Influence of Miscanthus Rhizome Pyrolysis Operating Conditions on Products Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Experimental Setup
2.3. Gas Characterization
2.4. Condensate Characterization
2.5. Biochar Characterization
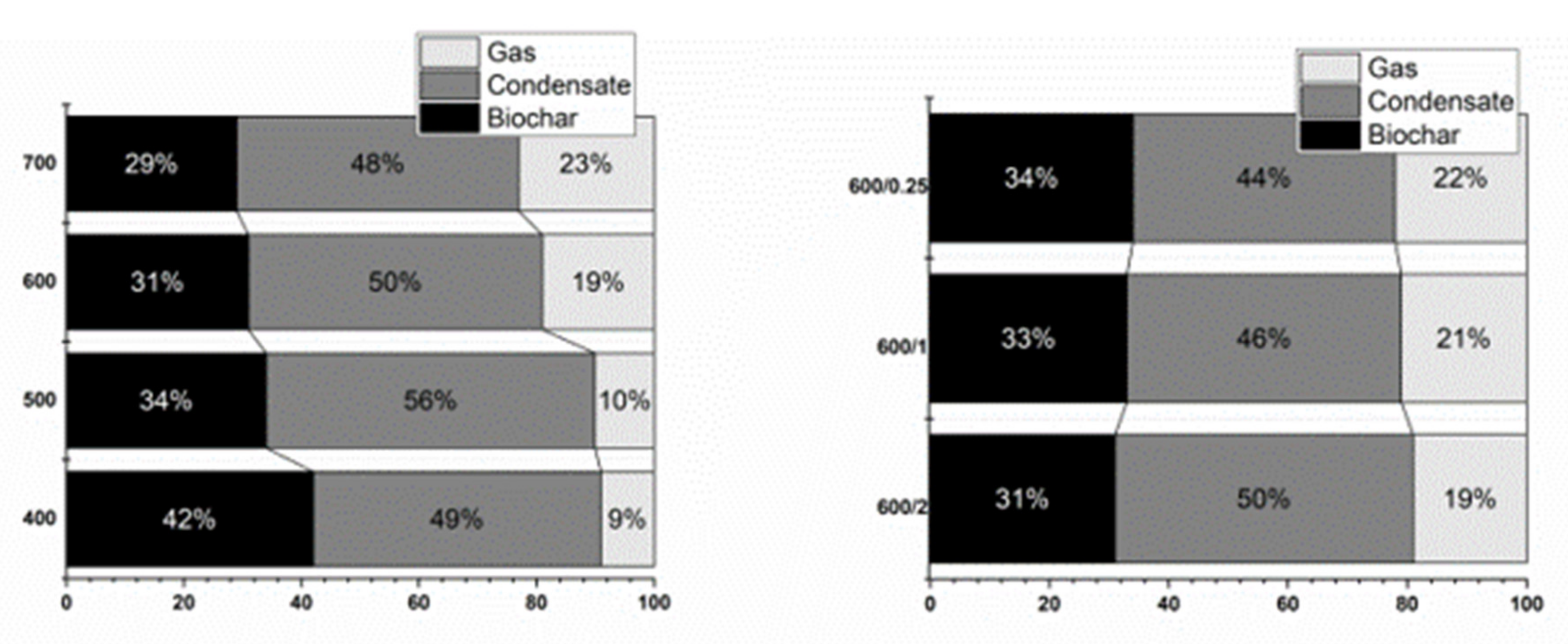
3. Results and Discussion
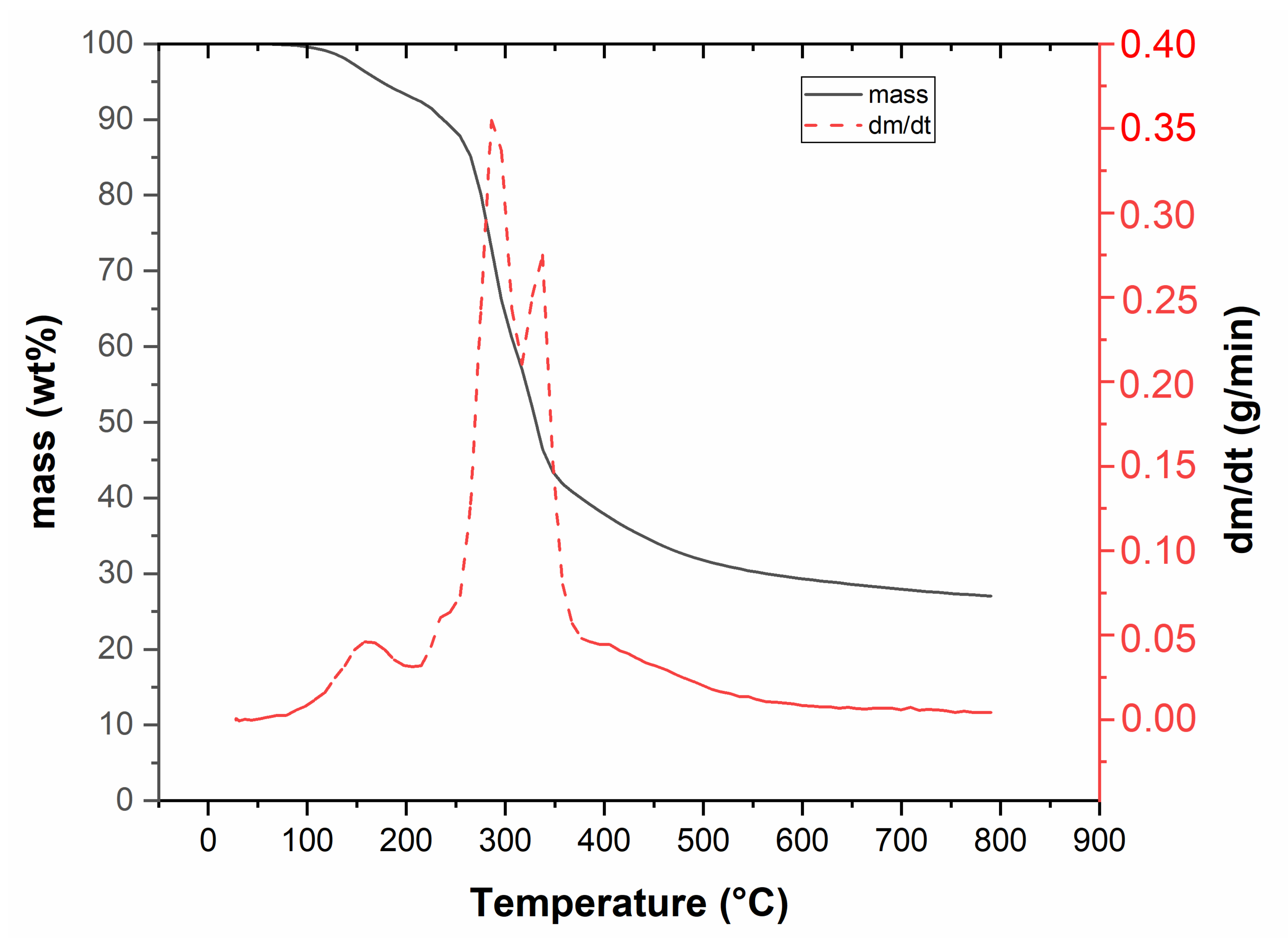
3.1. Feedstock Characterization
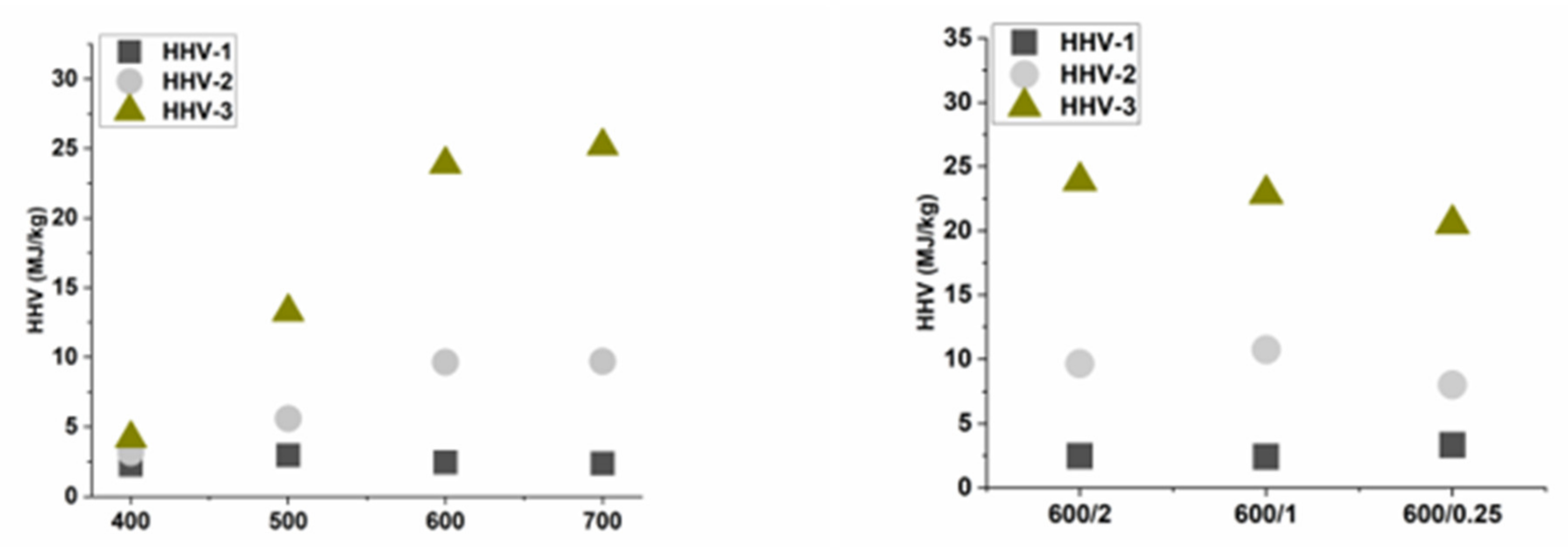
3.2. Gas Characterization
3.3. Condensate Characterization
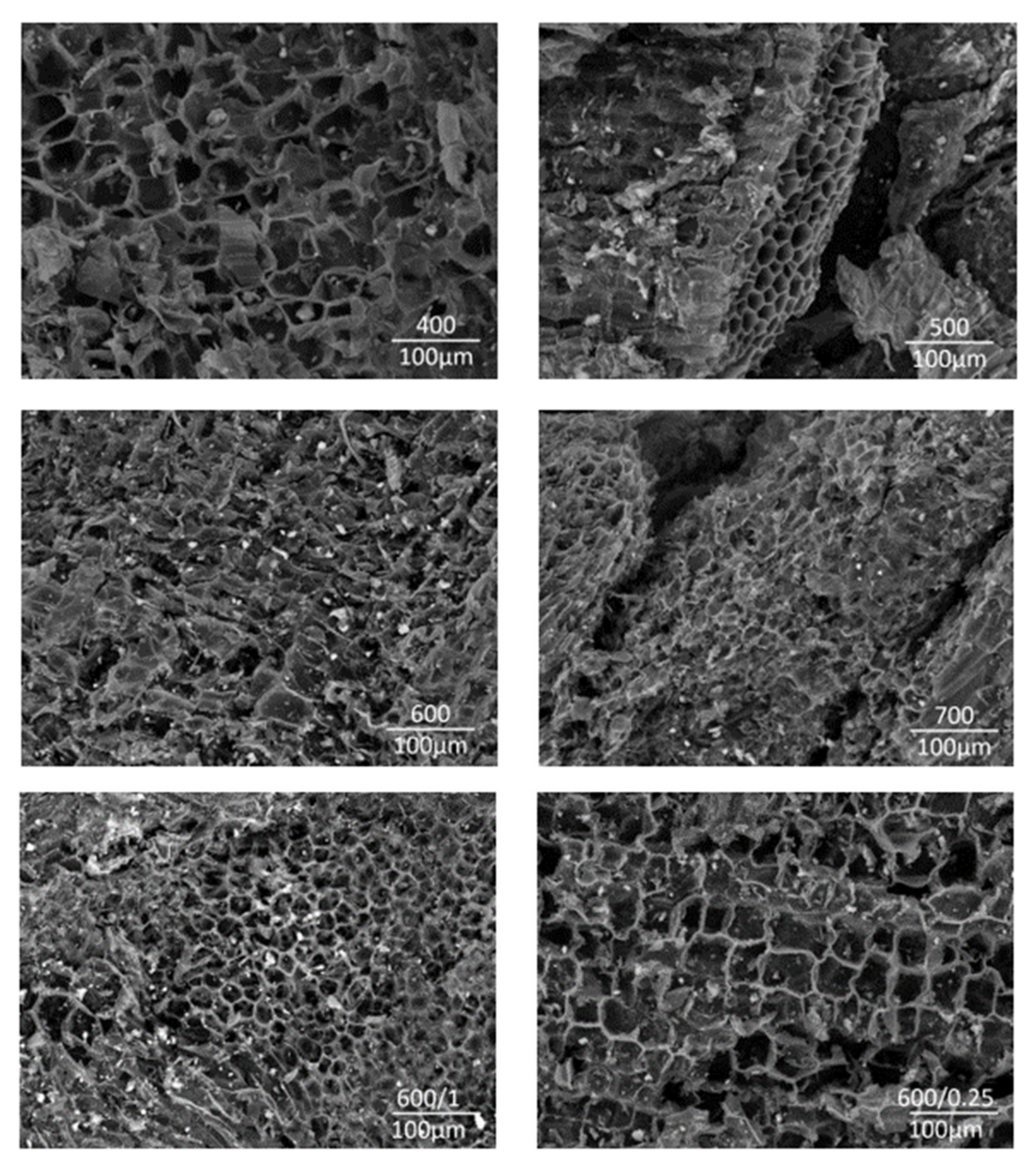
3.4. Biochar Characterization
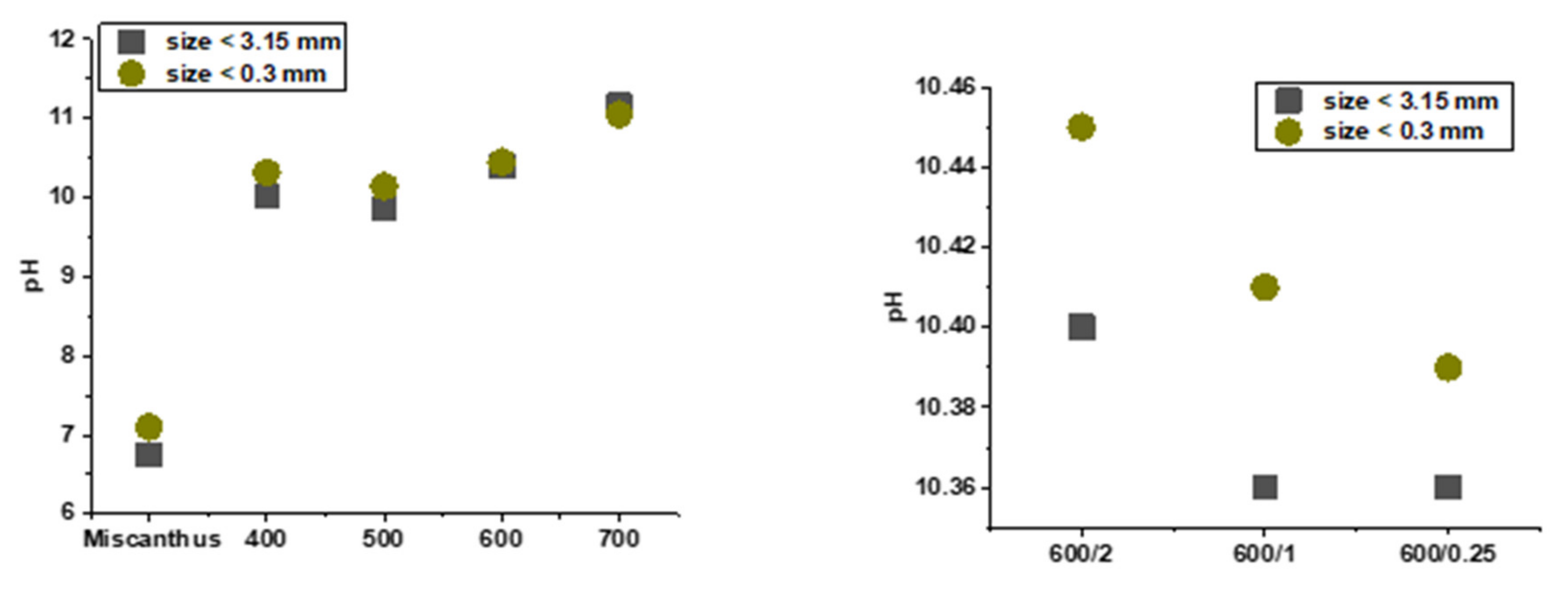
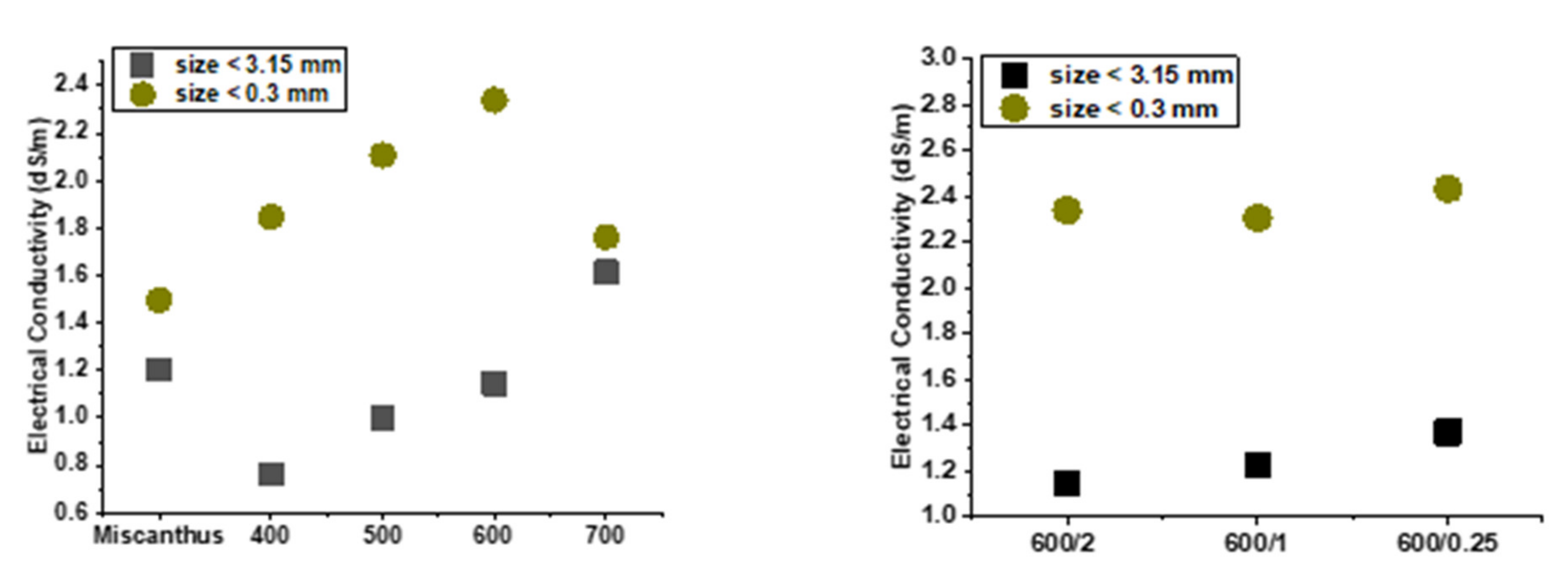
3.5. pH and Electrical Conductivity (EC)
3.6. Surface Area
3.7. PAHs and Heavy Metals
3.8. Concentration of Ca, K, Mg, S, N and P
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghalibaf, M.; Ullah, S.; Alén, R. Fast pyrolysis of hot-water-extracted and soda-AQ-delignified okra (Abelmoschus esculentus) and miscanthus (miscanthus x giganteus) stalks by Py-GC/MS. Biomass-Bioenergy 2018, 118, 172–179. [Google Scholar] [CrossRef]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Wafiq, A.; Reichel, D.; Hanafy, M. Pressure influence on pyrolysis product properties of raw and torrefied Miscanthus: Role of particle structure. Fuel 2016, 179, 156–167. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A. Hydrothermal carbonization, torrefaction and slow pyrolysis of Miscanthus giganteus. Energy 2017, 140, 1292–1304. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; Van Der Linden, G.C.; Schwarz, K.-U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.-L.; Dolstra, O.; Donnison, I.S.; et al. Progress on Optimizing Miscanthus Biomass Production for the European Bioeconomy: Results of the EU FP7 Project OPTIMISC. Front. Plant Sci. 2016, 7, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Parveen, I.; Threadgill, M.D.; Hauck, B.; Donnison, I.; Winters, A. Isolation, identification and quantitation of hydroxycinnamic acid conjugates, potential platform chemicals, in the leaves and stems of Miscanthus×giganteus using LC–ESI-MSn. Phytochemistry 2011, 72, 2376–2384. [Google Scholar] [CrossRef] [Green Version]
- Tew, T.L.; Cobill, R.M. Genetic Improvement of Sugarcane as an Energy Crop. In Genetic Improvement of Bioenergy Crops; Vermerris, W., Ed.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Da Costa, R.M.F.; Lee, S.J.; Allison, G.G.; Hazen, S.P.; Winters, A.; Bosch, M. Genotype, development and tissue-derived variation of cell-wall properties in the lignocellulosic energy crop Miscanthus. Ann. Bot. 2014, 114, 1265–1277. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, L.; Siuta, D.; Markowski, M. Pyrolysis of Rapeseed Oil Press Cake and Steam Gasification of Solid Residues. Energies 2020, 13, 4472. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology, 2nd ed.; Earthscan Routlegde: New York, NY, USA, 2015. [Google Scholar]
- Kim, J.; Hyun, S. Sorption of ionic and nonionic organic solutes onto giant Miscanthus-derived biochar from methanol-water mixtures. Sci. Total Environ. 2018, 615, 805–813. [Google Scholar] [CrossRef] [PubMed]
- He, K.; He, G.; Wang, C.; Zhang, H.; Xu, Y.; Wang, S.; Kong, Y.; Zhou, G.; Hu, R. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2019, 155, 103674. [Google Scholar] [CrossRef]
- Mimmo, T.; Panzacchi, P.; Baratieri, M.; Davies, C.A.; Tonon, G. Effect of pyrolysis temperature on miscanthus (Miscanthus × giganteus) biochar physical, chemical and functional properties. Biomass-Bioenergy 2014, 62, 149–157. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Tang, Y.; Zhang, Q. Long-term effects of biochar amendment on rhizosphere and bulk soil microbial communities in a karst region, southwest China. Appl. Soil Ecol. 2019, 140, 126–134. [Google Scholar] [CrossRef]
- Novak, J.M.; Ippolito, J.A.; Ducey, T.F.; Watts, D.W.; Spokas, K.; Trippe, K.M.; Sigua, G.C.; Johnson, M.G. Remediation of an acidic mine spoil: Miscanthus biochar and lime amendment affects metal availability, plant growth, and soil enzyme activity. Chemosphere 2018, 205, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.-U.D.; Ramzani, P.M.A.; Anjum, S.; Abbas, F.; Iqbal, M.; Yasar, A.; Ihsan, M.Z.; Anwar, M.N.; Baqar, M.; Tauqeer, H.M.; et al. Potential of miscanthus biochar to improve sandy soil health, in situ nickel immobilization in soil and nutritional quality of spinach. Chemosphere 2017, 185, 1144–1156. [Google Scholar] [CrossRef]
- Mete, F.Z.; Mia, S.; Dijkstra, F.A.; Abuyusuf, M.; Hossain, A.S.M.I. Synergistic Effects of Biochar and NPK Fertilizer on Soybean Yield in an Alkaline Soil. Pedosphere 2015, 25, 713–719. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Assil, Z.; Esegbue, O.; Mašek, O.; Gutierrez, T.; Free, A. Specific enrichment of hydrocarbonclastic bacteria from diesel-amended soil on biochar particles. Sci. Total Environ. 2020, 762, 143084. [Google Scholar] [CrossRef]
- Wang, C.; Lv, H.; Yang, W.; Li, T.; Fang, T.; Lv, G.; Han, Q.; Dong, L.; Jiang, T.; Jiang, B.; et al. SVCT-2 determines the sensitivity to ascorbate-induced cell death in cholangiocarcinoma cell lines and patient derived xenografts. Cancer Lett. 2017, 398, 1–11. [Google Scholar] [CrossRef]
- Hilber, I.; Bastos, A.C.; Loureiro, S.; Soja, G.; Marsz, A.; Cornelissen, G.; Bucheli, T.D. The different faces of biochar: Contamination risk versus remediation tool. J. Environ. Eng. Landsc. Manag. 2017, 25, 86–104. [Google Scholar] [CrossRef] [Green Version]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Lyu, H.; He, H.; Tang, J.; Hecker, M.; Liu, Q.; Jones, P.D.; Codling, G.; Giesy, J.P. Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ. Pollut. 2016, 218, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Herath, H.M.S.K. Polycyclic aromatic hydrocarbons (PAHs) in biochar—Their formation, occurrence and analysis: A review. Org. Geochem. 2017, 114, 1–11. [Google Scholar] [CrossRef]
- Cipullo, S.; Snapir, B.; Tardif, S.; Campo, P.; Prpich, G.; Coulon, F. Insights into mixed contaminants interactions and its implication for heavy metals and metalloids mobility, bioavailability and risk assessment. Sci. Total Environ. 2018, 645, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Zadel, U.; Nesme, J.; Michalke, B.; Vestergaard, G.; Płaza, G.A.; Schröder, P.; Radl, V.; Schloter, M. Changes induced by heavy metals in the plant-associated microbiome of Miscanthus x giganteus. Sci. Total Environ. 2020, 711, 134433. [Google Scholar] [CrossRef]
- Arduini, I.; Masoni, A.; Mariotti, M.; Ercoli, L. Low cadmium application increase miscanthus growth and cadmium translocation. Environ. Exp. Bot. 2004, 52, 89–100. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Mench, M.; Douay, F. Suitability of Miscanthus species for managing inorganic and organic contaminated land and restoring ecosystem services. A review. J. Environ. Manag. 2014, 143, 123–134. [Google Scholar] [CrossRef]
- Grycova, B.; Pryszcz, A.; Lestinsky, P.; Chamradova, K. Preparation and characterization of sorbents from food waste. Green Process. Synth. 2017, 6, 287–293. [Google Scholar] [CrossRef]
- Lakshman, V.; Brassard, P.; Hamelin, L.; Raghavan, V.; Godbout, S. Pyrolysis of Miscanthus: Developing the mass balance of a biorefinery through experimental tests in an auger reactor. Bioresour. Technol. Rep. 2021, 14, 100687. [Google Scholar] [CrossRef]
- Yan, Y.; Meng, Y.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. Miscanthus as a carbon precursor for graphene oxide: A possibility influenced by pyrolysis temperature. Bioresour. Technol. 2021, 331, 124934. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, A.D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Budai, A.; Wang, L.; Grønli, M.G.; Strand, L.T.; Antal, M.J., Jr.; Abiven, S.; Dieguez-Alonso, A.; Anca-Couce, A.; Rasse, D.P. Surface Properties and Chemical Composition of Corncob and Miscanthus Biochars: Effects of Production Temperature and Method. J. Agric. Food Chem. 2014, 62, 3791–3799. [Google Scholar] [CrossRef]
- Bergs, M.; Völkering, G.; Kraska, T.; Pude, R.; Do, X.T.; Kusch, P.; Monakhova, Y.; Konow, C.; Schulze, M. Miscanthus x giganteus Stem Versus Leaf-Derived Lignins Differing in Monolignol Ratio and Linkage. Int. J. Mol. Sci. 2019, 20, 1200. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Meng, Y.; Tang, L.; Kostas, E.T.; Lester, E.H.; Wu, T.; Pang, C.H. Ignition and Kinetic Studies: The Influence of Lignin on Biomass Combustion. Energy Fuels 2019, 33, 6463–6472. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Hwang, H.; Moon, Y.-H.; Choi, J.W. Assessment of miscanthus biomass (Miscanthus sacchariflorus) for conversion and utilization of bio-oil by fluidized bed type fast pyrolysis. Energy 2014, 76, 284–291. [Google Scholar] [CrossRef]
- Huang, C.-W.; Li, Y.-H.; Xiao, K.-L.; Lasek, J. Cofiring characteristics of coal blended with torrefied Miscanthus biochar optimized with three Taguchi indexes. Energy 2019, 172, 566–579. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A Synthesis of Its Agronomic Impact beyond Carbon Sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Yang, C.; Liu, J.; Lu, S. Pyrolysis temperature affects pore characteristics of rice straw and canola stalk biochars and biochar-amended soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Rangecroft, S.; Emmett, B.A.; DeLuca, T.H.; Jones, D.L. Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural soils? GCB Bioenergy 2013, 5, 96–103. [Google Scholar] [CrossRef]
- Dutta, T.; Kwon, E.; Bhattacharya, S.S.; Jeon, B.H.; Deep, A.; Uchimiya, M.; Kim, K.-H. Polycyclic aromatic hydrocarbons and volatile organic compounds in biochar and biochar-amended soil: A review. GCB Bioenergy 2017, 9, 990–1004. [Google Scholar] [CrossRef]
- Odinga, E.S.; Gudda, F.O.; Waigi, M.G.; Wang, J.; Gao, Y. Occurrence, formation and environmental fate of polycyclic aromatic hydrocarbons in biochars. Fundam. Res. 2021, 1, 296–305. [Google Scholar] [CrossRef]
- IBI. International Biochar Initiative. International Biochar Initiative. Copyright © 2018. Available online: https://biochar-international.org (accessed on 17 February 2022).
- Cho, D.-W.; Cho, S.-H.; Song, H.; Kwon, E.E. Carbon dioxide assisted sustainability enhancement of pyrolysis of waste biomass: A case study with spent coffee ground. Bioresour. Technol. 2015, 189, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Hu, Q.; Wang, J.; Chen, D.; Yang, Y.; Bridgwater, A.V. Kinetic study on the CO2 gasification of biochar derived from Miscanthus at different processing conditions. Energy 2021, 217, 119341. [Google Scholar] [CrossRef]

| Oven (°C) | 60 | Run Time (min) | 34.5 |
| Value (°C) | 70 | ||
| Injector | |||
| Temperature (°C) | 200 | ||
| Pressure (psi) | 50 | ||
| Detector | FID | TCD | |
| Temperature (°C) | 250 | Temperature (°C) | 200 |
| Air (mL/min) | 350 | Ref. (mL/min) | 20 |
| H2 (mL/min) | 40 | Make up (ml/min) | 25 |
| Make up (mL/min) | 5 | ||
| 400 | 500 | 600 | 700 | 600/1 | 600/0.25 | |
|---|---|---|---|---|---|---|
| H2 (vol.%) | 1.81 ± 0.08 | 1.13 ± 0.05 | 4.52 ± 0.12 | 4.92 ± 0.16 | 2.21 ± 0.10 | 1.93 ± 0.04 |
| CO (vol.%) | 27.66 ± 0.18 | 31.39 ± 0.11 | 23.22 ± 0.13 | 25.00 ± 0.20 | 26.27 ± 0.21 | 29.64 ± 0.19 |
| CO2 (vol.%) | 68.13 ± 0.77 | 59.88 ± 0.85 | 57.98 ± 0.74 | 56.49 ± 0.89 | 56.78 ± 0.70 | 56.70 ± 0.81 |
| CH4 (vol.%) | 1.99 ± 0.01 | 5.82 ± 0.01 | 11.62 ± 0.01 | 11.09 ± 0.16 | 12.01 ± 0.01 | 9.57 ± 0.01 |
| C2H4 (vol.%) | 0.15 ± 0.01 | 0.51 ± 0.02 | 0.53 ± 0.02 | 0.49 ± 0.03 | 0.52 ± 0.01 | 0.43 ± 0.02 |
| C2H6 (vol.%) | 0.17 ± 0.01 | 0.89 ± 0.07 | 1.48 ± 0.08 | 1.38 ± 0.14 | 1.56 ± 0.08 | 1.23 ± 0.11 |
| C3H6 (vol.%) | 0.04 ± 0.01 | 0.15 ± 0.01 | 0.24 ± 0.01 | 0.26 ± 0.01 | 0.24 ± 0.01 | 0.18 ± 0.01 |
| C3H8 (vol.%) | 0.05 ± 0.01 | 0.24 ± 0.01 | 0.41 ± 0.02 | 0.37 ± 0.01 | 0.40 ± 0.01 | 0.32 ± 0.01 |
| Condensate Compounds in Dichlormethane Extract | Peak Area/Total Area of All Peaks | Condensate Compounds in Diethyleether Extract | Peak Area/Total Area of All Peaks |
|---|---|---|---|
| 2-Propanone, 1-(acetyloxy)- | 10.58 | Acetic acid | 28.23 |
| Phenol, 2-methoxy- | 9.32 | 2-Propanone, 1-hydroxy- | 11.74 |
| Phenol | 8.80 | Phenol | 4.94 |
| 3-Furaldehyde | 6.75 | 2-Furanmethanol | 4.57 |
| Furfural | 5.86 | Hydroquinone | 4.44 |
| Benzofuran, 2,3-dihydro- | 5.73 | Propanoic acid | 3.80 |
| 1-Hydroxy-2-butanone | 5.10 | 1-Hydroxy-2-butanone | 3.61 |
| Phenol, 4-ethyl- | 4.86 | 2-Propanone, 1-(acetyloxy)- | 3.21 |
| Butyrolactone | 4.48 | Phenol, 2-methoxy- | 2.82 |
| 2-Furanmethanol | 3.89 | Furfural | 2.65 |
| Phenol, 2,6-dimethoxy- | 3.82 | 2,3-Butanedione | 2.63 |
| Propanoic acid, 2-methyl-, anhydride | 3.12 | Benzofuran, 2,3-dihydro- | 2.05 |
| Phenol, 4-ethyl-2-methoxy- | 3.08 | 1,2-Cyclopentanedione | 1.96 |
| 2-Methoxy-4-vinylphenol | 2.84 | Phenol, 4-ethyl- | 1.68 |
| Propanoic acid | 2.68 | Phenol, 2,6-dimethoxy- | 1.35 |
| 1,2-Cyclopentanedione, 3-methyl- | 2.33 | 1,2-Cyclopentanedione, 3-methyl- | 1.33 |
| Raw Miscanthus | Biochar 400 | Biochar 500 | Biochar 600 | Biochar 700 | Biochar 600/1 | Biochar 600/0.25 | |
|---|---|---|---|---|---|---|---|
| Proximate analysis (wt.%) | |||||||
| W | 8.53 ± 0.08 | 2.05 ± 0.02 | 1.84 ± 0.01 | 1.55 ± 0.01 | 1.53 ± 0.03 | 1.74 ± 0.00 | 1.85 ± 0.04 |
| VM d | 76.00 ± 1.05 | 22.82 ± 0.31 | 15.78 ± 0.24 | 9.92 ± 0.08 | 7.30 ± 0.07 | 11.07 ± 0.46 | 13.13 ± 0.42 |
| FC d | 20.58 ± 0.32 | 67.33 ± 0.28 | 74.04 ± 0.42 | 77.82 ± 0.13 | 78.71 ± 0.04 | 76.79 ± 0.73 | 76.50 ± 0.38 |
| A d | 3.42 ± 0.01 | 9.86 ± 0.01 | 10.17 ± 0.21 | 12.26 ± 0.04 | 13.98 ± 0.14 | 12.14 ± 0.27 | 10.38 ± 0.84 |
| Ultimate analysis (wt.%) | |||||||
| C d | 55.33 ± 0.29 | 74.73 ± 0.22 | 80.63 ± 0.32 | 81.42 ± 0.42 | 81.30 ± 0.06 | 80.90 ± 0.27 | 80.98 ± 0.47 |
| H d | 12.51 ± 0.21 | 4.41 ± 0.08 | 4.18 ± 0.05 | 2.61 ± 0.04 | 1.59 ± 0.01 | 2.84 ± 0.11 | 2.87 ± 0.03 |
| O d | 27.53 ± 0.23 | 8.58 ± 0.07 | 2.22 ± 0.04 | 1.32 ± 0.03 | 1.08 ± 0.01 | 1.68 ± 0.10 | 3.23 ± 0.02 |
| N d | 0.87 ± 0.10 | 1.57 ± 0.01 | 1.69 ± 0.01 | 1.58 ± 0.00 | 1.48 ± 0.01 | 1.59 ± 0.00 | 1.58 ± 0.02 |
| S d | 0.34 ± 0.00 | 0.85 ± 0.00 | 1.11 ± 0.00 | 0.81 ± 0.01 | 0.57 ± 0.00 | 0.85 ± 0.01 | 0.96 ± 0.02 |
| Atomic ratio | |||||||
| H/C | 2.71 ± 0.02 | 0.71 ± 0.01 | 0.62 ± 0.01 | 0.39 ± 0.01 | 0.24 ± 0.01 | 0.42 ± 0.01 | 0.43 ± 0.01 |
| O/C | 0.38 ± 0.00 | 0.09 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| Higher heating value (MJ/kg) | |||||||
| HHV d | 19.84 ± 0.01 | 30.00 ± 0.03 | 31.17 ± 0.02 | 30.37 ± 0.07 | 29.32 ± 0.03 | 30.70 ± 0.04 | 31.18 ± 0.10 |
| Sample | SBET (m2/g) | Smeso (m2/g) | Vmicro (mm3liq/g) | Vnet (mm3liq/g) |
|---|---|---|---|---|
| Miscanthus | 0.7 ± 0.01 | nd | nd | nd |
| 400 | 5 ± 0.01 | nd | nd | nd |
| 500 | 47 ± 0.08 | nd | nd | nd |
| 600 | 217 ± 6.21 | 22 ± 0.08 | 106 ± 4.03 | 132 ± 2.87 |
| 700 | 273 ± 4.24 | 18 ± 0.06 | 123 ± 1.89 | 146 ± 1.04 |
| 600/1 | 193 ± 0.28 | 19 ± 0.45 | 87 ± 1.14 | 113 ± 3.40 |
| 600/0.25 | 16 ± 0.02 | 6 ± 0.01 | 4 ± 0.01 | 10 ± 0.01 |
| Criteria (IBI) mg/kg Dry wt | 400 | 500 | 600 | 700 | 600/1 | 600/0.25 | |
|---|---|---|---|---|---|---|---|
| PAHs | 6–300 | 6.80 ± 0.02 | 17.40 ± 0.68 | 5.60 ± 0.13 | 4.10 ± 0.11 | 5.80 ± 0.08 | 5.00 ± 0.07 |
| Criteria (IBI) mg/kg Dry wt | Miscanthus | |
|---|---|---|
| As | 13–100 | <0.5 ± 0.00 |
| Cd | 1.4–39 | <0.4 ± 0.00 |
| Cr | 93–1200 | 9.57 ± 0.14 |
| Co | 34–100 | 1.26 ± 0.28 |
| Cu | 143–6000 | 10.6 ± 0.71 |
| Pb | 121–300 | 2.7 ± 0.08 |
| Hg | 1–17 | <0.2 ± 0.00 |
| Mo | 5–75 | 0.44 ± 0.02 |
| Ni | 47–420 | 7.5 ± 0.93 |
| Se | 2–200 | <2.0 ± 0.00 |
| Zn | 416–7400 | 102 ± 0.08 |
| 600/2 | |
|---|---|
| Total Ca (mg/kg) | 1910 ± 98.07 |
| Available Ca (mg/kg) | 1204 ± 37.44 |
| Total Mg (mg/kg) | 1190 ± 36.98 |
| Available Mg (mg/kg) | 272 ± 10.26 |
| Total K (mg/kg) | 25,400 ± 109.03 |
| Available K (mg/kg) | 7069 ± 13.91 |
| Total S (mg/kg) | 8100 ± 61.23 |
| Available SO42− (mg/kg) | 2558 ± 79.54 |
| Total N (mg/kg) | 15,800 ± 102.01 |
| Available NH3+ (mg/kg) | <2 ± 0.00 |
| Total P (mg/kg) | 2870 ± 75.08 |
| Available PO43− (mg/kg) | 1983 ± 26.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klemencova, K.; Grycova, B.; Lestinsky, P. Influence of Miscanthus Rhizome Pyrolysis Operating Conditions on Products Properties. Sustainability 2022, 14, 6193. https://doi.org/10.3390/su14106193
Klemencova K, Grycova B, Lestinsky P. Influence of Miscanthus Rhizome Pyrolysis Operating Conditions on Products Properties. Sustainability. 2022; 14(10):6193. https://doi.org/10.3390/su14106193
Chicago/Turabian StyleKlemencova, Katerina, Barbora Grycova, and Pavel Lestinsky. 2022. "Influence of Miscanthus Rhizome Pyrolysis Operating Conditions on Products Properties" Sustainability 14, no. 10: 6193. https://doi.org/10.3390/su14106193
APA StyleKlemencova, K., Grycova, B., & Lestinsky, P. (2022). Influence of Miscanthus Rhizome Pyrolysis Operating Conditions on Products Properties. Sustainability, 14(10), 6193. https://doi.org/10.3390/su14106193






