Enhance Systemic Resistance Significantly Reduces the Silverleaf Whitefly Population and Increases the Yield of Sweet Pepper, Capsicum annuum L. var. annuum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. The Tested Formulations
2.3. The Experimental Procedure
2.4. Field and Laboratory Inspection of B. tabaci
2.5. Morphological Identification of B. tabaci
2.6. Enzymes’ Assay
2.7. The Economic Efficiency
2.8. Statistical Analysis
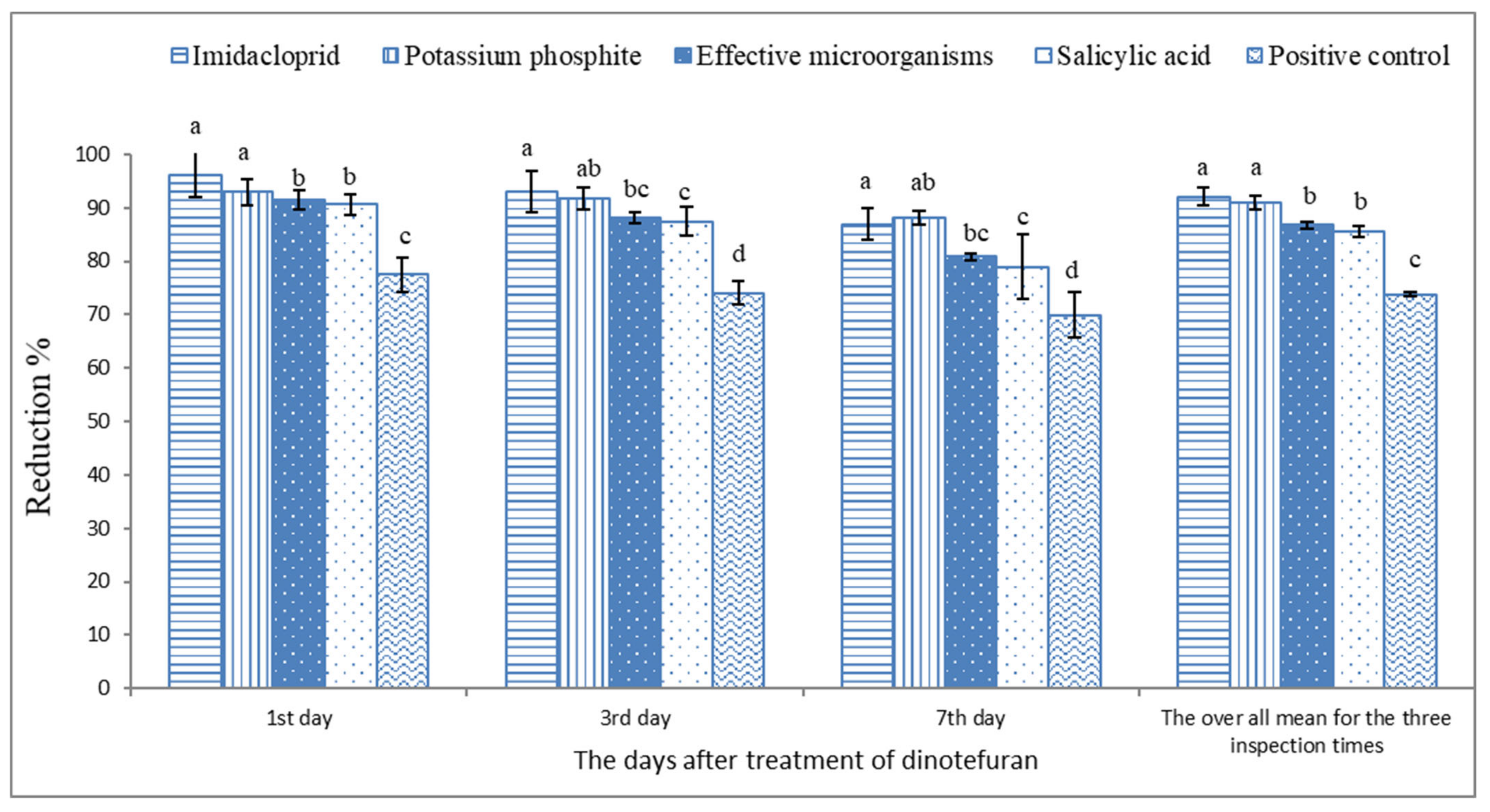
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rohini, N.; Lakshmanan, V. Evaluation studies of hot pepper hybrids (Capsicum annuum L.) for yield and quality characters. J. Electron. J. Plant Breed. 2017, 8, 643–651. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization (FAO) of the United Nation Crops and Livestock Products Department. 2018. Available online: https://www.fao.org/statistics/en (accessed on 2 May 2022).
- Ridzuan, R.; Rafii, M.Y.; Ismail, S.I.; Yusoff, M.M.; Miah, G.; Usman, M.G. Breeding for anthracnose disease resistance in chili: Progress and prospects. Int. J. Mol. Sci. 2018, 19, 3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ma, Y.; Zhang, B. Market demand and breeding trend of pepper varieties in China. J. China Veg. 2019, 8, 1–4. [Google Scholar]
- Lapidot, M.; Legg, J.P.; Wintermantel, W.M.; Polston, J.E. Management of whitefly-transmitted viruses in open-field production systems. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2014; Volume 90, pp. 147–206. [Google Scholar]
- Prabhaker, N.; Castle, S.J.; Merten, P. Comparative susceptibility of Bemisia tabaci to imidacloprid in field- and laboratory-based bioassays. J. Pest Manag. Sci. 2014, 70, 1538–1546. [Google Scholar]
- Kumar, P.; Naqvi, A.R.; Meena, R.S.; Mahendra, M. Seasonal incidence of whitefly, Bemisia tabaci (Gennadius) in tomato (Solanum lycopersicum Mill). Int. J. Chem. Stud. 2019, 7, 185–188. [Google Scholar]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E.; Webb, S.E. Suppression of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), and incidence of cucurbit leaf crumple virus, a whitefly-transmitted virus of zucchini squash new to Florida, with mulches and imidacloprid. Fla. Entomol. 2008, 91, 460–465. [Google Scholar] [CrossRef]
- De Marchi, B.R.; Smith, H.; Turechek, W.; Riley, D. A Maximum Dose Bioassay to Assess Efficacy of Key Insecticides against Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2021, 13, 914–921. [Google Scholar] [CrossRef]
- El-Nabawy, E.-S.M.; Tsuda, K.; Sakamaki, Y. Attractiveness of spiders and insect predators and parasitoids to flowering plants. Egypt. J. Biol. Pest Cont. 2015, 25, 245–250. [Google Scholar]
- Pérez-Hedo, M.; Urbaneja, A. Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. J. Pest Sci. 2015, 88, 65–73. [Google Scholar] [CrossRef] [Green Version]
- El-Nabawy, E.S.M.; Tsuda, K.; Sakamaki, Y.; Oda, A.; Ushijima, Y. The effect of organic fertilizers and flowering plants on sheet-web and wolf spider populations (Araneae: Lycosidae and Linyphiidae) and its importance for pest control. J. Insect Sci. 2016, 16, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonetti, P.; Zonno, M.C.; Molinari, S.; Altomare, C. Induction of SA signaling pathway and ethylene biosynthesis in Trichoderma harzianum treated tomato plants after infection of the root-knot nematode Meloidogyne incognita. J. Plant Cell Rep. 2017, 36, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Walsh, D.; Newton, A.; Lyon, G. Induced resistance for plant disease control: Maximizing the efficacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, A.M.N.M.; Rodrigues, F.A.; Berger, P.G.; Barros, A.F.; Silva, Y.C.R.; Lima, T.C. Aspectos bioquimicos da resistencia do algodoeiro a ramulose potencializada pelo silicio. Bragantia 2013, 72, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.C.; Sun, W.C.; Si, J.; Romheld, V. Effects of foliar-and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005, 54, 678–685. [Google Scholar] [CrossRef]
- Jafarbeigi, F.; Samih, M.A.; Alaei, H.; Shirani, H. Induced tomato resistance against Bemisia tabaci Triggered by Salicylic Acid, β-Aminobutyric Acid, and Trichoderma. Neotrop Entomol. 2020, 49, 456–467. [Google Scholar] [CrossRef]
- Araujo, L.; Bispo, W.M.S.; Rios, V.S.; Fernandes, S.A.; Rodrigues, F.A. Induction of the phenylpropanoid pathway by acibenzolar-s-methyl and potassium phosphite increases mango resistance to Ceratocystis fimbriata infection. Plant Dis. 2015, 99, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Dalio, R.J.D.; Fleischmann, F.; Humez, M.; Osswald, W. Phosphite protects Fagus sylvatica seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLoS ONE 2014, 9, e87860. [Google Scholar] [CrossRef] [Green Version]
- Siegel, M.R.; Latch, G.C.M.; Bush, L.P.; Fannin, F.F.; Rowan, D.D.; Tapper, B.A.; Bacon, C.W.; Johnson, M.C. Fungal endophyte-infected grasses: Alkaloid accumulation and aphid response. J. Chem. Ecol. 1990, 16, 3301–3316. [Google Scholar] [CrossRef]
- Ndona, R. Einfluss von Behandlungen Mit EMs. Effektiven Mikroorganismen auf Tomato in Geschützten Anbau. Ph.D. Thesis, Universität für Bodenkultur, Vienna, Austria, 2008. [Google Scholar]
- Filipp, M.; Spornberger, A.; Keppel, H.; Brunmayer, R. Influence of effective microorganisms (EM) on yield and quality in organic apple production. Univ. Nat. Res. Appl. Life Sci. J. Crop Prod. 2009, 281–284. Available online: https://www.researchgate.net/profile/Andreas-Spornberger/publication/268299040_Influence_of_effective_microorganisms_EM_on_yield_and_quality_in_organic_apple_production/links/551937c20cf2d241f355d0ea/Influence-of-effective-microorganisms-EM-on-yield-and-quality-in-organic-apple-production.pdf (accessed on 2 May 2022).
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Akbulut, G.B.; Yigit, E.; Bayram, D. Investigation of the effects of salicylic acid on some biochemical parameters in Zea mays to glyphosate herbicide. J. Environ. Anal. Toxicol. 2015, 5, 271. [Google Scholar] [CrossRef] [Green Version]
- Ryan, C.A. The systemin signaling pathway: Differential activation of plant defensive genes. Biochem. Biophys. Acta 2000, 1477, 112–121. [Google Scholar] [CrossRef]
- Chehab, E.W.; Kaspi, R.; Savchenko, T.; Rowe, H.; Negre-Zakarov, N.; Kliebenstein, D.; Dehesh, K. Distinct roles of jasmonates and aldehydes in plantdefense responses. PLoS ONE 2008, 3, e1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sherbeni, A.E.; Khaleid, M.S.; AbdAllah, S.A.; Ali, O.S.M. Effect of some insecticides alone and in combination with salicylic acid against aphid, Aphis gossypii, and whitefly Bemisia tabaci on the cotton field. Bull. Nat. Res. Cen. 2019, 43, 57. [Google Scholar] [CrossRef] [Green Version]
- Walling, L.L. The myriad plants responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Iverson, A.L.; Iverson, L.R.; Eshita, S. The effects of surface-applied jasmonic and salicylic acids on caterpillar growth and damage to tomato plants. Ohio J. Sci. 2001, 101, 90–94. [Google Scholar]
- Tomlin, C.D.S. The Pesticide Manual, a World Compendium, 14th ed.; British Crop Protection Council: Surry, UK, 2006; pp. 598–599. [Google Scholar]
- Talcott, M.S. Small Animal Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780323241984. [Google Scholar]
- Dalefield, R. Veterinary Toxicology for Australia and New Zealand, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780127999128. [Google Scholar]
- Kranthi, K.R. Bt-Cotton Question and Answer; Indian Society for Cotton Improvement: Mumbai, India, 2012. [Google Scholar]
- Oosterhuis, D.M.; Brown, R.S.; Gonias, E.D. Effects of Trimax™ insecticide application under water-deficit stress conditions on the lint yield and physiology of field-grown cotton. In Summaries of Arkansas Cotton Research 2003; Series 521; Arkansas Agricultural Experiment Station: Fayetteville, AR, USA, 2003. [Google Scholar]
- Francis, M.I.; Redondo, A.; Burns, J.K.; Graham, J.H. Soil application of imidacloprid and related SAR-inducing compounds produce effective and persistent control of citrus canker. Eur. J. Plant Pathol. 2009, 124, 283–292. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E.; Chandran, D.; Gulevich, A.G.; Okrent, R.A.; Durkin, K.A.; Sarpong, R.; Bunnelle, E.M.; Wildermuth, M.C. Neonicotinoid insecticides induce salicylate- associated plant defense responses. Proc. Nat. Acad. Sci. USA 2010, 107, 17527–17532. [Google Scholar] [CrossRef] [Green Version]
- Gonias, E.D.; Oosterhuis, D.M.; Bibi, A.C. How the insecticide TRIMAXTM improves growth and yield of cotton. In Proceedings of the Beltwide Cotton Conferences, San Antonio, TX, USA, 3–6 January 2006. [Google Scholar]
- Gonias, E.D.; Oosterhuis, D.M.; Bibi, A.C. Physiologic response of cotton to the insecticide imidacloprid under high-temperature stress. J. Plant Growth Regul. 2008, 27, 77–82. [Google Scholar] [CrossRef]
- Kumar, V.; Garima, K.; Cindy, L.M.; Lance, S.O. Effect of Dinotefuran on Bemisia tabaci and Amblyseius swirskii, SALVIA: Salvia nemorosa (L.), ‘New Dimension Blue. Arthropod Manag. Tests 2016, 41, tsw100. [Google Scholar] [CrossRef] [Green Version]
- Bin Hamid, M.S. Assessment of Imidacloprid and Dinotefuran on Whitefly Bemisia tabaci of Chilli. Bachelor‘s Thesis, Faculty of Plantation and Agrotechnology, Universiti Teknologi MARA, Shah Alam, Malaysia, 2015; p. 48. [Google Scholar]
- Derbalah, A.S.; Khidr, A.A.; Moustafa, H.Z.; Taman, A. Laboratory evaluation of some non-conventional pest control agents against the pink bollworm Pectinophora gossypiella (Saunders). Egypt. Biol. Pest Control 2014, 24, 363–368. [Google Scholar]
- Henderson, C.F.; Tilton, E.W. Test with acaricides against the brown wheat mite in Gossypium hirsutum and isolation of mutants with improved yield and fiber characters. Actainduction of mutation in cotton. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Bink-Moenen, R.M. Revision of the African Whiteflies (Aleyrodidae) Mainly Bases on a Collection from Tchad; CABI: Wallingford, UK, 1983; p. 201. [Google Scholar]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Role of salicylic acid in induction of plant defence system in chickpea (Cicer arientum L.). Plant Signal. Behav. 2011, 6, 1787–1792. [Google Scholar] [CrossRef] [Green Version]
- Moran, P.J.; Cipolini, D.F. Effect of fungal infection and mechanical stress on peroxidase activity and resistance to pests in cucumber. J. Phytopathol. 1998, 147, 313–316. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E. Polyphenol oxidases in plant. J. Phytochem. 1979, 18, 193–215. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. J. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Harman, G.E.; Hayes, C.K.; Lorito, M.; Broadway, R.M.; Di Pietro, A.; Peterbauer, C.; Tronsmo, A. Chitinolytic enzymes of Trichoderma harzianum, purification of chitobiosidase and endo chitinases. Phytopathology 1993, 83, 313–318. [Google Scholar] [CrossRef]
- Flohé, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1985, 105, 114–121. [Google Scholar]
- Warholm, M.; Guthenberg, C.; von Bahr, C.; Mannervik, B. Glutathione transferases from human liver. J. Methods Enzymol. 1985, 113, 449–503. [Google Scholar]
- Goldberg, D.M.; Spooner, R.J. Assay of Glutathione Reductase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyen, H.V., Ed.; Verlog Chemie: Weinheim, Germany, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Costat. Costat Statistical Software: Micro Computer Program Analysis Version 4.20; Cohort Software: Berkeley, CA, USA, 2006. [Google Scholar]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. J. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defence-related proteins in infected plants. J. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid induced a biotic stress tolerance and underlying mechanisms in plants. J. Plant Sci. 2015, 6, 462. [Google Scholar]
- Bargaus-Lars, N.; Rebecca, L.; Parry, J. Systemic resistance in sugar beet is SA independent and NPR1 dependent. J. Sugar Beet Res. 2007, 44, 2–17. [Google Scholar]
- Ramiro, D.A.; Guerreiro-Filho, O.; Mazzafera, P. Phenol contents, oxidase activities, and the resistance of coffee to the leaf miner Leucoptera coffeella. J. Chem. Ecol. 2006, 32, 1977–1988. [Google Scholar] [CrossRef]
- Mena, F.; Fernandez, S.J.M.; Campos, B.; Sánchez-Ávila, J.; Faria, M. Pesticide residue analyses and biomarker responses of native Costa Rican fish of the Poeciliidae and Cichlidae families to assess environmental impacts of pesticides in Palo Verde National Park. J. Environ. Biol. 2014, 35, 19–27. [Google Scholar]
- Livingstone, D.R. Contaminated-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Hakeem, K.U.R.; Chandna, R.; Ahmad, P. Role of glutathione reductase in plant abiotic stress BT-Abiotic Stress Responses. In Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 149–158. [Google Scholar]
- Tisler, T.; Jemec, A.; Mozetic, B.; Trebse, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immune competence of honey bees (Apis mellifera L.). J. Insect. Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Zinovieva, S.V.; Vasyukova, N.I.; Udalova, Z.V.; Gerasimova, N.G. The Participation of salicylic and jasmonic acids in genetic and induced resistance of tomato to Meloidogyne incognita (Kofoid and White, 1919). Biol. Bull. 2013, 40, 297–303. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, D.E.; Plyuta, V.A.; Padiy, D.A.; Kupriyanova, E.V.; Roshina, N.V.; Koksharova, O.A.; Khmel, I.A. The Effect of Volatile Organic Compounds on Different Organisms: Agrobacteria, Plants and Insects. Microorganisms 2022, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, R.G.; Soares, C.M.; Capdeville, G.; Jones, G.; Martins, E.S.; Praça, L.; Cordeiro, B.A.; Braz, S.V.; dos Santos, R.C.; Berry, C. Translocation and insecticidal activity of Bacillus thuringiensis living inside of plants. Microb. Biotechnol. 2009, 2, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila, F.W.; Faquin, V.; Da Silva Lobato, A.K.; Ávila, P.A.; Marques, D.J.; Guedes, E.M.S.; Tan, D.K.Y. Effect of phosphite supply in nutrient solution on yield, phosphorus nutrition and enzymatic behavior in common bean (Phaseolus vulgaris L.) plants. Aust. J. Crop Sci. 2013, 7, 713–722. [Google Scholar]
- Eltelib, H.A.; Hamad, M.A.; Ali, E. The effect of nitrogen and phosphorus fertilization on growth, yield and quality of forage maize (Zea mays L.). J. Agron. 2006, 5, 515–518. [Google Scholar]
- Al-Kahtani, S.N.; Taha, E.K.A.; Al-Abdulsalam, M. Alfalfa (Medicago sativa L.) seed yield in relation to phosphorus fertilization and honeybee pollination. Saudi J. Biol. Sci. 2017, 24, 1051–1055. [Google Scholar] [CrossRef]
- El-Bassiouny, A.M.; Ghoname, A.A.; El-Awadi, M.E.; Fawzy, Z.F.; Gruda, N. Ameliorative Effects of Brassinosteroids on Growth and Productivity of Snap Beans Grown Under High Temperature. Gesunde Pflanzen 2012, 64, 175–182. [Google Scholar] [CrossRef]




| Treatment | POX (nMole g−) ± SE | PPO (μMol g−) ± SE | CAT (nMol g−) ± SE | CHI (nMol g−) ± SE | GSHpx (nMol g−) ± SE | GSHtf ± SE (μMol g−) | GSHrd (nMole g−) ± SE |
|---|---|---|---|---|---|---|---|
| IMI | 488.62 ± 0.16 a | 733.06 ± 0.73 b | 699.60 ± 10.12 a | 703.00 ± 0.99 b | 588.17 ± 0.57 d | 101.80 ± 0.56 d | 179.10 ± 0.35 e |
| PK | 322.19 ± 0.64 c | 840.11 ± 3.11 a | 333.09 ± 1.84 d | 723.00 ± 0.13 a,b | 612.17 ± 0.79 b | 115.04 ± 0.12 b | 201.88 ± 0.36 c |
| EMs | 184.55 ± 0.73 d | 532.13 ± 0.50 d | 433.52 ± 1.21 c | 766.31 ± 1.02 a | 601.99 ± 0.75 c | 111.60 ± 0.58 c | 267.28 ± 0.51 b |
| SA | 458.22 ± 0.93 b | 714.43 ± 1.20 c | 618.33 ± 1.23 b | 719.15 ± 0.58 a,b | 644.12 ± 0.64 a | 119.90 ± 0.57 a | 288.55 ± 0.12 a |
| Control | 128.15 ± 0.41 e | 399.98 ± 1.14 f | 214.55 ± 1.10 e | 703.84 ± 0.38 b | 509.00 ± 0.27 e | 94.82 ± 0.21 e | 187.40 ± 2.89 d |
| Variable | POX | PPO | CAT | CHI | GSHpx | GSHtf | GSHrd |
|---|---|---|---|---|---|---|---|
| SS | 307,385.16 | 376,128.39 | 450,381.56 | 7524.53 | 30,580.83 | 1290.19 | 29,316.28 |
| MS | 76,846.29 | 94,032.09 | 112,595.39 | 1881.13 | 7645.20 | 322.54 | 7329.07 |
| F value | 52,712.43 | 11,153.24 | 1705.91 | 1245.83 | 6298.71 | 522.22 | 1369.04 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment | Variable | Adults | Nymphs | ||
|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | ||
| First inspection | SS | 406.48 | 476.95 | 794.56 | 621.11 |
| MS | 101.62 | 119.24 | 198.64 | 155.27 | |
| F value | 35.27 | 189.25 | 62.02 | 54.97 | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Second inspection | SS | 719.35 | 639.59 | 651.35 | 685.04 |
| MS | 179.83 | 159.89 | 162.83 | 171.26 | |
| F value | 25.10 | 305.75 | 87.08 | 80.12 | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Third inspection | SS | 1546.78 | 1884.46 | 713.26 | 636.93 |
| MS | 386.69 | 471.11 | 178.31 | 159.23 | |
| F value | 150.38 | 1118.34 | 30.63 | 36.47 | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| The overall average for the three inspection times | SS | 740.28 | 884.31 | 692.66 | 630.81 |
| MS | 185.07 | 221.08 | 173.16 | 157.70 | |
| F value | 105.92 | 1924.60 | 88.76 | 378.71 | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Treatment | Price (L.E)/kg | Percentage Amount Losses (Ton) | Crop Yield (L.E)/ha | Control Costs (L.E)/ha | Sprays No. during The Season |
|---|---|---|---|---|---|
| IMI | 7.15 a | 3.15 c | 95,265.07 c | 5030.95 d | 8.11 d |
| PK | 7.15 a | 3.12 d | 101,102.70 a | 4478.41 e | 7.23 e |
| EMs | 7.15 a | 3.07 e | 96,242.55 b | 5489.41 b,c | 9.12 c |
| SA | 6.75 b | 3.11 d,e | 83,052.36 d | 5487.83 c | 9.01 c |
| Positive control | 6.01 d | 10.11 a | 75,133.36 f | 6907.48 a | 14.02 a |
| Variable | Price (L.E)/kg | Percentage Amount Losses (Ton) | Productivity Yield (L.E)/ha | Control Costs (L.E)/Ha | Sprays No. during The Season |
|---|---|---|---|---|---|
| SS | 3.04 | 118.08 | 243,476,934.95 | 1,742,076.99 | 87.04 |
| MS | 0.76 | 29.52 | 60,869,234 | 435,519.20 | 21.76 |
| F value | 1151.803 | 340,629.70 | 49,172.56 | 631.63 | 1169.54 |
| p-value | <0.0001 | <0.001 | <0.0001 | <00.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, M.S.; Taha, E.-K.A.; Hassan, M.M.; Elnabawy, E.-S.M. Enhance Systemic Resistance Significantly Reduces the Silverleaf Whitefly Population and Increases the Yield of Sweet Pepper, Capsicum annuum L. var. annuum. Sustainability 2022, 14, 6583. https://doi.org/10.3390/su14116583
Zayed MS, Taha E-KA, Hassan MM, Elnabawy E-SM. Enhance Systemic Resistance Significantly Reduces the Silverleaf Whitefly Population and Increases the Yield of Sweet Pepper, Capsicum annuum L. var. annuum. Sustainability. 2022; 14(11):6583. https://doi.org/10.3390/su14116583
Chicago/Turabian StyleZayed, Mohamed S., El-Kazafy A. Taha, Montaser M. Hassan, and El-Said M. Elnabawy. 2022. "Enhance Systemic Resistance Significantly Reduces the Silverleaf Whitefly Population and Increases the Yield of Sweet Pepper, Capsicum annuum L. var. annuum" Sustainability 14, no. 11: 6583. https://doi.org/10.3390/su14116583
APA StyleZayed, M. S., Taha, E.-K. A., Hassan, M. M., & Elnabawy, E.-S. M. (2022). Enhance Systemic Resistance Significantly Reduces the Silverleaf Whitefly Population and Increases the Yield of Sweet Pepper, Capsicum annuum L. var. annuum. Sustainability, 14(11), 6583. https://doi.org/10.3390/su14116583






