2.1. Water Harvesting from Fog
Fog is a common natural phenomenon defined as a cloud made up of abundant water droplets close to the ground, the diameters of which typically range from 1–30
m, reducing the visibility below one kilometer [
13,
14]. Fog can form in multiple processes, such as advection of moist over cold water bodies and the adiabatic cooling of moist water during uphill transport [
15]. Since oceans are filled with tremendous water content, fog can easily form near the coastline especially combined with mountainous areas.
Figure 1 marked these costal locations with plenty of foggy days to implement the fog harvesting technology. Even though fog water might contain marine salt or dust, it is expected to meet the drinking water standards after simple treatment [
14,
16]. Therefore, water harvesting from fog has been proposed as a feasible solution to relieving water shortage, especially in areas where fog is prevalent but rainfall is scarce, such as the fog harvesting projects achieved in Chile [
17].
The research on fog harvesting has been developed from traditional mesh designs to state-of-art collectors with bio-inspired materials to achieve the advanced functions and high performance. Initial fog harvesters were designed as simply as a single or double layers of vertical meshes equipped with a water tank, given the horizontal traveling pattern of fog in the atmosphere [
5,
18]. Materials such as polypropylene were used in practical applications at an early time. The standard fog collector (SFC) (
Figure 2a) was proposed, further developed and fabricated based on the specific environmental conditions, in order to relieve local water shortage [
19,
20,
21]. For example, Schemenauer [
19] reported one of the earliest SFCs with an average water collection rate of 1.26 L m
h
located in El Tofo, Chile. In the past two decades, the natural biological structures with advanced capability of fog capture, coalescence, and directional transportation, attracted researchers’ attention to spark new ideas and designs. For example, the coexistence of hydrophobic and hydrophilic surfaces on the back enables desert beetles to harvest fog water from the air [
10]. Inspired by the alternating pattern, Wang et al. [
22] reported a superhydrophobic-superhydrophilic hybrid (SSH) fabric coated by Co, Fe and n-octadecyl thiol (
Figure 2b). The fabric with a Co and Fe concentration ratio of 3:1 was tested to achieve the fog collection rate of 0.52 L m
h
under the laboratory conditions, while the water collection efficiency was only 0.16%. The special aligned structures of spines, barbs and trichomes on cactus sparked a new idea of directional water transportation [
11]. Cao et al. [
23] fabricated a cactus-inspired fog collector with the approximate diameter of 2 cm, by integrating cactus spine-like hydrophobic conical micro-tip arrays with the hydrophilic cotton matrix (
Figure 2c). At a normal fog velocity of 45–50 cm/s, one such cactus-inspired fog collector was able to harvest about 3 mL water per 10 min. Additionally, some advanced harvesters were inspired by spider silk with the special alternating structures of spindle-knots and joints (
Figure 2d), to achieve the directional water transportation [
12]. The artificial spider silk was fabricated to mimic the structure of wet rebuilt spider silk and proved to show its directional water collection capability.
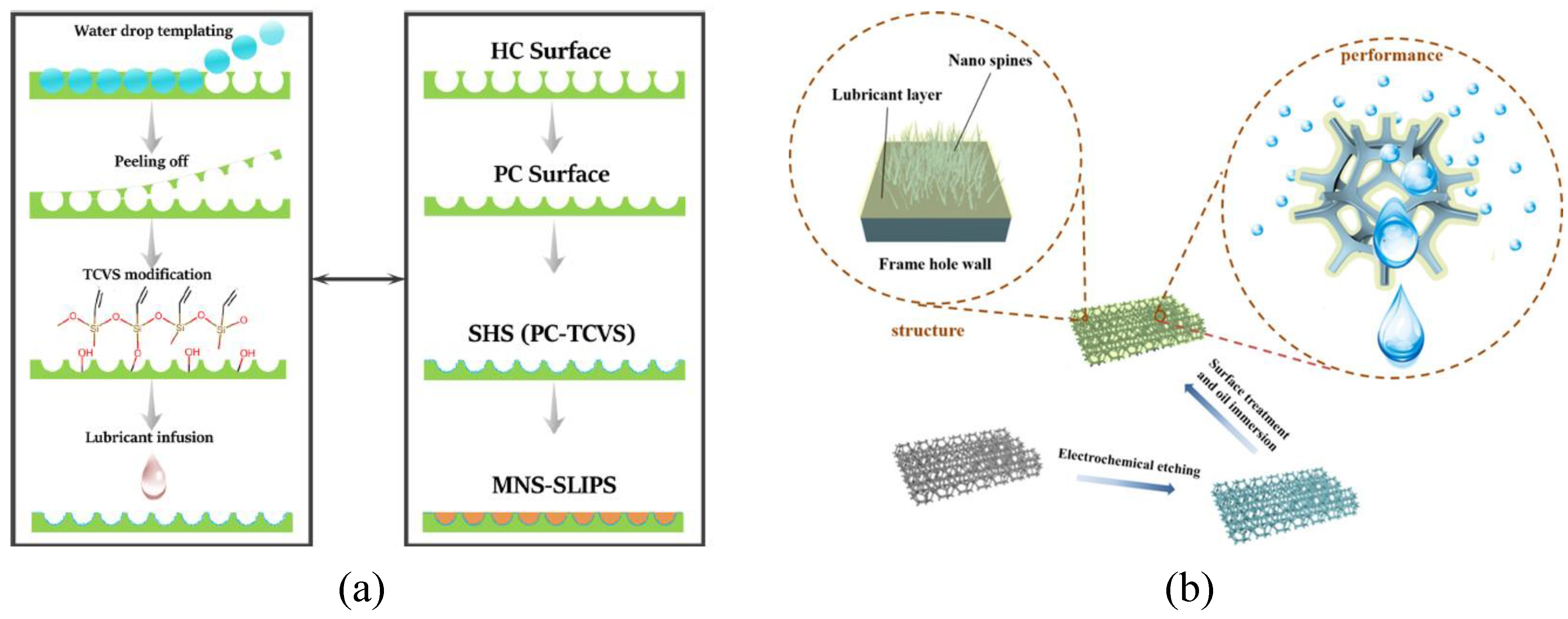
Recent research has developed more advanced bio-inspired materials in order to resolve problems in practical applications. A slippery liquid-infused porous surface (SLIPS) proposed by Wong et al. [
24] in 2011, achieved omniphobicity, high-pressure stability, and self-healing capability, compared with a traditional superhydrophobic surface (SHS). SLIPS is beneficial to water coalescence and transportation, raising the interest of groups of researchers. The latest research proposed several polished structures to improve the applicability of SLIPS. Feng et al. [
25] recently infused SHS with silicone oil to create a SLIPS with hierarchical micro-/nanostructures (MNS-SLIPS) shown in
Figure 3a. MNS-SLIPs successfully reduced lubricant loss and improved the water collection rate, up to 8.52 L m
h
, more efficient than the traditional design of SLIPS. Han and Guo [
26] fabricated a three-dimensional ball-cluster structure used as the substrate of lubricate-infused surface (
Figure 3b). Due to the inner 3D grid system and the supportive nano-structures, the new design was endowed with a good durability and achieved a water collection rate of approximately 45.18 L m
h
.
Previous research aiming at improving fog harvesting efficiency was mainly focused on the development of one- or two-dimensional materials. However, the 1D or 2D structures are not ideal enough within the real fog environment. Although fewer investigations have been conducted on 3D fog harvesting designs, the interest in this specific direction is rising. Early in 2011, Andrews et al. [
27] reported the 3D hierarchical structures of a plant,
Cotula fallax (
Figure 4a), and demonstrated that the compact structures greatly assisted in water harvesting from fog, where such 3D structures increased the surface-to-volume ratio and hence potentially improved the efficiency of water capture. The air plasma treatment changed the surface chemistry of the plant and further increased the water collection efficiency. Lee et al. [
28] recently reported a new cactus-inspired 3D fog harvester (
Figure 4b) integrated with a developed temperature-responsive material, poly-(N-isopropylacrylamide) (PNIPAAm). The PNIPAAm enabled the interchange of absorbing and releasing water via the interpenetrating polymer network (IPN) hydrogel, in response to the temperature change which has a potential application value in water management. In addition, the omnidirectional water capture of the design contributed to improving water capture rate, up to 2.09 L m
h
, higher than that of the superhydrophilic IPN hydrogel, superhydrophobic copper mesh (SHPM), and pristine copper mesh (PTM).
The integration of multiple bio-inspired features in the design and fabrication of materials or devices for fog harvesting has been hot topics. Inspired by Namib desert beetles and leaf veins, Yu et al. [
29] reported a dual-biomimetic knitted fabric (SHL-SHB@TF) fabricated by modern weaving technology (
Figure 5a). SHL–SHB@TF is featured for its excellent stretching, anti-aging, anti-bacterial, and water capture ability. Wang et al. [
30] successfully fabricated an integrated bio-inspired surface (IBS) by combining features of beetles, spider silk, cactus, and
Sarracenia (
Figure 5b), by an innovative capillary-induced selective oxidation method. To engage SLIPS in a complete fog harvesting process, Feng et al. [
31] developed a quadruple-biomimetic surface (SLIPS-SHIS) inspired by desert beetles, pitcher plants, cactus and
Nephrolepis auriculata (
Figure 5c). SLIPS-SHIS contained all four fog-harvest processes, including fog capture, coalescence, transportation, and collection; together, the synergetic functions of the upgraded processes improved the overall fog harvesting efficiency.
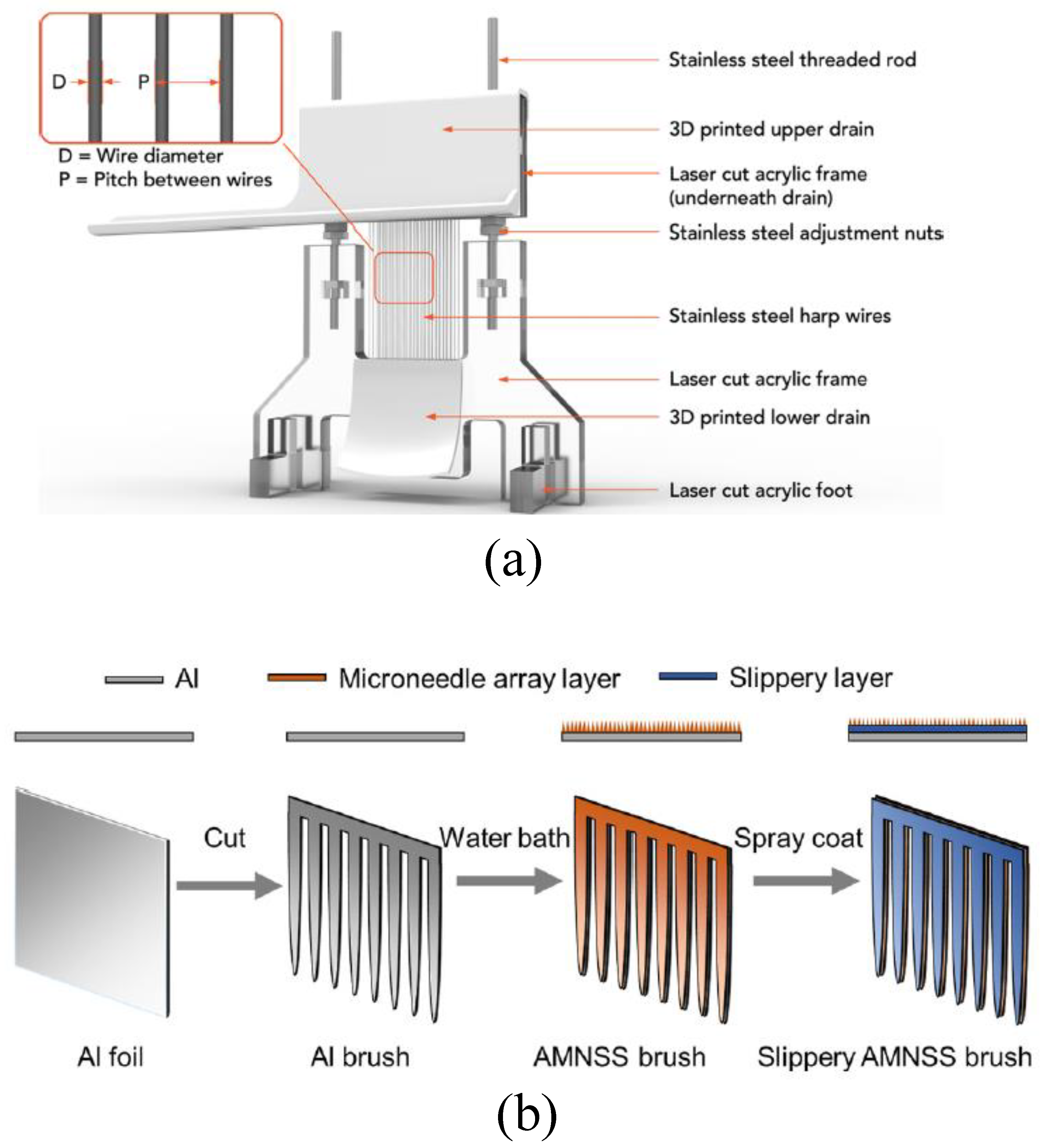
Although bio-inspired materials raised much attention regarding the subject of improving the fog-harvesting rate, inventions regarding the physical structure of fog harvesters remained a promising solution to difficulties induced by the application of new materials. Given the blockage issue of traditional net-like meshes, Shi et al. [
32] invented fog harps, comprised of vertical fibers (
Figure 6a), where the acrylic frames were cut by laser and the water drainage was fabricated by 3D-printing. In the lab testing, the fog harps with the stainless steel wires could harvest up to 3 times more water compared to the traditional mesh netting of the same size. Considering the similar shortcomings, Cheng et al. [
33] proposed an arc-shape fog catcher brushes (
Figure 6b) made from aluminum foil and coated with polydimethylhydrosiloxane (PMHS) to achieve a better performance of water collection. The arc-shape brushes with the unit pattern width of 3.2 mm had the best fog-harvesting rate of 4.45 L m
h
, in their study.
The development of fog harvesting techniques was rapid in the past few years. More advanced and feasible designs of new materials, novel structures, and innovative devices have been continuously investigated in order to improve collection efficiency. However, imperfections exist in fog harvesting technologies. Firstly, most of the work done by now were carried out in laboratory conditions while the real fog environment can be more complicated. Ultraviolet radiations from sunlight, temperature changes, air pollutants, extreme weather and other environmental factors are likely to affect the actual efficiency of current designs. Secondly, even though some novel designs showed a greater water capture efficiency, limitations of certain properties may become significant issues in practical applications. For example, the cactus-inspired 3D fog harvester reported by Lee et al. [
28] can work more than ten times in response to temperature change before it suffers from morphological deformation. Nevertheless, their durability outdoors may need investigating, given the cost of maintenance. Thirdly, apart from improving the quality of a single part of the fog harvesting process, investigations on the integration of components with different functions are necessary to achieve overall high efficiency. For example, a well-designed component for water capture may not fit into a device for efficient water transportation as a whole piece of water harvester. Fourthly, the quality of water from fog harvesting should be considered. Since fresh water is of high demand, the quality of harvested water is expected to have a criterion as important as efficiency when evaluating a new device in the future. Last but not least, beyond the current scope as it might be, the mass production of a prototype also needs more attention, and the possible points include economic manufacture technology and maintenance, the cost of raw materials and labor. Overall, fog harvesting technology is a promising solution to relieving water shortage in the regions where fog is plentiful but rain is rare, indispensable in the family of water harvesters.
2.2. Water Harvesting from Dew
Dew is defined as water droplets condensed from vapor in the air onto a surface whose temperature is lower than the dew point [
13]. The formation of dew normally goes through the two main steps: nucleation and growth [
34]. Heterogeneous nucleation (
Figure 7b), defined as condensation of water vapor on a substrate, can lower or even suppress the energy barriers compared with homogeneous nucleation (
Figure 7a) [
34]. Therefore, heterogeneous nucleation is more commonly observed than homogeneous nucleation in life. Given the widespread formation of dew [
35], more research on water harvesting from dew has been one of hot topics in the past few years, as an option to resolve global water shortage.
The traditional design of dew harvesting can date back to more than two thousands years ago when the artificial condensers—aerial wells were built [
37], attributed to the temperature difference between the voids on the stone walls and the incoming air [
38]. With regard to the formation conditions required for dew, rapid nucleation at a surface is the first and the essential step to achieve efficient dew harvesting. The first key factor influencing the nucleation rate is the temperature difference between the substrate (
) and the surrounding atmosphere (
) [
34]. Due to this characteristic of dew formation, the design idea of efficiently cooling down the surface below the dew point is proposed. Passive radiative cooling could be the most straightforward methodology that does not need high-technology or external energy input [
38]. The rationale of the technology is that the emitting infrared radiation from a surface at night tends to exceed the radiation absorption from the ambient environment, resulting in the lower surface temperature than that of the surroundings [
39]. When daytime comes and more water moisture enters the atmosphere, dew forms at the surface, with this idea motivating research on low emissivity materials [
6]. Alnaser and Barakat [
40] tested the dew collection efficiency of aluminum, glass and polyethylene foils, respectively, and reported that aluminum achieved the highest collection rate of 1.3 L m
h
among the three types of materials. As more interest was cast on the passive radiative cooling, different types of designs were quickly developed, i.e., film-based, nanoparticle-based, or photonic radiators, contributing to a more efficient cooling process [
41]. Given the low yield and their reliance on environmental conditions for passive radiative cooling, active condensation also attracted people’s attention, although external energy is normally input in the systems [
42]. The traditional design of active condensation is to keep the surface cool for a longer time than passive ways by integrating the surface with a cooling system. Apart from passive radiative cooling and active condensation, designs combining dew condensation with water extraction from the air were integrated to achieve a higher water harvesting efficiency [
6].
Surface property is another key factor that would impact the dew harvesting efficiency. Hydrophilic surfaces were proposed and designed as the condensation substrates in early studies. By testing the dew condensation ability on surfaces with the alternating hydrophobic and hydrophilic regions, Varanasi et al. [
43] found that water droplets would preferentially condense on the hydrophilic regions due to the lower energy barriers (
Figure 8). Lee et al. [
44] also reported that hydrophilic surfaces could condense approximately 30 mg more water per hour compared with moderately hydrophobic surfaces, whose surface areas were both 9 cm
. The observation was consistent with the dew harvesting behaviour of green tree frogs,
Litoria caerulea, living in northern Australia [
45]. Green tree frogs are living without the direct access to the nearby water resources because the hydrophilic skin cooled at night can nucleate water droplets when frogs come back to the warm humid tree hollows [
44]. Instead of drinking water, frogs can directly absorb condensed water by skin [
46]. Despite the remarkable capture ability at hydrophilic surfaces, they were still not the ideal design in the application of dew harvesting due to the low mobility of water droplets on such surfaces. Rapid removal of water droplets on the surface hence became the crucial step for continuous dropwise condensation. To solve this problem, superhydrophobic surfaces turned out to be a promising candidate [
47]. Inspired by lotus leaves, Chen et al. [
47] designed a two-tier surface which can remain superhydrophobic before and after dew condensation. The structure enabled water droplets to stand in a sustained Cassie’s state and have a higher mobility. As the nanotechnology developed, opportunities were provided for researchers to fabricate more micro/nanostructures to achieve the superhydrophobic property, which raised great interest in the past years [
48,
49]. However, even though the advent of superhydrophobic surfaces increased the overall dew collection rate via rapid water transportation compared with hydrophilic surfaces, the (super)hydrophobic surfaces generally generate a high energy barrier for nucleation which is unfavourable for capturing water vapour [
50]. Aware of the dilemma, Hou et al. [
50] developed biphilic structured surfaces composed of nanoscale hydrophilic bumps and superhydrophobic substrates inspired by desert beetles and lotus effect (
Figure 9a). When the water droplets were condensed and coalesced on the hydrophilic bumps, the droplets would be ejected by the superhydrophobic surface in time. The biphilic surface was reported to have 349% water collection rate and 184% heat transfer coefficient approximately as compared to the state-of-the-art superhydrophobic surface in a dry environment. In a recent research, Hou et al. [
51] designed a superhydrophilic/superhydrohobic hybrid surfaces by using laser ablation (
Figure 9b). With a spacing of 1.5 mm between triangles, the water collection rate of the hybrid surfaces was measured to be 1.107 ± 0.057 L m
h
, which was about 54% and 21% higher than that of superhydrophobic and superhydrophilic surfaces respectively, indicating the great potential of biphilic structured surfaces in increasing the dew harvesting efficiency.
Dew harvesting has the advantage over fog harvesting in that dew requires less atmospheric formation conditions. However, some issues in fog harvesting technologies discussed in the previous section happen to dew harvesting. In addition, there are some distinct drawbacks that make it even farther from practical application. Nioras et al. [
35] carried out both theoretical and experimental analysis on the water collection rate and efficiency summarized in
Table 1. Although the efficiency of dew harvesting is approximately two times as large as that of fog harvesting, the absolute water collection rate of dew harvesting is over one order of magnitude smaller. Increasing water collection rate of dew harvesters is hence the essential step currently. As reported by Hou et al. [
50], the combination of different wettability by using micro/nanotechnology has proved to be a feasible way to resolve this issue. Apart from improving the efficiency of a separate part, the integrated systems such as combining passive radiative cooling, active cooling or dew harvesting materials of high efficiency together may work as a promising direction and lead to unexpected positive synergetic effects.
2.3. Water Harvesting from Rain
Rain is probably the most well-known type of precipitation in our daily life. In meteorology, rain is defined as liquid water droplets with diameters ranging from 0.5 to 5 mm that fall from the atmosphere [
13]. In a global scale, the amount of precipitation varies from place to place. As shown in
Figure 10a, Central Africa, South America, Europe, East Asia and Eastern North America are rich of annual precipitation on average while North Africa, Central Asia, Western Asia and Central Australia experience the least rainfall throughout the year. Compared with the global map of water scarcity risks estimated by Gassert et al. [
3] (
Figure 10b), the overlapping areas of large precipitation and relatively high water scarcity risks such as East Asia indicates the great potential application of rainwater harvesting (RWH) in order to relieve local water shortage. In general, RWH technology can also be applied to other areas where water scarcity risks are high such as Africa even though the low annual precipitation may result in a relatively low yearly yield of collected rainwater.
Rainwater harvesting has a long history for domestic and agricultural purposes, but its unified definition has always been under discussion by the scientific community [
53]. Given the widespread classical structure of an RWH system, it is commonly accepted that rainwater harvesting is a process of collecting and storing rainwater for future use, e.g., irrigation, cooking, washing and drinking [
8]. The domestic rainwater harvesting (DRWH) in urban and rural areas is one of the most popular RWH technology all over the world. The most common DRWH structure includes the rooftops while other types of catchment are also available [
54]. Abdulla and Al-Shareef [
55] introduced the importance of rooftops for RWH set-up in Jordan, one of the most water-stressed semi-arid countries in the world. The typical RWH system used in Jordan was composed of a collection roof, a conveyance system and a storage tank (
Figure 11a). It was reported that a maximum of 15.5 Mm
every year of rainwater could be collected if all rooftop surfaces were used, which was equivalent to 5.6% of the total domestic water supply in 2005. The water quality of rooftop-collected water was also tested and was considered to be drinkable in a usual case. Melville-Shreeve et al. [
56] designed a similar set-up of rooftop rainwater harvesters used in UK. Based on the fundamental structures, more complicated innovative RWH systems were integrated in practical applications for a more intelligent control of rainwater supply.
Figure 11b demonstrated a real-time control system used in Australia, Korea and USA that enables the control of the storage volume of water in the tank based on the weather forecast data so that enough capacity can be available if a storm hits.
Rainwater harvesting technologies are also well adopted in agriculture such as irrigations. The proper design of an RWH system can greatly improve the rainwater-use efficiency and sustain the rainfed crops [
57]. Xiao et al. [
58] compared two types of micro-catchment rainwater harvesting systems combined with supplemental irrigation used in a semiarid region of China. According to the experimental data, the field cultivation of rainwater harvesting with a sowing in the furrow between film-covered ridges (SFFCR) (
Figure 12a) and with a sowing in the holes on film-covered ridges (SHFCR) (
Figure 12b) can save water at the efficiency of 5.5–5.8% and 9.4–9.6% respectively, which showed that the combination of water harvesting and supplemental irrigation can play a significant role in improving the crop yield and water use in this region. Apart from directly increasing the rainwater capture efficiency by soil and plants, storage of rainwater is also common in agriculture. Bruins et al. [
59] introduced an easy design of the liman terrace used in Kenya, a pond that can store runoff originated from rain (
Figure 12c). Interestingly, limans can also be built in a series and water will fill out limans one by one by transporting through the spillway when runoff is of a large amount. Aware of the shortcomings in traditional ponds, Pari et al. [
60] recently proposed an innovative flexible water storage system (FWSS) for macro-catchment (
Figure 12d). FWSS can be easily folded and moved, and no concrete base was needed for installation. FWSS turned out to have lower costs (€16.94/m
vs. €20.41/m
) and lower environmental impacts (17.04 g per m
CO
vs. 28.2 g per m
CO
) than traditional ponds, for which FWSS can be a good alternative component of the rainwater harvesting system in agriculture.
The recent research on rainwater harvesting starts to develop a more integrated method and technology in order to further improve the quality of water and the efficiency of enlarging the scale of rainwater harvesting. Given that most research on RWH was focused on non-potable usage, Alim et al. [
61] developed an integrated rainwater harvesting unit (IRWHU) (
Figure 13a). IRWHU adopted classical rooftop rainwater harvesting system while combined with a filtration unit that could generate drinkable water, capable of producing drinking water at a capacity of 348 ± 20 L/day. In China, Sponge City Concept (SCC) is proposed to mimic the natural hydrological cycle to absorb, infiltrate, store, purify and manage the rainwater. It was planned by the State Council that 20% of urban cities in China should reuse 80% of the rainwater by 2030. Zevenbergen et al. [
62] pointed out that SCC is operated widely all over the conutry (
Figure 13b). High technology is also integrated into the harvesting systems for a more accurate control of rainwater collection. Adham et al. [
63] developed a GIS-based approach to identifying potential sites for rainwater harvesting in Iraq by considering five biophysical factors. The suitability map of potential rainwater harvesting sites (
Figure 13c) provided a valuable guideline to optimize the efficiency of RWH technology.
Compared with fog and dew harvesting, the concept of rainwater harvesters is more intuitive given the way of collecting and storage. The simpler design of rainwater harvesting renders this topic more popular in a local scale, and rainfall will primarily determine the yearly yields on average, which makes the comparison of different designs difficult in a global scale. However, even though rainfall and atmospheric conditions vary from place to place, improving the efficiency of rainwater harvesting by learning from the experience from all projects is still valuable while it is important to stay active to modify the design based on the local ecological environment. In addition, the practical applications of rainwater harvesting technology in more places is effective in increasing the overall yield of collected rainwater globally. One key point to achieve this goal is to lower the cost, including materials and labour. Last but not least, collected rainwater still needs purification process for drinking and cooking. With the higher quality, the water may be of greater value in more application fields.
2.4. Water Desalination Technology
Earth is usually called a blue planet because approximately 71% of the surface is covered by ocean. However, only a small proportion of the water on Earth can be directly used by human beings. Shiklamonov [
2] reported that only 2.5% of total global water is fresh water, and the rest is not directly accessed mainly due to the high salinity (
Figure 14). Given the rapid consumption of fresh water and that fresh water becomes increasingly brackish, desalination, a process of removing salts and other particles from seawater or brackish water, attracts people’s attention as a feasible technology to alleviate water shortage [
4]. The major desalination market is along the coastal areas, such as the Gulf Region and the Mediterranean Region, and has been extended to America and Asia [
64]. Desalination technology is one of the most well-developed topics in water harvesting during the past decades. The dominant desalination technology includes thermal and membrane-based desalination while many other methods such as chemical-based or absorption desalination are also under investigation [
7].
Thermal desalination typically requires input of thermal energy to heat or evaporate seawater for the purpose of separating water and salts. One of the most popular thermal desalination technology is multi-stage flash (MSF) [
66] (
Figure 15a). Seawater input into the system is heated to 90–110 °C and travels through different stages in which water evaporates from seawater, condenses on the pipe of preheated flowing seawater and is collected as fresh water. There are normally 4 to 40 stages in an MSF unit and 10,000–35,000 m
3 of fresh water can be produced per day by one unit. Another traditional thermal desalination technology is multi-effect distillation (MED) [
66] (
Figure 15b), typically with 2–16 stages. Seawater input is sprayed on horizontal tubes filled with steam, then evaporates and moves into the horizontal tubes of the next stage, collected as fresh water. Meanwhile, the hot water vapor in the horizontal tubes also evaporates seawater spray in this stage, and the whole system is driven by decreasing pressure in different stages. Depending on the length of series, one MED unit could produce fresh water at the capacity of 600–30,000 m
3/day.
Given the high energy consumption of the traditional thermal desalination, the renewable energy has raised people’s attention [
67]. Solar-thermal desalination is one of clean energy applications, and can be either in direct or indirect way. Indirect solar-thermal desalination is converting solar energy into thermal or electrical energy for driving the traditional thermal desalination systems such as MSF and MED. Direct solar-thermal desalination absorbs the energy from sun for distillation but more suitable for small production. Recently, Lei et al. [
68] reported an MXene-decorated 3D honeycomb-fabric design for direct solar-thermal desalination (
Figure 15c). Bulk seawater was drawn up through 1D hydrophilic fibrous rods to the patterned surface, and the water can evaporate efficiently by solar energy due to the high light absorbency of MXene and light-trapping ability of honeycomb-fabric structures. The MXene-decorated 3D honeycomb-fabric design demonstrated a high solar efficiency of 93.5% and the evaporation rate of 1.62 kg m
−2 h
−1. Membrane distillation (MD) (
Figure 15d) is also a promising thermally-driven separation method combined with the renewable energy [
69,
70]. MD is driven by thermally-induced pressure difference between the two sides of the hydrophobic microfiltration membrane which only allows gas (e.g., water vapor) to permeate. Although MD may consume more than ten times of thermal energy than MSF or MED, the low-grade operational temperature made it more compatible to the renewable energy. Other thermal desalination technologies such as compression distillation (MVC), humidification-dehumidification desalination (HDH), solar distillation (SD) have also been gradually developed, for a better performance of thermal desalination [
7].
Membrane-based desalination is the dominant desalination technology all over the world (
Figure 16), and the main subtypes include reverse osmosis (RO), forward osmosis (FO) and electrodialysis (ED) [
71]. RO is the most widespread desalination technology and shares more than half of the total capital of the desalination market as shown in
Figure 16 [
71]. RO produces fresh water by motivating permeation of pure water through semi-permeable membrane under pressure difference, and the pressure on the feed side commonly ranges from 55–68 bar [
64] (
Figure 17a). Compared with other desalination technology, RO has the advantage of saving energy and is typically considered to be the most energy-efficient desalination technology so far [
72]. ED is a common desalination technology driven by electrical energy, typically composed of anion and cation exchange membranes between which is a spacer gasket [
73]. Two electrodes are semi-separated by the membranes. When voltage is applied on the electrodes, reduction and oxidation will occur on the cathode and anode respectively. When seawater enters the ED stack, cations such as Na
will move towards the cathode and anions such as Cl
will move towards anode through membranes, leaving fresh water in the stack. The microbial desalination cell (MDC) (
Figure 17b) is a novel ED design utilizing microorganisms to convert organic compounds into electricity [
74], considered as an eco-friendly and sustainable technology by producing and using bioenergy. FO also evolves membrane-based desalination technology, which draws water from low salinity to high salinity through semi-permeable membranes and involves two main steps: the osmotic dilution of draw solution and extraction of fresh water from diluted draw solution [
75]. FO is usually combined with other desalination technology to achieve a more energy-efficient performance. Yuan et al. [
76] reported a hybrid system of MDC and FO (
Figure 17c) in 2015, showing that MDC-FO had a better performance in removing organic concentration and desalination than standalone MDC, resulting in a lower wastewater volume by 64%.
Apart from thermal and membrane-based desalination technologies which are dominant in the current market, research on other technologies is also on the way. Recently, Guo et al. [
77] reported the [emim][Tf2N] ionic liquid, better than the state-of-the-art directional solvent (decanoic acid) in directional solvent extraction (DSE), a developing non-membrane desalination technology (
Figure 18a). DSE uses the directional solvent, which can not dissolve in water but can absorb water and reject salts, to separate water from seawater in response to different conditions such as temperature. It was demonstrated that [emim][Tf2N] can yield more than ten times of water than the current decanoic acid. Zhang et al. [
78] compared the efficiency of two LiCl supported composite sorbents in adsorption desalination (AD) technology. AD is typically composed of an evaporator, a reactor and a condenser (
Figure 18b). Seawater evaporates in the evaporator by heating and temporarily is absorbed by dry sorbents in the reactor. By using solar energy or waste heat, water can be released from the sorbents, enter the condenser and condense as fresh water. AD attracts people’s attention because of several advantages such as its lower corrosion and fouling on tube materials. The predicted specific daily water production (SDWP) of LiCl@SG
30 and LiCl@EVM
45 showed that AD can be a promising desalination technology, and the SDWP of LiCl@SG
30 ranges from 43 to 60 m
/(tonne day).
Desalination has been systematically studied in the past few decades, and many technologies have emerged, such as RO, MSF and MED, widely used in practical applications. Improving the performance and reducing the energy required for these technologies may deserve more investigation, but development of combined solar energy collection systems, integrated to current desalination technologies, can play a more significant role. In addition, more types of renewable energy such as wind can also be alternative energy sources, taken into considerations for future designs. Last but not least, the modification on the mature desalination technologies for the application of a wider scale such as desalination of continental brackish water deserves more research.
2.5. Extracting Water from Atmosphere
Approximate 12,900 km
of water is stored in the atmosphere [
2], especially in the form of water vapor. Aware of the increasing demand of fresh water, scientists started to conceive simple and practical methods to extract water directly from air [
79], even in the desert. The idea attracted much attention and sorbent-based water harvesting technology became the popular topic for atmospheric water extraction [
80]. The development of sorbents with high water sorption ability, low regeneration cost and fine hydrolytic stability is one of the main directions of research on atmospheric water extraction.
Generally, sorbents can be categorized into absorbents and adsorbents [
80]. Absorbents are substances that take up water into their bulk phase [
80]. Elashmawy [
9] designed a tubular solar still (TSS) using CaCl
as the absorbent (
Figure 19a), where TSS absorbed water at night and went through regeneration process via solar energy at daytime during which evaporation of water from the absorbent happened. Five separate stills were tested under different wind speeds, and the results showed that the module at 4 m/s air speed had the maximum evaporation and efficiency, 467 mL/m
per day and 25%, respectively. Adsorbents are substances with porous structures whose inner surfaces can bind water molecules [
80]. Essa et al. [
81] designed a double-slope half-cylindrical basin solar still (DS-HCBSS) using silica gel, one of the most popular adsorbents (
Figure 19b). In this design, the silica gel was spread on the still with a thickness of 1.5 cm and a parabolic trough solar collector was used to enhance the distillation of water in the morning. As a result, at most 400 mL/m
of water per day, can be produced by the DS-HCBSS with silica gel, longitudinal fins, and gravels.
Metal organic frameworks (MOFs) are a unique class of adsorbent, which have the great potential applications in water extraction [
80]. MOFs are composed of inorganic cores linked by organic linkers, and the porous structures allow uptake of water molecules. The development of optimal chemical and physical structures of MOFs for water harvesting has become an emerging research topic. Furukawa et al. [
82] systematically compared the water adsorption properties of zeolite 13X, BPL carbon, MCM-41 and 20 types of MOFs. The results revealed that MOF-801 and MOF-841 (
Figure 20a,b), two Zirconium MOFs, achieved the highest water adsorption performance. Specifically, MOF-801-P realized a water uptake capacity of 280 cm
/g at the relative humidity
and MOF-841 could take up water at the capacity of 550 cm
/g when the relative humidity reached
. Due to the excellent water-extraction ability from air, water harvesters based on MOFs materials were further invented. Fathieh et al. [
83] fabricated a MOF-based water harvester which went through one adsorption/desorption cycle per day (
Figure 20c). The instrument shared the similar idea with TSS and DS-HCBSS while MOFs would not corrode apparatus due to deliquescence such as hygroscopic salts and possess good hydrothermal stability. To enhance the thermalphysical and adsorptive properties of MOF-801, 33
of porous graphite was blended together with MOF-801, and the results demonstrated that 100 g/day of water can be produced by 1 kg MOF-801 in the desert of Arizona USA, illustrating the value of MOF-based water harvester in practical applications. In addition, a newly designed aluminum-based MOF-303 showed around 64.5% higher water production capability in the laboratory conditions than MOF-801. Recently, Hanikel [
84] reported a multi-cycle MOF-based water harvester by using photovoltaic module to heat the MOF mildly, achieving multiple adsorption/desorption cycles during day and night (
Figure 20d). MOF-303 and three other hydrophilic materials were tested, where MOF-303 even showed better water extracting performance than others, with 1 kg MOF-303 could produce 0.7 L/day of water in Mojave Desert, more efficient than the reported one-cycle MOF-based water harvester.
Water extraction from atmosphere is an ideal technology to obtain fresh water, for air is ubiquitous everywhere. If the technology is developed well, it is striking to produce water in the deserts. However, there exist some issues in current water extraction systems [
80]. One key challenge is that the water productivity is still too low to meet the basic demand of water consumption for both active and passive routes. In addition, the current designs of sorbent-based water harvesters are in small scales, far from industrial mass production and practical applications in life. Moreover, the durability of sorbents needs to be largely improved for a sustainable purpose. Although many challenges remain in water extraction technology, especially the applications of MOFs in this scenario, the high performance of new types of adsorbents indicates a bright future of achieving direct water extraction from air in real life.