Characteristics of Hydro-Geochemistry and Groundwater Pollution in Songnen Plain in Northeastern China
Abstract
1. Introduction
2. Materials and Methods
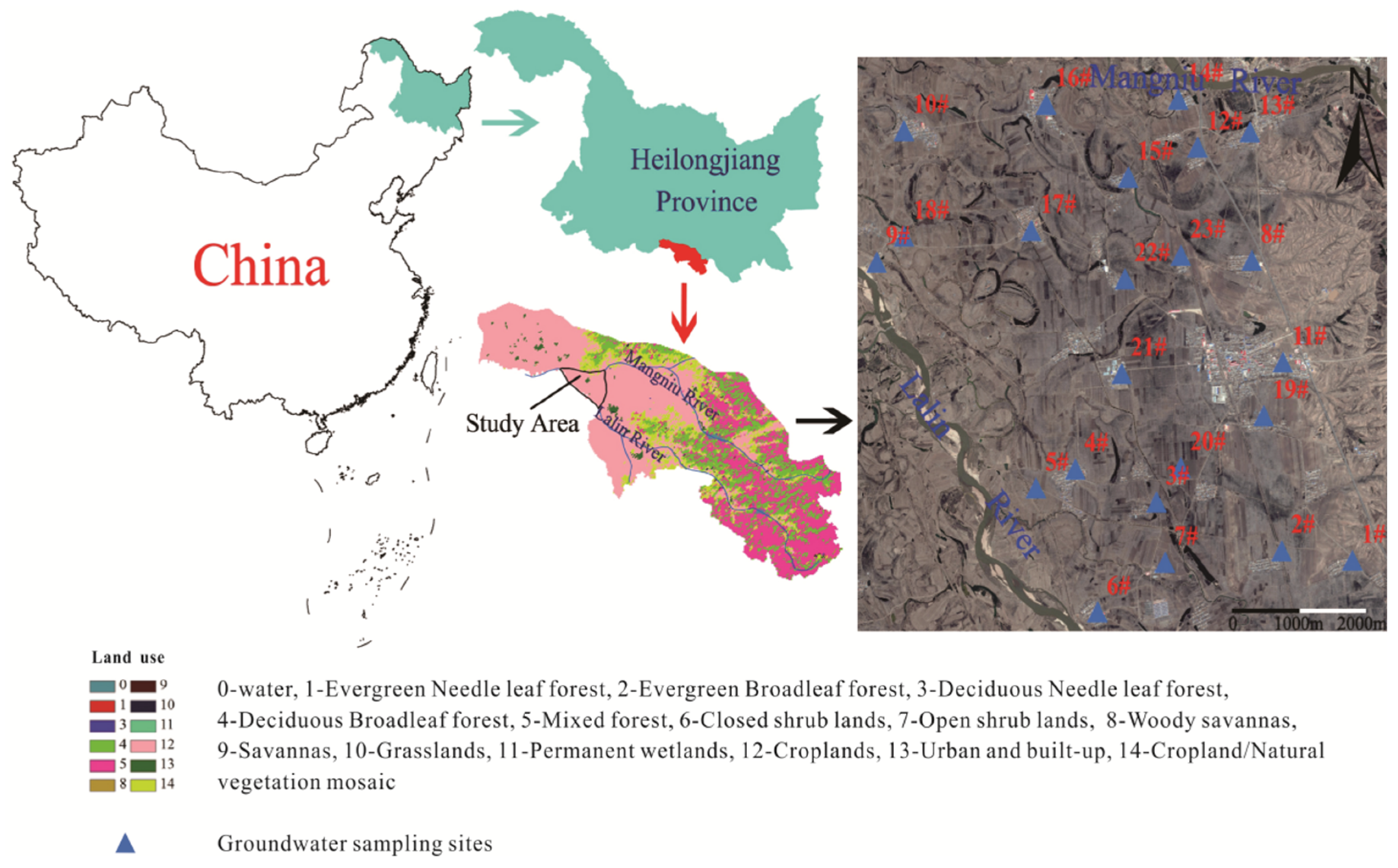
2.1. Description of Study Area
2.2. Sampling Collection and Analysis
2.3. Chemical Analyses
2.4. Groundwater Quality Assessment Method
2.4.1. Comprehensive Evaluation Method
2.4.2. Improved Fuzzy Mathematical Method
2.4.3. Gray Correlation Method
3. Results
3.1. Hydrogeochemical Characteristics
3.1.1. Geochemical Characteristics of Major Ions
3.1.2. Spatial Distribution of Major Ions
3.2. Source of Major Components
3.2.1. Dissolution of Minerals Analysis
3.2.2. Water–Rock Interaction and Correlation Analysis
4. Discussion
4.1. Groundwater Pollution Assessment
4.2. Groundwater Quality Priority Control and Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Xiao, C.; Liang, X.; Wei, H. Response of groundwater chemical characteristics to land use types and health risk assessment of nitrate in semi-arid areas: A case study of Shuangliao City, Northeast China. Ecotoxicol. Environ. Saf. 2022, 236, 113473. [Google Scholar] [CrossRef]
- Ma, J.; Ding, Z.; Wei, G.; Zhao, H.; Huang, T. Sources of water pollution and evolution of water quality in the Wuwei basin of Shiyang river, Northwest China. J. Environ. Manag. 2009, 90, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, Z.H.; Xi, B.D.; He, X.S.; Li, Q.L.; Qi, Y.J.; Jin, M.Y.; Guo, Y. Characteristics of groundwater pollution in a vegetable cultivation area of typical facility agriculture in a developed city. Ecol. Indic. 2019, 105, 709–716. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, Z.; Liu, Y. An overview of in-situ remediation for nitrate in groundwater. Sci. Total Environ. 2022, 804, 149981. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, D.; Xu, H.; Ding, Z.; Shi, Y.; Lu, Z.; Cheng, Z. Groundwater pollution risk assessment based on groundwater vulnerability and pollution load on an isolated island. Chemosphere 2022, 289, 133134. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Lei, Y.; Zhou, J.; Li, M.; Wang, J.; Teng, Y. The spatial and seasonal variability of the groundwater chemistry and quality in the exploited aquifer in the Daxing District, Beijing, China. Environ. Monit. Assess. 2015, 187, 43. [Google Scholar] [CrossRef]
- Zhang, B.; Song, X.; Zhang, Y.; Han, D.; Tang, C.; Yu, Y.; Ma, Y. Hydrochemical characteristics and water quality assessment of surface water and groundwater in Songnen plain, Northeast China. Water Res. 2012, 46, 2737–2748. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, S.; Li, H.; Fu, K.; Xu, Y. Groundwater pollution source identification and apportionment using PMF and PCA-APCA-MLR receptor models in a typical mixed land-use area in Southwestern China. Sci. Total Environ. 2020, 741, 140383. [Google Scholar] [CrossRef]
- Böhlke, J.-K. Groundwater recharge and agricultural contamination. Hydrogeol. J. 2002, 10, 153–179. [Google Scholar] [CrossRef]
- Dhimmar, J.B.; Singh, S.S. Assessment of groundwater quality and it’s suitability for drinking and agriculture uses. Int. J. Adv. Res. 2014, 2, 77–87. [Google Scholar]
- Adimalla, N.; Qian, H.; Nandan, M.J. Groundwater chemistry integrating the pollution index of groundwater and evaluation of potential human health risk: A case study from hard rock terrain of south India. Ecotoxicol. Environ. Saf. 2020, 206, 111217. [Google Scholar] [CrossRef] [PubMed]
- El Alfy, M.; Lashin, A.; Abdalla, F.; Al-Bassam, A. Assessing the hydrogeochemical processes affecting groundwater pollution in arid areas using an integration of geochemical equilibrium and multivariate statistical techniques. Environ. Pollut. 2017, 229, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chi, B.; Li, H.; Jiang, J.; Qin, W.; Wang, H. Assessment of groundwater quality and identification of contaminant sources of Liujiang basin in Qinhuangdao, North China. Environ. Earth Sci. 2015, 73, 6477–6493. [Google Scholar] [CrossRef]
- Alaa, A.; Masoud, A.A. Groundwater quality assessment of the shallow aquifers west of the Nile Delta (Egypt) using multivariate statistical and geostatistical techniques. J. Afr. Earth Sci. 2014, 95, 123–137. [Google Scholar]
- Arumugam, K.; Elangovan, K. Hydrochemical characteristics and groundwater quality assessment in Tirupur Region, Coimbatore District, Tamil Nadu, India. Environ. Earth Sci. 2009, 58, 1509. [Google Scholar] [CrossRef]
- Augustsson, A.; Söderberg, T.U.; Fröberg, M.; Kleja, D.B.; Åström, M.; Svensson, P.A.; Jarsjö, J. Failure of generic risk assessment model framework to predict groundwater pollution risk at hundreds of metal contaminated sites: Implications for research needs. Environ. Res. 2020, 185, 109252. [Google Scholar] [CrossRef] [PubMed]
- Haghnazar, H.; Johannesson, K.H.; González-Pinzón, R.; Pourakbar, M.; Aghayani, E.; Rajabi, A.; Hashemi, A.A. Groundwater geochemistry, quality, and pollution of the largest lake basin in the Middle East: Comparison of PMF and PCA-MLR receptor models and application of the source-oriented HHRA approach. Chemosphere 2022, 288, 132489. [Google Scholar] [CrossRef]
- Moussa, A.B.; Mzali, H.; Zouari, K.; Hezzi, H. Hydrochemical and isotopic assessment of groundwater quality in the Quaternary shallow aquifer, Tazoghrane region, north-eastern Tunisia. Quat. Int. 2014, 338, 51–58. [Google Scholar] [CrossRef]
- Nagaraju, A.; Sreedhar, Y.; Kumar, K.S.; Thejasw, A.; Sharifi, Z. Assessment of groundwater quality and evolution of hydrochemical facies around Tummalapalle Area, Cuddapah District, Andhra Pradesh, South India. Environ. Anal. Chem. 2014, 1, 1–6. [Google Scholar]
- Liu, L.; Zhou, J.; An, X.; Zhang, Y.; Yang, L. Using fuzzy theory and information entropy for water quality assessment in Three Gorges region, China. Expert Syst. Appl. 2010, 37, 2517–2521. [Google Scholar] [CrossRef]
- Nazzal, Y.; Ahmed, I.; Al-Arifi, N.; Ghrefat, H.; Zaidi, F.K.; El-Waheidi, M.M.; Batayneh, A.; Zumlot, T. A pragmatic approach to study the groundwater quality suitability for domestic and agricultural usage, Saq aquifer, northwest of Saudi Arabia. Environ. Monit. Assess. 2014, 186, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.R.; Bhandary, N.P. Evaluation of groundwater vulnerability to nitrate in shallow aquifer using multi-layer fuzzy inference system within GIS environment. Groundw. Sustain. Dev. 2020, 11, 100470. [Google Scholar] [CrossRef]
- Purushotham, D.; Prakash, M.R.; Rao, A.N. Groundwater depletion and quality deterioration due to environmental impacts in Maheshwaram watershed of R.R. district, AP (India). Environ. Earth Sci. 2011, 62, 1707–1721. [Google Scholar] [CrossRef]
- Jasmin, I.; Mallikarjuna, P. Physicochemical quality evaluation of groundwater and development of drinking water quality index for Araniar River Basin, Tamil Nadu, India. Environ. Monit. Assess. 2014, 186, 935–948. [Google Scholar] [CrossRef]
- Li, L.; Zou, S. Comparison of comprehensive index method and fuzzy comprehensive method in the evaluation of groundwater quality: A case study in Zunyi City. Carsologica Sin. 2014, 33, 22–30. [Google Scholar]
- Rodriguez-Galiano, V.F.; Luque-Espinar, J.A.; Chica-Olmo, M.; Mendes, M.P. Feature selection approaches for predictive modelling of groundwater nitrate pollution: An evaluation of filters, embedded and wrapper methods. Sci. Total Environ. 2018, 624, 661–672. [Google Scholar] [CrossRef]
- Schot Paul, P.; Pieber Simone, M. Spatial and temporal variations in shallow wetland groundwater quality. J. Hydrol. 2012, 422–423, 43–52. [Google Scholar] [CrossRef]
- Sege, J.; Ghanem, M.; Ahmad, W.; Bader, H.; Rubin, Y. Distributed data collection and web-based integration for more efficient and informative groundwater pollution risk assessment. Environ. Model. Softw. 2018, 100, 278–290. [Google Scholar] [CrossRef]
- Huan, H.; Hu, L.; Yang, Y.; Jia, Y.; Lian, X.; Ma, X.; Xi, B. Groundwater nitrate pollution risk assessment of the groundwater source field based on the integrated numerical simulations in the unsaturated zone and saturated aquifer. Environ. Int. 2020, 137, 105532. [Google Scholar] [CrossRef]
- Jankowski, J.; Acworth, R.I.; Shekarforoush, S. Reverse ion exchange in deeply weathered porphyritic dacite fractured aquifer system, Yass, New South Wales, Australia. In Water-Rock Interaction, Proceedings of the 9th International Symposium on Water-Rock Interaction, Taupo, New Zealand, 30 March–3 April 1998; Arehart, G.B., Hulston, J.R., Eds.; AA Balkema: Rotterdam, The Netherlands, 1998; pp. 243–246. [Google Scholar]
- Rajendra Prasad, D.S.; Sadashivaiah, C.; Rangnna, G. Hydrochemical characteristics and evaluation of groundwater quality of Tumkur Amanikere lake Watershed, Karnataka. India. E-J. Chem. 2009, 6 (Suppl. S1), S211–S218. [Google Scholar] [CrossRef][Green Version]
- Ramesh, S.; Sukumaran, N.; Murugesan, A.; Rajan, M. An innovative approach of Drinking Water Quality Index—A case study from Southern Tamil Nadu, India. Ecol. Indic. 2010, 10, 857–868. [Google Scholar] [CrossRef]
- Ranjan, R.K.; Ramanathan, A.L.; Parthasarathy, P.; Kumar, A. Hydrochemical characteristics of groundwater in the plains of Phalgu River in Gaya, Bihar, India. Arab. J. Geosci. 2013, 6, 3257–3267. [Google Scholar] [CrossRef]
- Sharif, M.; Davis, R.; Steele, K.; Kim, B.; Kresse, T.; Fazio, J. Inverse geochemical modeling of groundwater evolution with emphasis on arsenic in the Mississippi River Valley alluvial aquifer, Arkansas (USA). J. Hydrol. 2008, 350, 41–55. [Google Scholar] [CrossRef]
- Singh, P.; Tiwari, A.; Singh, P.K. Assessment of groundwater quality of Ranchi township area, Jharkhand, India by using Water Quality Index method. Int. J. Chem. Tech. Res. 2015, 7, 73–79. [Google Scholar]
- Zhang, Q.; Li, P.; Lyu, Q.; Ren, X.; He, S. Groundwater contamination risk assessment using a modified DRATICL model and pollution loading: A case study in the Guanzhong Basin of China. Chemosphere 2022, 291, 132695. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, C.; Sun, X. Analysis and evaluation of groundwater quality of Qijia reserve water source of Changchun city, Jilin provice. Jilin Geol. 2014, 33, 98–102. [Google Scholar]
- Sitambuk-Giljanovic, N. Water quality evaluation by index in Dalmatia. Water Res. 1999, 33, 3423–3440. [Google Scholar] [CrossRef]
- Subba Rao, N.; Dinakar, A.; Sun, L. Estimation of groundwater pollution levels and specific ionic sources in the groundwater, using a comprehensive approach of geochemical ratios, pollution index of groundwater, unmix model and land use/land cover—A case study. J. Contam. Hydrol. 2022, 248, 103990. [Google Scholar] [CrossRef]
- Wu, W.; Liao, R.; Hu, Y.; Wang, H.; Liu, H.; Yin, S. Quantitative assessment of groundwater pollution risk in reclaimed water irrigation areas of northern China. Environ. Pollut. 2020, 261, 114173. [Google Scholar] [CrossRef]
- Yu, L.; Zheng, T.; Zheng, X.; Hao, Y.; Yuan, R. Nitrate source apportionment in groundwater using Bayesian isotope mixing model based on nitrogen isotope fractionation. Sci. Total Environ. 2020, 718, 137242. [Google Scholar] [CrossRef]
- Zghibi, A.; Merzougui, A.; Zouhri, L.; Tarhouni, J. Understanding groundwater chemistry using multivariate statistics techniques to the study of contamination in the Korba unconfined aquifer system of Cap-Bon (North-east of Tunisia). J. Afr. Earth Sci. 2014, 89, 1–15. [Google Scholar] [CrossRef]





| No. | SI of Minerals | Minimum | Average | Maximum | Standard Deviation | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2014 | 2000 | 2014 | 2000 | 2014 | 2000 | 2014 | ||
| 1 | Calcite | −2.65 | −1.90 | −0.95 | −0.78 | 0.87 | 0.46 | 1.03 | 0.65 |
| 2 | Dolomite | −5.58 | −4.18 | −2.26 | −2.08 | 1.24 | 0.46 | 2.00 | 1.26 |
| 3 | Gypsum | −3.38 | −3.16 | −2.46 | −2.07 | −1.41 | −1.22 | 0.54 | 0.50 |
| 4 | Halite | −9.09 | −8.74 | −7.93 | −7.55 | −6.31 | −5.88 | 0.77 | 0.74 |
| 5 | Aragonite | −2.80 | −2.06 | −1.10 | −0.94 | 0.72 | 0.31 | 1.03 | 0.65 |
| Site | Affiliation | A * | B * | C * | D * | E * | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||||||
| 1 | 0.394 | 0.333 | 0.218 | 0.058 | 0 | 0.394 | IV | 3 | III | 3.549 |
| 2 | 0.579 | 0.226 | 0.067 | 0.018 | 0.112 | 0.579 | III | 3 | II | 3.016 |
| 3 | 0.188 | 0.296 | 0.288 | 0.121 | 0.107 | 0.296 | III | 3 | III | 3.608 |
| 4 | 0 | 0.202 | 0.091 | 0.205 | 0.507 | 0.507 | V | 4 | IV | 3.902 |
| 5 | 0 | 0 | 0.333 | 0.455 | 0.212 | 0.455 | III | 3 | III | 2.678 |
| 6 | 0.012 | 0.496 | 0.123 | 0.369 | 0 | 0.496 | III | 3 | III | 3.142 |
| 7 | 0 | 0.268 | 0.396 | 0.082 | 0.254 | 0.396 | II | 3 | III | 3.401 |
| 8 | 0.089 | 0.471 | 0 | 0.219 | 0.621 | 0.221 | II | 3 | III | 2.223 |
| 9 | 0.109 | 0 | 0 | 0.672 | 0.219 | 0.219 | I | 2 | III | 2.759 |
| 10 | 0.315 | 0.175 | 0.302 | 0.088 | 0.195 | 0.315 | III | 3 | IV | 3.402 |
| 11 | 0.241 | 0.351 | 0.074 | 0 | 0.335 | 0.335 | IV | 3 | III | 3.215 |
| 12 | 0.743 | 0.063 | 0.136 | 0.058 | 0 | 0.743 | I | 2 | II | 2.000 |
| 13 | 0.481 | 0.352 | 0.149 | 0.018 | 0 | 0.481 | III | 3 | III | 3.423 |
| 14 | 0.125 | 0.379 | 0.343 | 0.153 | 0 | 0.379 | II | 3 | II | 1.643 |
| 15 | 0.187 | 0.212 | 0.601 | 0 | 0 | 0.601 | I | 2 | I | 1.025 |
| 16 | 0.315 | 0.585 | 0.089 | 0.011 | 0 | 0.585 | III | 3 | III | 2.907 |
| 17 | 0.305 | 0.212 | 0.387 | 0.096 | 0 | 0.387 | III | 3 | III | 2.659 |
| 18 | 0.254 | 0.128 | 0 | 0.116 | 0.546 | 0.546 | I | 2 | I | 1.074 |
| 19 | 0.521 | 0.081 | 0 | 0 | 0.398 | 0.521 | II | 3 | II | 1.686 |
| 20 | 0.709 | 0 | 0 | 0.185 | 0.106 | 0.709 | III | 3 | III | 3.566 |
| 21 | 0.423 | 0.312 | 0.058 | 0.207 | 0 | 0.423 | II | 3 | II | 2.341 |
| 22 | 0.305 | 0.212 | 0.387 | 0.096 | 0 | 0.387 | III | 3 | III | 2.659 |
| 23 | 0.179 | 0.629 | 0 | 0.177 | 0.015 | 0.629 | IV | 3 | III | 3.335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Liu, L.; Li, Y.; Zhai, Y.; Chen, H.; Hu, B.; Zhang, Q.; Teng, Y. Characteristics of Hydro-Geochemistry and Groundwater Pollution in Songnen Plain in Northeastern China. Sustainability 2022, 14, 6527. https://doi.org/10.3390/su14116527
Chen R, Liu L, Li Y, Zhai Y, Chen H, Hu B, Zhang Q, Teng Y. Characteristics of Hydro-Geochemistry and Groundwater Pollution in Songnen Plain in Northeastern China. Sustainability. 2022; 14(11):6527. https://doi.org/10.3390/su14116527
Chicago/Turabian StyleChen, Ruihui, Linmei Liu, Yi Li, Yuanzheng Zhai, Haiyang Chen, Bin Hu, Qianru Zhang, and Yanguo Teng. 2022. "Characteristics of Hydro-Geochemistry and Groundwater Pollution in Songnen Plain in Northeastern China" Sustainability 14, no. 11: 6527. https://doi.org/10.3390/su14116527
APA StyleChen, R., Liu, L., Li, Y., Zhai, Y., Chen, H., Hu, B., Zhang, Q., & Teng, Y. (2022). Characteristics of Hydro-Geochemistry and Groundwater Pollution in Songnen Plain in Northeastern China. Sustainability, 14(11), 6527. https://doi.org/10.3390/su14116527









