch/nanofibre |
|
| [219,220,221,222] |
| Starch/natural rubber |
|
| [223] |
| Starch-based foam processes/fiber/fillers/resins |
|
| [224,225,226] |
| Starch-based controlled-release devices |
|
| [227] |
| Chitosan-starch beads |
|
| [228] |
| Starch/Charcoal/Urea |
|
| [229,230,231] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamage, A.; Liyanapathiranage, A.; Manamperi, A.; Gunathilake, C.; Mani, S.; Merah, O.; Madhujith, T. Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability 2022, 14, 6085. https://doi.org/10.3390/su14106085
Gamage A, Liyanapathiranage A, Manamperi A, Gunathilake C, Mani S, Merah O, Madhujith T. Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability. 2022; 14(10):6085. https://doi.org/10.3390/su14106085
Chicago/Turabian StyleGamage, Ashoka, Anuradhi Liyanapathiranage, Asanga Manamperi, Chamila Gunathilake, Sudhagar Mani, Othmane Merah, and Terrence Madhujith. 2022. "Applications of Starch Biopolymers for a Sustainable Modern Agriculture" Sustainability 14, no. 10: 6085. https://doi.org/10.3390/su14106085
APA StyleGamage, A., Liyanapathiranage, A., Manamperi, A., Gunathilake, C., Mani, S., Merah, O., & Madhujith, T. (2022). Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability, 14(10), 6085. https://doi.org/10.3390/su14106085










