Experimental Investigation of Mass Transfer Intensification for CO2 Capture by Environment-Friendly Water Based Nanofluid Solvents in a Rotating Packed Bed
Abstract
:1. Introduction
- (a)
- (b)
- Optimization of RPB operations by improving contact surface area or mass transfer by changing the hydrodynamic features through:
- Modifications to RPB configurations such as “liquid distributor(s).” For instance, Hacking et al. [35,36] examined novel redistribution rings in a bed. Wu et al. used a multi-inlet to distribute liquid in a bed [37] whose efficiency was examined by Zhang et al. [38], and Wang et al. [39] evaluated the contacting angle of fluids,
- Modifications to the bulk zone by adding baffles/blades, as in Yang et al. [40], or mesh pin, as in Liu et al. [41,42]; or changing the number of mesh screen layers, as in Su et al. [43]; changing the packing wettability, as Lu et al. [44]; or changing the kind of packing structure, as reported in [45].
- (c)
- (d)
- Focusing on the micro-scale or micro-mixing in a heat-sensible process, controlling polymerization processes, and packing optimization [51].
2. Experimental Setup and Data Processing
2.1. Materials and Solvent Preparation
2.2. Setup Characteristics, Operating, Data Gathering, and Processing
- Introducing the mixed gas stream to the RPB under a flow control and design limitation of system, then venting it out to a safe location.
- Turning on the RPB driver at low speed and checking the operating condition.
- Turning on the solution pump, introducing the solvent to the RPB slowly, setting the operating conditions with the instrument devices, and keeping the proper situation (Table 3).
- Starting the regeneration section and recycling the regenerated solution stream to the solution pump suction when a steady-state situation is reached, then closing the outlet of the solvent vessel and keeping the circulation circuit.
- At this stage, the rotating speed can be changed when stability of the system is reached.
- Sampling for titration is caried out after 10 min.
2.3. Experimental Mass Transfer Modeling and Analysis
- Rotating speed, (affecting liquid elements);
- Gas and liquid rate;
- Packing form and configuration;
- Liquid distribution;
- Temperature.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature and Greek Symbols
| Nomenclature | |
| a or ae | Gas–liquid interfacial effective surface area per unit volume of bed, 1/m or m2/m3 |
| ac | Centrifugal acceleration, m/s2, |
| ap or at | Total packing surface area per unit volume of bed, 1/m |
| A | Gas–liquid MT interfacial area, m2 |
| CL,inlet | CO2 concentration at inlet stream of RPB, mol/m3 |
| CL,outlet | CO2 concentration at outlet stream of RPB, mol/m3 |
| DCO2, w | Diffusivity of CO2 in water, m2/s |
| h | Ion constant (Na+ = 0.091, OH− = 0.066, CO32− = 0.021, and hG = −0.019) |
| HE | Henry coefficient, mol/m3 Pa |
| kB | Boltzmann constant = 1.382×10−23 J/K |
| KGa | Overall volumetric gas phase MT coefficient, 1/s |
| k1 | Pseudo-first-order reaction rate constant, 1/s |
| k2 | Pseudo-second-order reaction rate constant between CO2 and OH−, m3/kmol s |
| kLa | Local volumetric liquid phase MT coefficient, 1/s |
| Experimental value of liquid MT coefficient | |
| Predicted value of liquid MT coefficient by Equation (17) | |
| m | Mass, kg |
| MNaOH | Molarity of NaOH, mol/L |
| MH2SO4 | Molarity of H2SO4, mol/L |
| N | rpm (revolutions per minute) |
| NCO2 | CO2 absorption flux, mol/m2 s |
| N’CO2 | CO2 absorption rate, mol/s |
| n | Number of data series |
| n’CO2 | Inlet molar flow rate of CO2, mol/L |
| QG | Volumetric gas flow rate, m3/s |
| QL | Volumetric liquid flow rate, m3/s |
| Ri | Inner radius of packing, m |
| Ro | Outer radius of packing, m |
| R2 | Model’s goodness of fit |
| Yi | Inlet CO2 concentration, % |
| Yo | Outlet CO2 concentration, % |
| Z | RPB packing height, m |
| Greek Symbols | |
| ϕ | Pore diameter (mm) and volume fraction of NF (Equations (16) and (17)) |
| ρ | Density, kg/m3 |
| μ | Dynamic viscosity |
| σ | Surface tension, kg/s2 or N/m |
| σc | Critical surface tension of packing material, Kg/s2 or N/m |
| γ | Contact angle, degree |
| Shape parameter of packing | |
| ν | Kinematic viscosity, m2/s |
| νNaOH | Consumed NaOH in titration, mL |
| Dimensionless Groups | |
Abbreviations
| BF | Base fluid |
| DLS | Dynamic light scattering |
| DW | Distillated water |
| GLR | Gas–liquid ratio |
| MEA | Monoethanolamine |
| MT | Mass transfer |
| nm | Nanometer |
| NF | Nanofluid |
| NP | Nanoparticle |
References
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Memb. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Tan, L.S.; Shariff, A.M.; Lau, K.K.; Bustam, M.A. Factors affecting CO2 absorption efficiency in packed column: A review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]
- Budzianowski, W.M. (Ed.) Energy Efficient Solvents for CO2 Capture by Gas-Liquid Absorption: Compounds, Blends and Advanced Solvent Systems, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Li, B.; Duan, Y.; Luebke, D.; Morreale, B. Advances in CO2 capture technology: A patent review. Appl. Energy 2013, 102, 1439–1447. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, Y.; Song, Y.; Li, H. Alternative pathways for efficient CO2 capture by hybrid processes—A review. Renew. Sustain. Energy Rev. 2017, 82, 215–231. [Google Scholar] [CrossRef]
- Tong, D.; Maitland, G.C.; Trusler, M.J.P.; Fennell, P.S. Solubility of carbon dioxide in aqueous blends of 2-amino-2-methyl-1-propanol and piperazine. Chem. Eng. Sci. 2013, 101, 851–864. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Gao, H.; Xu, B.; Liang, Z. Mass transfer performance studies of aqueous blended DEEA-MEA solution using orthogonal array design in a packed column. Sep. Purif. Technol. 2017, 183, 117–126. [Google Scholar] [CrossRef]
- Reay, D.; Ramshaw, C.; Harvey, A. Process Intensification: Engineering for Efficiency, Sustainability and Flexibility, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Feng, D.; Gao, J.; Zhang, Y.; Li, H.; Du, Q.; Wu, S. Mass transfer in ammonia-based CO2 absorption in bubbling reactor under static magnetic field. Chem. Eng. J. 2018, 338, 450–456. [Google Scholar] [CrossRef]
- Boodhoo, K.; Harvey, A. Process Intensification for Green Chemistry: Engineering Solutions for Sustainable Chemical Processing; Wiley Online Library: Hoboken, NJ, USA, 2013; p. 413. [Google Scholar] [CrossRef]
- Yu, C.H.; Chen, M.T.; Chen, H.; Tan, C.S. Effects of process configurations for combination of rotating packed bed and packed bed on CO2 capture. Appl. Energy 2016, 175, 269–276. [Google Scholar] [CrossRef]
- Chamchan, N.; Chang, J.Y.; Hsu, H.C.; Kang, J.L.; Wong, D.S.H.; Jang, S.S.; Shen, J.F. Comparison of rotating packed bed and packed bed absorber in pilot plant and model simulation for CO2 capture. J. Taiwan Inst. Chem. Eng. 2017, 73, 20–26. [Google Scholar] [CrossRef]
- Stankiewicz, A.I.; Moulijn, J.A. Process intensification: Transforming chemical engineering. Chem. Eng. Prog. 2000. Available online: https://www.aiche.org/sites/default/files/docs/news/010022_cep_stankiewicz.pdf (accessed on 20 April 2022).
- Ashrafmansouri, S.S.; Esfahany, M.N. Mass transfer into/from nanofluid drops in a spray liquid-liquid extraction column. AIChE J. 2015, 62, 852–860. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Pan, Z.; Zhang, Z. A brief review of enhanced CO2 absorption by nanoparticles. Int. J. Energy Clean Environ. 2018, 19, 201–215. [Google Scholar] [CrossRef]
- Pineda, I.T.; Lee, J.W.; Jung, I.; Kang, Y.T. CO2 absorption enhancement by methanol-based Al2O3 and SiO2 nanofluids in a tray column absorber. Int. J. Refrig. 2012, 35, 1402–1409. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, Y.T. CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution. Energy 2013, 53, 206–211. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, B.; Zhuo, Y.; Wang, S. Experimental study of CO2 absorption in aqueous MEA and MDEA solutions enhanced by nanoparticles. Int. J. Greenh. Gas Control 2014, 29, 135–141. [Google Scholar] [CrossRef]
- Haghtalab, A.; Mohammadi, M.; Fakhroueian, Z. Absorption and solubility measurement of CO2 in water-based ZnO and SiO2 nanofluids. Fluid Phase Equilibria 2015, 392, 33–42. [Google Scholar] [CrossRef]
- Lu, S.; Song, J.; Li, Y.; Xing, M.; He, Q. Improvement of CO2 absorption using AL2O3 nanofluids in a stirred thermostatic reactor. Can. J. Chem. Eng. 2015, 93, 935–941. [Google Scholar] [CrossRef]
- Rahmatmand, B.; Keshavarz, P.; Ayatollahi, S. Study of Absorption Enhancement of CO2 by SiO2, Al2O3, CNT, and Fe3O4 Nanoparticles in Water and Amine Solutions. J. Chem. Eng. Data 2016, 61, 1378–1387. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Esfahany, M.N.; Esmaeili-Faraj, S.H. Investigation of the effects of nanoparticle size on CO2 absorption by silica-water nanofluid. Sep. Purif. Technol. 2018, 195, 208–215. [Google Scholar] [CrossRef]
- Salimi, J.; Salimi, F. CO2 capture by water-based Al2NO3 and Al2O3-SiO2 mixture nanofluids in an absorption packed column. Rev. Mex. Ing. Quim. 2016, 15, 185–192. [Google Scholar]
- Samadi, Z.; Haghshenasfard, M.; Moheb, A. CO2 absorption using nanofluids in a wetted-wall column with external magnetic field. Chem. Eng. Technol. 2014, 37, 462–470. [Google Scholar] [CrossRef]
- Salimi, J.; Haghshenasfard, M.; Etemad, S.G. CO2 absorption in nanofluids in a randomly packed column equipped with magnetic field. Heat Mass Transf. Stoffuebertragung. 2014, 51, 621–629. [Google Scholar] [CrossRef]
- Komati, S.; Suresh, A.K. CO2 absorption into amine solutions: A novel strategy for intensification based on the addition of ferrofluids. J. Chem. Technol. Biotechnol. 2008, 83, 1094–1100. [Google Scholar] [CrossRef]
- Reddy, K.J.; Gupta, A.; Rao, D.P.; Rama, O.P. Process intensification in a HIGEE with split packing. Ind. Eng. Chem. Res. 2006, 45, 4270–4277. [Google Scholar] [CrossRef]
- Wang, G.Q.; Xu, O.G.; Xu, Z.C.; Ji, J.B. New HIGEE-rotating zigzag bed and its mass transfer performance. Ind. Eng. Chem. Res. 2008, 47, 8840–8846. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, J.Z.; Yue, X.J.; Song, Y.J.; Chu, G.W.; Liu, Y.; Le, Y.; Chen, J.F. Feasibility studies of micromixing and mass-transfer in an ultrasonic assisted rotating packed bed reactor. Chem. Eng. J. 2018, 331, 510–516. [Google Scholar] [CrossRef]
- Dashti, M.S.A.; Abolhasani, M. Intensification of CO2 capture by monoethanolamine solution containing TiO2 nanoparticles in a rotating packed bed. Int. J. Greenh. Gas Control 2019, 94, 102933. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, L.; Gao, L.; Chen, J.; Liu, Y.; Zhao, H. Pilot Scale Applied Research on CO2 Removal of Natural Gas Using a Rotating Packed Bed with Propylene Carbonate. Chem. Eng. Res. Des. 2019, 150, 33–39. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Y.; Zhang, L.; Chu, G.; Zou, H.; Sun, B.; Zeng, X. Carbon dioxide capture by non-aqueous blend in rotating packed bed reactor: Absorption and desorption investigation. Sep. Purif. Technol. 2021, 269, 118714. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wu, W.; Liu, Y.; Li, B.B.; Luo, Y.; Chu, G.W.; Zou, H.K.; Chen, J.F. Desulfurization intensification by ionic liquid in a rotating packed bed. Chem. Eng. Processing-Process Intensif. 2019, 148, 107793. [Google Scholar] [CrossRef]
- Hacking, J.A.; Delsing, N.F.E.J.; de Beer, M.M.; van der Schaaf, J. Improving liquid distribution in a rotating packed bed. Chem. Eng. Processing-Process Intensif. 2020, 149, 107861. [Google Scholar] [CrossRef]
- Hacking, J.A.; de Beer, M.M.; van der Schaaf, J. Gas-liquid mass transfer in a rotating liquid redistributor. Chem. Eng. Processing-Process Intensif. 2021, 163, 108377. [Google Scholar] [CrossRef]
- Wu, W.; Luo, Y.; Chu, G.W.; Su, M.J.; Cai, Y.; Zou, H.K.; Chen, J.F. Liquid flow behavior in a multiliquid-inlet rotating packed bed reactor with three-dimensional printed packing. Chem. Eng. J. 2019, 386, 121537. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, P.; Li, Y.; Teng, L.; Zhu, J. CFD analysis of the hydrodynamic characteristics in a rotating packed bed with multi-nozzles. Chem. Eng. Processing-Process Intensif. 2020, 158, 108107. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.B.; Su, M.J.; Chu, G.W.; Sun, B.C.; Luo, Y. Liquid droplet dispersion in a rotating packed bed: Experimental and numerical studies. Chem. Eng. Sci. 2021, 240, 116675. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Y.; Li, Y.; Chu, G.; Zou, H.; Arowo, M.; Chen, J. 3D CFD modelling and optimization of single-phase flow in rotating packed beds. Can. J. Chem. Eng. 2015, 93, 1138–1148. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Liu, Y.Z.; Chu, G.W. Scale-Up of a Rotating Packed Bed Reactor with a Mesh-Pin Rotor: (I) Hydrodynamic Studies. Ind. Eng. Chem. Res. 2020, 59, 5114–5123. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Li, Y.B.; Chu, G.W. Scale-Up of a Rotating Packed Bed Reactor with a Mesh-Pin Rotor: (II) Mass Transfer and Application. Ind. Eng. Chem. Res. 2020, 59, 5124–5132. [Google Scholar] [CrossRef]
- Su, M.J.; Le, Y.; Chu, G.W.; Li, Y.B.; Zhang, L.L.; Luo, Y. Intensification of Droplet Dispersion by Using Multilayer Wire Mesh and Its Application in a Rotating Packed Bed. Ind. Eng. Chem. Res. 2020, 59, 3584–3592. [Google Scholar] [CrossRef]
- Lu, Y.-Z.; Liu, W.; Xu, Y.-C.; Luo, Y.; Chu, G.-W.; Chen, J.-F. Initial liquid dispersion and mass transfer performance in a rotating packed bed. Chem. Eng. Processing-Process Intensif. 2019, 140, 136–141. [Google Scholar] [CrossRef]
- Yuan, Z.-G.; Wang, Y.-X.; Liu, Y.-Z.; Wang, D.; Jiao, W.-Z.; Liang, P.-F. Research and development of advanced structured packing in a rotating packed bed. Chinese J. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Groß, K.; de Beer, M.; Dohrn, S.; Skiborowski, M. Scale-Up of the Radial Packing Length in Rotating Packed Beds for Deaeration Processes. Ind. Eng. Chem. Res. 2020, 59, 11042–11053. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Z.; Liu, G. High-gravity deoxygenation of jet fuels using rotating packed bed. Fuel 2022, 314, 123080. [Google Scholar] [CrossRef]
- Ng, Y.S.; Tan, Y.T.; Chua, A.S.M.; Hashim, M.A.; Gupta, B.S. Removal of nickel from water using rotating packed bed contactor: Parametric studies and mode of operations. J. Water Process Eng. 2020, 36, 101286. [Google Scholar] [CrossRef]
- Liu, Z.; Esmaeili, A.; Zhang, H.; Wang, D.; Lu, Y.; Shao, L. Modeling and Experimental Studies on Carbon Dioxide Absorption with Sodium Hydroxide Solution in a Rotating Zigzag Bed. Processes 2022, 10, 614. [Google Scholar] [CrossRef]
- Jiao, W.; Yang, P.; Qi, G.; Liu, Y. Selective absorption of H2S with High CO2 concentration in mixture in a rotating packed bed. Chem. Eng. Processing-Process Intensif. 2018, 129, 142–147. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Chu, G.W.; Larachi, F.; Zou, H.K.; Chen, J.F. Liquid microflow inside the packing of a rotating packed bed reactor: Computational, observational and experimental studies. Chem. Eng. J. 2019, 386, 121134. [Google Scholar] [CrossRef]
- Chen, W.C.; Fan, Y.W.; Zhang, L.L.; Sun, B.C.; Luo, Y.; Zou, H.K.; Chu, G.W.; Chen, J.F. Computational fluid dynamic simulation of gas-liquid flow in rotating packed bed: A review. Chinese J. Chem. Eng. 2021, 41, 85–108. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Y.; Chu, G.W.; Wu, W.; Yu, X.; Sun, B.C.; Chen, J.F. NOx removal in a rotating packed bed: Oxidation and enhanced absorption process optimization. Sep. Purif. Technol. 2019, 227, 115682. [Google Scholar] [CrossRef]
- Liu, L. VOC removal in rotating packed bed: ANN model vs empirical model. Alexandria Eng. J. 2021, 61, 4507–4517. [Google Scholar] [CrossRef]
- Jiao, W.Z.; Liu, Y.Z.; Qi, G.S. Gas pressure drop and mass transfer characteristics in a cross-flow rotating packed bed with porous plate packing. Ind. Eng. Chem. Res. 2010, 49, 3732–3740. [Google Scholar] [CrossRef]
- Xing, Y.Q.; Liu, Y.Z.; Cui, L.J. Experimental study on intensification absorption of carbon dioxide from simulation flue gas by high-gravity rotary packed bed. Mod. Chem. Ind. 2007, 470–473. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-XDHG2007S2139.htm (accessed on 20 April 2022).
- Lin, C.-C.; Chen, B.-C. Carbon Dioxide Absorption into NaOH Solution in a Cross-flow Rotating Packed Bed. J. Ind. Eng. Chem. 2007, 13, 1083–1090. Available online: https://www.cheric.org/PDF/JIEC/IE13/IE13-7-1083.pdf (accessed on 20 April 2022).
- Yu, C.H.; Cheng, H.H.; Tan, C.S. CO2 capture by alkanolamine solutions containing diethylenetriamine and piperazine in a rotating packed bed. Int. J. Greenh. Gas Control 2012, 9, 136–147. [Google Scholar] [CrossRef]
- Kang, J.L.; Sun, K.; Wong, D.S.H.; Jang, S.S.; Tan, C.S. Modeling studies on absorption of CO2 by monoethanolamine in rotating packed bed. Int. J. Greenh. Gas Control 2014, 25, 141–150. [Google Scholar] [CrossRef]
- Gao, X.Y.; Liu, L.; Hu, M.L.; Xiang, Y.; Chu, G.W.; Zou, H.K.; Sun, B.C.; Chen, J.F. Numerical simulation for mass transfer characteristics of CO2 capture in a rotating packed bed. Chem. Eng. Processing-Process Intensif. 2016, 109, 68–79. [Google Scholar] [CrossRef]
- Wu, T.W.; Hung, Y.T.; Chen, M.T.; Tan, C.S. CO2 capture from natural gas power plants by aqueous PZ/DETA in rotating packed bed. Sep. Purif. Technol. 2017, 186, 309–317. [Google Scholar] [CrossRef]
- Sheng, M.; Xie, C.; Zeng, X.; Sun, B.; Zhang, L.; Chu, G.; Luo, Y.; Chen, J.F.; Zou, H. Intensification of CO2 capture using aqueous diethylenetriamine (DETA) solution from simulated flue gas in a rotating packed bed. Fuel 2018, 234, 1518–1527. [Google Scholar] [CrossRef]
- Zhao, B.; Tao, W.; Zhong, M.; Su, Y.; Cui, G. Process, performance and modeling of CO2 capture by chemical absorption using high gravity: A review. Renew. Sustain. Energy Rev. 2016, 65, 44–56. [Google Scholar] [CrossRef]
- Mohammaddoost, H.; Azari, A.; Ansarpour, M.; Osfouri, S. Experimental investigation of CO2 removal from N2 by metal oxide nanofluids in a hollow fiber membrane contactor. Int. J. Greenh. Gas Control 2018, 69, 60–71. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Oerations, 3rd ed.; McGraw-Hill Book Company: Singapore, 1981. [Google Scholar] [CrossRef] [Green Version]
- Munjal, S.; Dudukovć, M.P.; Ramachandran, P. Mass-transfer in rotating packed beds-I. Development of gas-liquid and liquid-solid mass-transfer correlations. Chem. Eng. Sci. 1989, 44, 2245–2256. [Google Scholar] [CrossRef]
- Ghadyanlou, F.; Azari, A.; Vatani, A. A Review of Modeling Rotating Packed Beds and Improving Their Parameters: Gas–Liquid Contact. Sustainability 2021, 13, 8046. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, C.C.; Liu, H.S. Mass transfer in a rotating packed bed with various radii of the bed. Ind. Eng. Chem. Res. 2005, 44, 7868–7875. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, F.Y.; Lin, C.C.; der Tai, C.Y.; Liu, H.S. Packing characteristics for mass transfer in a rotating packed bed. Ind. Eng. Chem. Res. 2006, 45, 6846–6853. [Google Scholar] [CrossRef]
- Rajan, S.; Kumar, M.; Ansari, M.J.; Rao, D.P.; Kaistha, N. Limiting gas liquid flows and mass transfer in a novel rotating packed bed (HiGee). Ind. Eng. Chem. Res. 2011, 50, 986–997. [Google Scholar] [CrossRef]
- Kim, S.; Xu, R.; Lee, W.; Kang, Y.T. Mass transfer performance enhancement by nanoabsorbents during CO2 absorption process. Int. J. Heat Mass Transf. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Fukushima, S.; Kusaka, K. Liquid-phase volumetric and mass-transfer coefficient, and boundary of hydrodynamic flow region in packed column with cocurrent downward flow. J. Chem. Eng. JAPAN 1977, 10, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Chu, G.; Zou, H.; Sun, B.; Shao, L.; Chen, J.F. Determination of the effective interfacial area in rotating packed bed. Chem. Eng. J. 2011, 168, 1377–1382. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, Z.; Luo, H.; Dang, J.; Qian, Z. An innovative approach of the effective mass transfer area in the rotating packed bed. Ind. Eng. Chem. Res. 2014, 53, 4052–4058. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Chen, Y.S. Effective interfacial area and liquid-side mass transfer coefficients in a rotating bed equipped with baffles. Sep. Purif. Technol. 2015, 144, 139–145. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Chu, G.W.; Luo, Y.; Sang, L.; Zhang, L.L.; Zou, H.K.; Chen, J.F. Polytetrafluoroethylene wire mesh packing in a rotating packed bed: Mass-Transfer studies. Ind. Eng. Chem. Res. 2016, 55, 11606–11613. [Google Scholar] [CrossRef]
- Versteeg, G.F.; van Swaal, W.P.M. Solubility and Diffusivity of Acid Gases (CO2, N2O) in Aqueous Alkanolamine Solutions. J. Chem. Eng. Data 1988, 33, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Dehghan, P.; Azari, A.; Azin, R. Measurement and correlation for CO2 mass diffusivity in various metal oxide nanofluids. J. Environ. Chem. Eng. 2019, 8, 103598. [Google Scholar] [CrossRef]
- Ramshaw, C.; Mallinson, R.H. Mass Transfer Process. U.S. Patent US4283255A, 11 August 1981. [Google Scholar]
- Saeednia, L.; Hashemipour, H.; Afzali, D. Study on Mass Transfer Enhancement in a Gas-Liquid System Using Nanomaterials. Transp. Phenom. Nano Micro Scales 2015, 3, 46–53. [Google Scholar] [CrossRef]
- Karamian, S.; Mowla, D.; Esmaeilzadeh, F. The effiect of various nanofluids on absorption intensification of CO2/SO2 in a single-bubble column. Processes 2019, 7, 393. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, L.; Pavani, V.; Rao, D.P.; Kaistha, N. Process intensification in HiGee absorption and distillation: Design procedure and applications. Ind. Eng. Chem. Res. 2010, 49, 10046–10058. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, C.W.; Kang, Y.T. Mass transfer enhancement during CO2 absorption process in methanol/Al2O3 nanofluids. Int. J. Heat Mass Transf. 2014, 76, 484–491. [Google Scholar] [CrossRef]
- Jeong, M.; Lee, J.W.; Lee, S.J.; Kang, Y.T. Mass transfer performance enhancement by nanoemulsion absorbents during CO2 absorption process. Int. J. Heat Mass Transf. 2017, 108, 680–690. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Bhattacharya, P.; Phelan, P.E.; Prasher, R.S. Enhanced mass transport in nanofluids. Nano Lett. 2006, 6, 419–423. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.; Pineda, I.T.; Kang, Y.T. Review of nanoabsorbents for capture enhancement of CO2 and its industrial applications with design criteria. Renew. Sustain. Energy Rev. 2020, 138, 110524. [Google Scholar] [CrossRef]
- Ali, F.M.; Yunus, W.M.M.; Talib, Z.A. Study of the effect of particles size and volume fraction concentration on the thermal conductivity and thermal diffusivity of Al2O3 nanofluids. Int. J. Phys. Sci. 2013, 8, 1442–1457. [Google Scholar]
- Kristiawan, B.; Rifa’i, A.I.; Enoki, K.; Wijayanta, A.T.; Miyazaki, T. Enhancing the thermal performance of TiO2/water nanofluids flowing in a helical microfin tube. Powder Technol. 2020, 376, 254–262. [Google Scholar] [CrossRef]
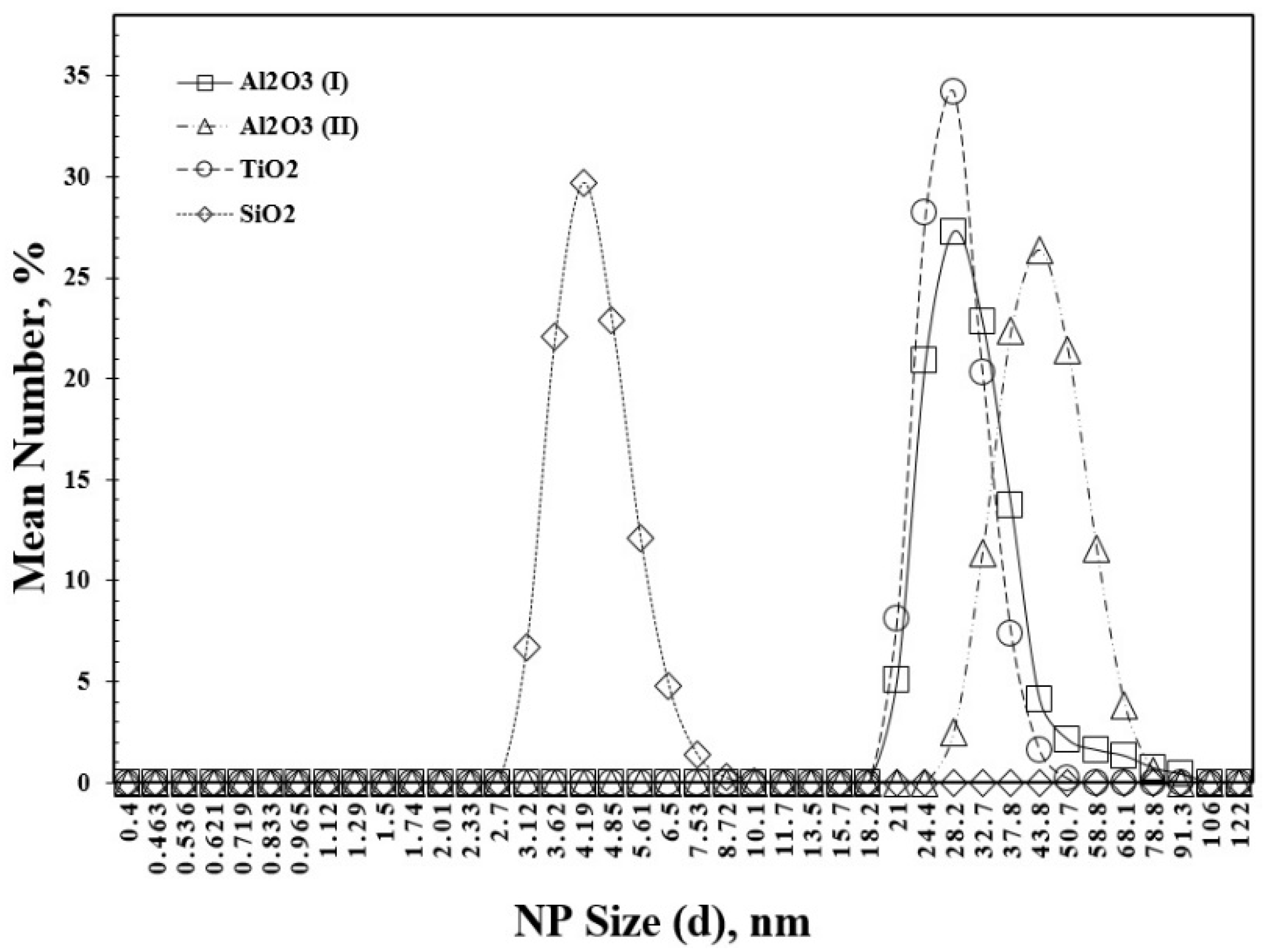
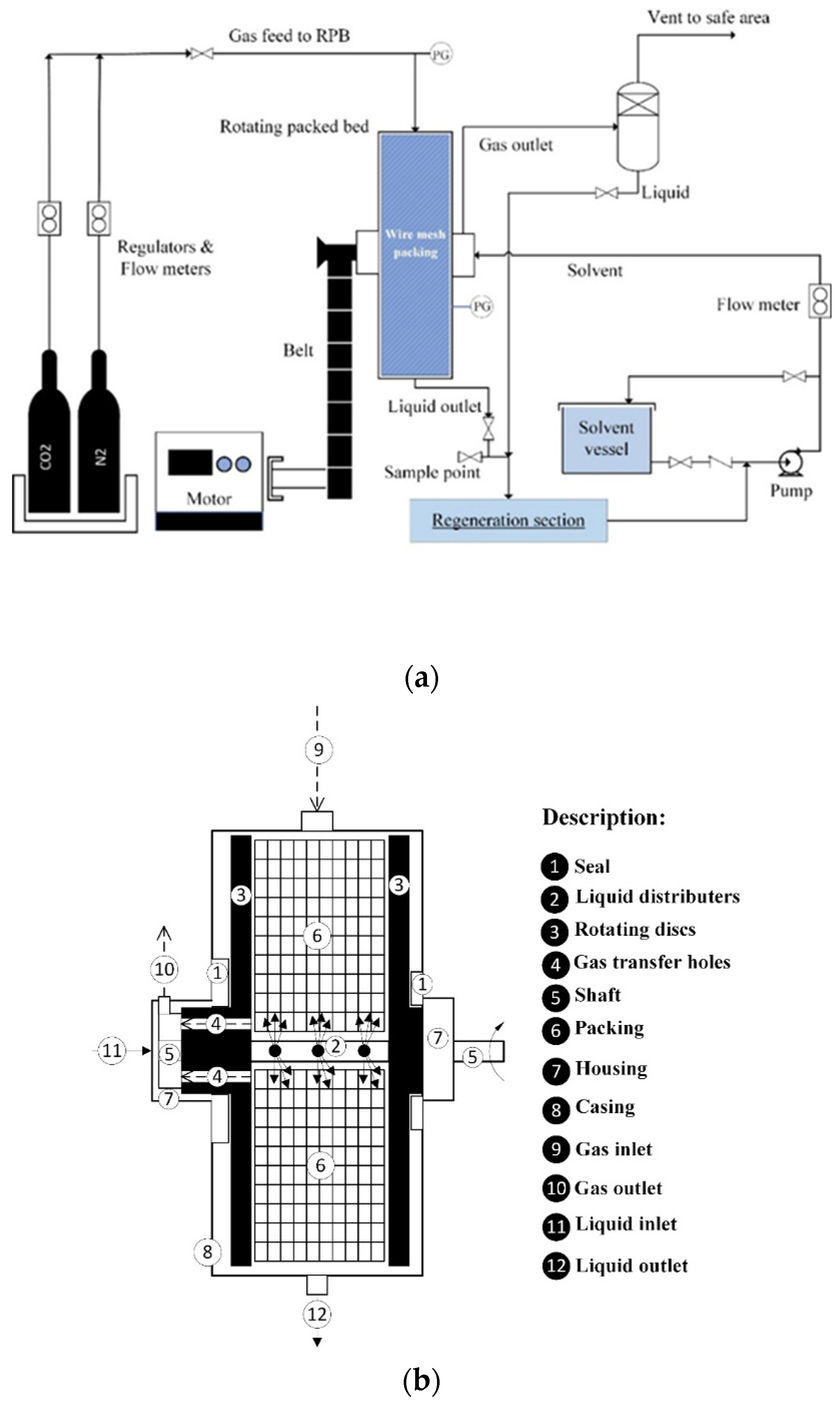
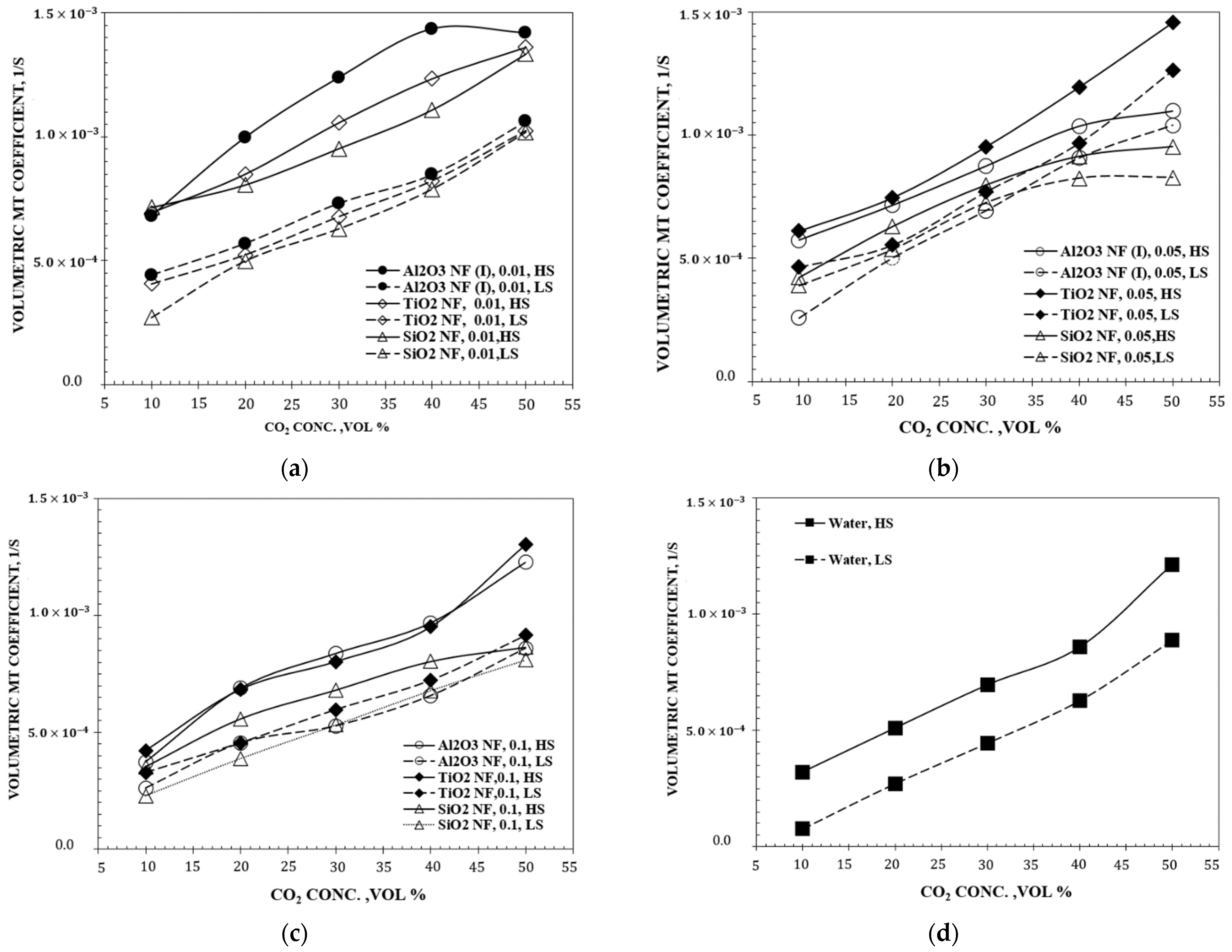

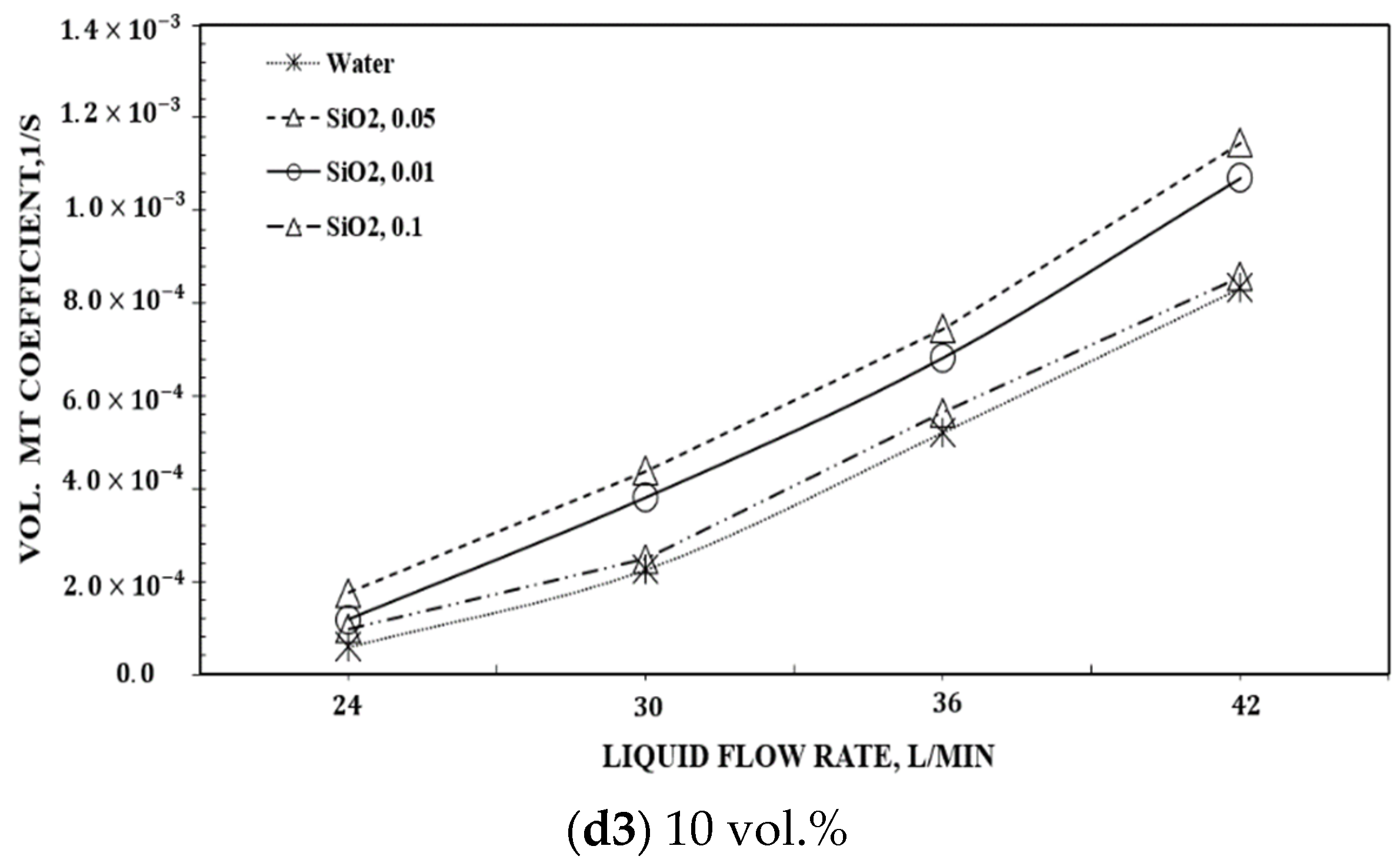
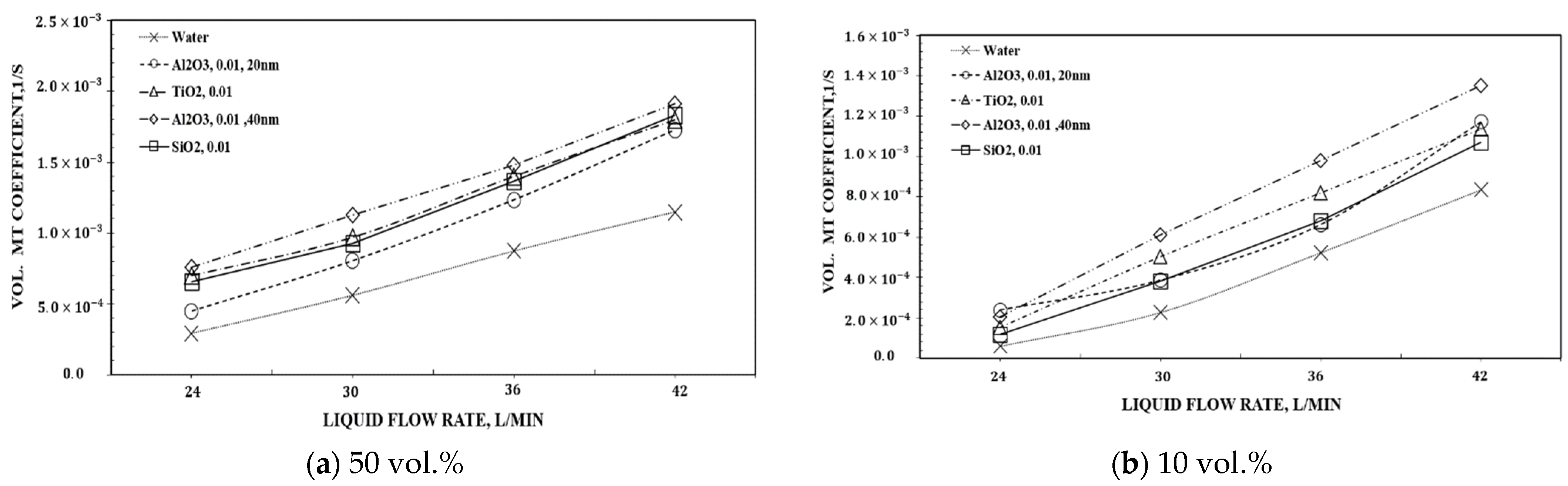
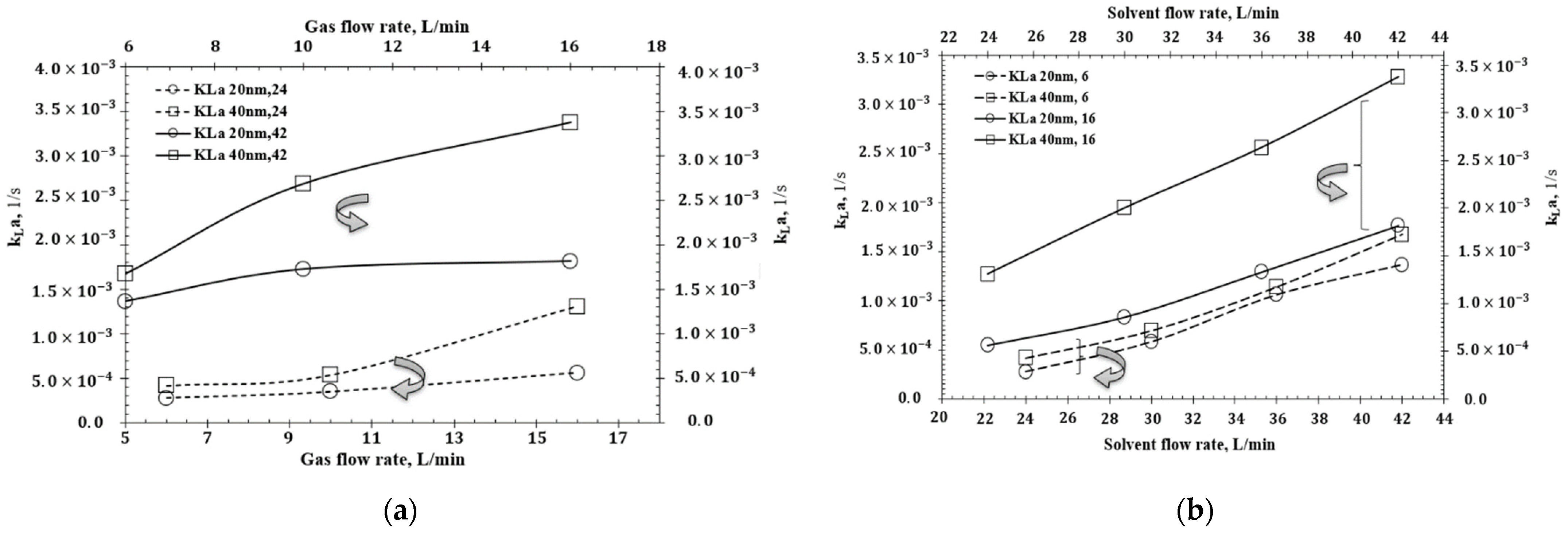

| Authors | Temp., K | GLR |
|---|---|---|
| Wang et al. [33] | 298–333 | 100–260 |
| Lin et al. [57] | 301 | 20–350 |
| Yu et al. [58] | 313–323 | 300–1200 |
| Kang et al. [59] | 333 | 67–700 |
| Sheng et al. [60] | 303–333 | 72–700 |
| Wu et al. [61] | 323 | 75–300 |
| Sheng et al. [62] | 303–323 | 138–242 |
| Solvent | Base Fluid | Silica | Titanium Oxide | Alumina | ||
|---|---|---|---|---|---|---|
| Parameter | ||||||
| Color | Colorless | White | White | White | ||
| Formula | H2O | SiO2 | TiO2 | Al2O3 (I) | Al2O3 (II) | |
| Density, Kg/m3 | 998.2 | 2400 | 3800 | 3890 | 3920 | |
| Thermal conductivity, W/m.K | 0.61 | 1.4 | 11.7 | 30 | 30 | |
| Molecular weight, g/mol | 18.02 | 60.08 | 79.87 | 159.69 | ||
| Mean size of particles (nm) | --- | 3–10 | 25 | 20 | 40 | |
| Status or morphology | liquid | Spherical solid powder | ||||
| Purity | DM | >99.9% | ||||
| Cp (J/Kg.K) | 4182 | 730 | 689.3 | 840 | 880 | |
| Surface tension (mN/m) | 72.03 | 52 | 67.4 | 57.4 | 68.9 | |
| Packing Type | Stacked Layers of Wire Mesh |
|---|---|
| Inner diameter of rotor (shaft diameter), m | 0.035 |
| Outer diameter of rotor (disc diameter), m | 0.255 |
| Packing height, m | 0.034 |
| Total surface area (m2. m−3) | 563.04 |
| Surface tension of packing, mN/m | 75 |
| Porosity | 0.8958 |
| Rotational speed (rpm) | 300 & 500 |
| Liquid flow rate, L/hr | 24–42 |
| Gas flow rate, L/min | 6–16 |
| CO2 present range, % | 10–50 |
| Temperature, °C | 35 |
| Casing pressure, bar | 3.3 |
| Instrument Name | Instrument Range | Measured Variable | Accuracy | Min and Max Values Measured in Experiment | Uncertainty (U%) |
|---|---|---|---|---|---|
| Gas flow meter | 0–20 L/min | Volume flow rate of gas | 0.1 L/min | 6–16 L/min | 0.01–0.03 |
| Liquid flow meter | 0–200 L/h | Volume flow rate of liquid | 0.6 L/h | 24–42 L/h | 0.99–2.32 |
| Titration glassware | 0–50 ml | Volume of liquid | 0.1 mL | 0–30 ml | 0.15–10 |
| Thermometer | −200–850 °C | Inlet and casing temperatures | 0.1 °C | 30–35 °C | 0.44 |
| Scale | 0–300 g | Weight of NPs | 0.0001 g | 0.025–0.25 g | 0.05–0.15 |
| Variable | Uncertainty Error (U%) |
|---|---|
| kLa | 6.82 |
| ReL | 0.89 |
| ReG | 0.96 |
| We | 1.04 |
| Gr | 1.56 |
| Sc | 0.08 |
| kLadp/DL.at | 1.36 |
| ReNP | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghadyanlou, F.; Azari, A.; Vatani, A. Experimental Investigation of Mass Transfer Intensification for CO2 Capture by Environment-Friendly Water Based Nanofluid Solvents in a Rotating Packed Bed. Sustainability 2022, 14, 6559. https://doi.org/10.3390/su14116559
Ghadyanlou F, Azari A, Vatani A. Experimental Investigation of Mass Transfer Intensification for CO2 Capture by Environment-Friendly Water Based Nanofluid Solvents in a Rotating Packed Bed. Sustainability. 2022; 14(11):6559. https://doi.org/10.3390/su14116559
Chicago/Turabian StyleGhadyanlou, Farhad, Ahmad Azari, and Ali Vatani. 2022. "Experimental Investigation of Mass Transfer Intensification for CO2 Capture by Environment-Friendly Water Based Nanofluid Solvents in a Rotating Packed Bed" Sustainability 14, no. 11: 6559. https://doi.org/10.3390/su14116559
APA StyleGhadyanlou, F., Azari, A., & Vatani, A. (2022). Experimental Investigation of Mass Transfer Intensification for CO2 Capture by Environment-Friendly Water Based Nanofluid Solvents in a Rotating Packed Bed. Sustainability, 14(11), 6559. https://doi.org/10.3390/su14116559








