Spatial Vegetation Patch Patterns and Their Relation to Environmental Factors in the Alpine Grasslands of the Qilian Mountains
Abstract
:1. Introduction
- How soil moisture affects spatial vegetation patch patterns;
- How topographic conditions affect spatial vegetation patch patterns.
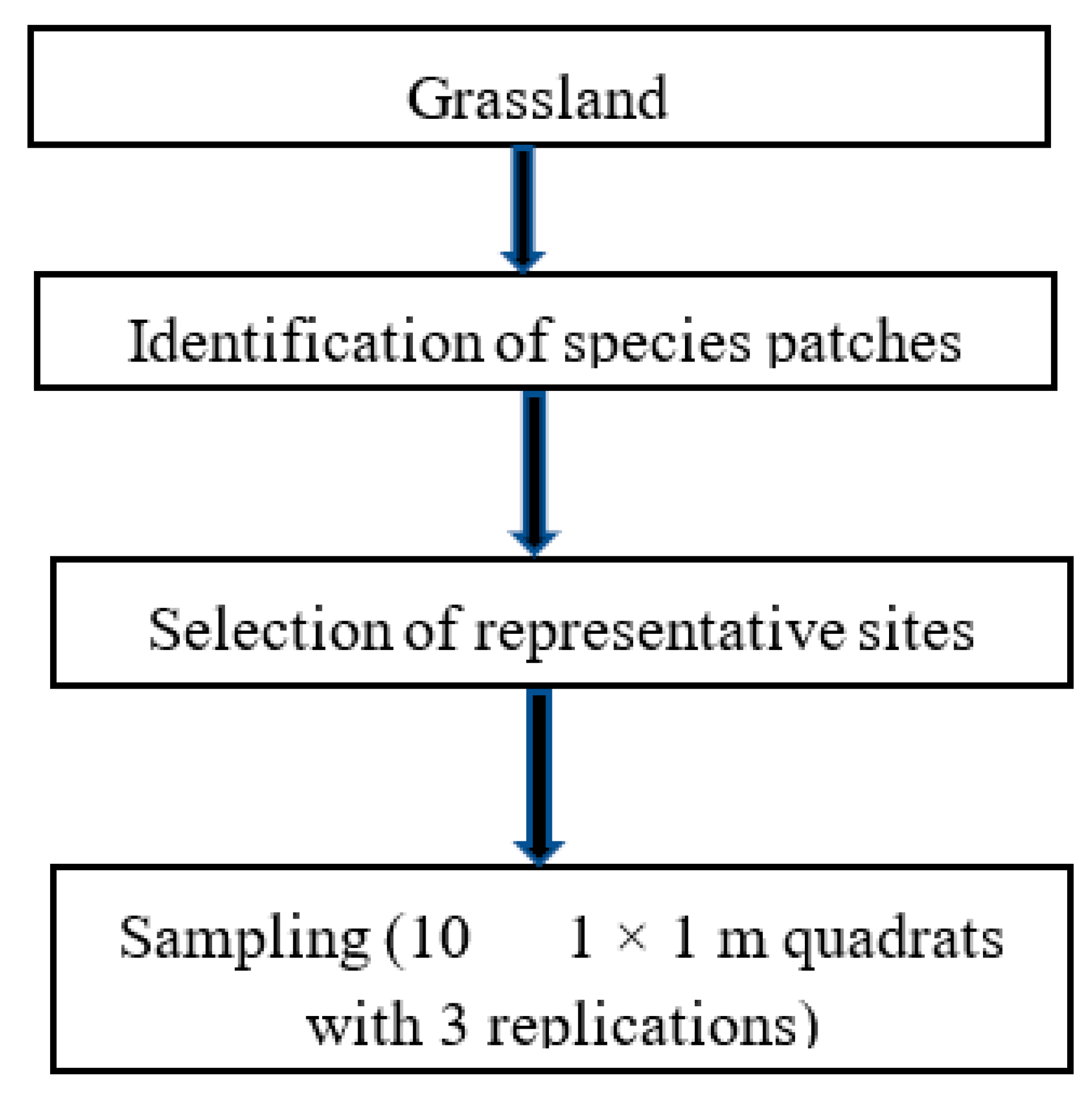
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Field Sampling
2.3. Soil Physical and Chemical Property Analysis
2.4. Statistical Analysis
3. Results
3.1. Patch Distributions
3.2. Numbers, Cover, and Perimeters of Vegetation Patches within Selected Environmental Gradients
3.3. Impact of Slope Aspect on Vegetation Patch Distribution
3.4. Changes in Vegetation Biomass as a Result of Soil Moisture
3.5. Variations in Soil Properties within the Selected Sites
4. Discussion
4.1. Effect of Habitat Conditions on Spatial Vegetation Patch Patterns
4.2. Impact of Soil Properties on Vegetation Patches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schleuss, P.-M.; Heitkamp, F.; Sun, Y.; Miehe, G.; Xu, X.; Kuzyakov, Y. Nitrogen uptake in an alpine Kobresia pasture on the Tibetan Plateau: Localization by 15 N labeling and implications for a vulnerable ecosystem. Ecosystems 2015, 18, 946–957. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; You, Q.; Xue, X.; Guo, J.; Wang, T. Evapotranspiration and its source components change under experimental warming in alpine meadow ecosystem on the Qinghai-Tibet plateau. Ecol. Eng. 2015, 84, 653–659. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, H.; Mi, Z.; Zhang, Z.; Wang, Y.; Xu, W.; Jiang, L.; He, J.-S. Climate warming reduces the temporal stability of plant community biomass production. Nat. Commun. 2017, 8, 15378. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lassoie, J.P.; Morreale, S.J.; Dong, S. A critical review of socioeconomic and natural factors in ecological degradation on the Qinghai-Tibetan Plateau, China. Rangel. J. 2015, 37, 1–9. [Google Scholar] [CrossRef]
- Umali, B.P.; Oliver, D.P.; Forrester, S.; Chittleborough, D.J.; Hutson, J.L.; Kookana, R.S.; Ostendorf, B. The effect of terrain and management on the spatial variability of soil properties in an apple orchard. Catena 2012, 93, 38–48. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Zhao, X.; Li, Y.; Zhao, W.; Li, A.; Chen, S.; Chen, S.; Han, X.; Huang, J. Topography and grazing effects on storage of soil organic carbon and nitrogen in the northern China grasslands. Ecol. Indic. 2018, 93, 45–53. [Google Scholar] [CrossRef]
- Lozano-García, B.; Parras-Alcántara, L.; Brevik, E.C. Impact of topographic aspect and vegetation (native and reforested areas) on soil organic carbon and nitrogen budgets in Mediterranean natural areas. Sci. Total Environ. 2016, 544, 963–970. [Google Scholar] [CrossRef]
- Song, C.J.; Qu, L.; Yishui, T.; Ma, K.M.; Zao, L.; Xianli, X. Impact of multiple soil nutrients on distribution patterns of shrubs in an arid valley, in southwest china. Pak. J. Bot. 2014, 46, 1621–1629. [Google Scholar]
- Liang, W.L.; Hung, F.X.; Chan, M.C.; Lu, T.H. Spatial structure of surface soil water content in a natural forested headwater catchment with a subtropical monsoon climate. J. Hydrol. 2014, 516, 210–221. [Google Scholar] [CrossRef]
- Pan, T.; Hou, S.; Liu, Y.; Tan, Q.; Liu, Y.; Gao, X. Influence of degradation on soil water availability in an alpine swamp meadow on the eastern edge of the Tibetan Plateau. Sci. Total Environ. 2020, 722, 137677. [Google Scholar] [CrossRef]
- Dong, S.; Wen, L.; Li, Y.; Wang, X.; Zhu, L.; Li, X. Soil-quality effects of grassland degradation and restoration on the Qinghai-Tibetan Plateau. Soil Sci. Soc. Am. J. 2012, 76, 2256–2264. [Google Scholar] [CrossRef]
- Medeiros, A.; Fernandes, C.; Goncalves, J.F.; Farinha-Marques, P. Research trends on integrative landscape assessment using indicators—A systematic review. Ecol. Indic. 2021, 129, 107815. [Google Scholar] [CrossRef]
- Ge, X.; Dong, K.; Luloff, A.; Wang, L.; Xiao, J.; Wang, S.; Wang, Q. Correlation between landscape fragmentation and sandy desertification: A case study in Horqin Sandy Land, China. Environ. Monit. Assess. 2016, 188, 62. [Google Scholar] [CrossRef]
- Li, X.L.; Gao, J.; Brierley, G.; Qiao, Y.M.; Zhang, J.; Yang, Y.W. Rangeland degradation on the Qinghai-Tibet plateau: Implications for rehabilitation. Land Degrad. Dev. 2013, 24, 72–80. [Google Scholar] [CrossRef]
- Qin, Y.; Yi, S.; Chen, J.; Ren, S.; Ding, Y. Effects of gravel on soil and vegetation properties of alpine grassland on the Qinghai-Tibetan plateau. Ecol. Eng. 2015, 74, 351–355. [Google Scholar] [CrossRef]
- Qin, Y.; Yi, S.; Ren, S.; Li, N.; Chen, J. Responses of typical grasslands in a semi-arid basin on the Qinghai-Tibetan Plateau to climate change and disturbances. Environ. Earth Sci. 2014, 71, 1421–1431. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Sun, Y. Effects of a thaw slump on active layer in permafrost regions with the comparison of effects of thermokarst lakes on the Qinghai–Tibet Plateau, China. Geoderma 2018, 314, 47–57. [Google Scholar] [CrossRef]
- Chen, J.; Yi, S.; Qin, Y. The contribution of plateau pika disturbance and erosion on patchy alpine grassland soil on the Qinghai-Tibetan Plateau: Implications for grassland restoration. Geoderma 2017, 297, 1–9. [Google Scholar] [CrossRef]
- Qin, Y.; Yi, S.; Ding, Y.; Zhang, W.; Qin, Y.; Chen, J.; Wang, Z. Effect of plateau pika disturbance and patchiness on ecosystem carbon emissions in alpine meadow in the northeastern part of Qinghai–Tibetan Plateau. Biogeosciences 2019, 16, 1097–1109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yi, S.; Chen, J.; Qin, Y.; Chang, L.; Sun, Y.; Shangguan, D. Characteristics and controlling factors of alpine grassland vegetation patch patterns on the central Qinghai-Tibetan plateau. Ecol. Indic. 2021, 125, 107570. [Google Scholar] [CrossRef]
- Song, M.-H.; Cornelissen, J.H.; Li, Y.-K.; Xu, X.-L.; Zhou, H.-K.; Cui, X.-Y.; Wang, Y.-F.; Xu, R.-Y.; Feng, Q. Small-scale switch in cover–perimeter relationships of patches indicates shift of dominant species during grassland degradation. J. Plant Ecol. 2020, 13, 704–712. [Google Scholar] [CrossRef]
- Kéfi, S.; Rietkerk, M.; Alados, C.L.; Pueyo, Y.; Papanastasis, V.P.; ElAich, A.; De Ruiter, P.C. Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature 2007, 449, 213–217. [Google Scholar] [CrossRef]
- Oñatibia, G.R.; Boyero, L.; Aguiar, M.R. Regional productivity mediates the effects of grazing disturbance on plant cover and patch-size distribution in arid and semi-arid communities. Oikos 2018, 127, 1205–1215. [Google Scholar] [CrossRef]
- Augustine, D.J.; Te Booth, D.R.; Cox, S.E.; Derner, J.D. Grazing intensity and spatial heterogeneity in bare soil in a grazing-resistant grassland. Rangel. Ecol. Manag. 2012, 65, 39–46. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.; Mortimore, M.; Batterbury, S.P.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffer, M.; Bascompte, J.; Brock, W.A.; Brovkin, V.; Carpenter, S.R.; Dakos, V.; Held, H.; Van Nes, E.H.; Rietkerk, M.; Sugihara, G. Early-warning signals for critical transitions. Nature 2009, 461, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Wu, J.; Joergensen, R.; Pommerening, B.; Chaussod, R.; Brookes, P. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Jenkinson, D. Determination of microbial biomass carbon and nitrogen in soil. In Advances in Nitrogen Cycling in Agricultural Ecosystems; CABI: Wallingford, UK, 1988; pp. 368–386. [Google Scholar]
- Guo, X.; Fu, Q.; Hang, Y.; Lu, H.; Gao, F.; Si, J. Spatial variability of soil moisture in relation to land use types and topographic features on hillslopes in the black soil (Mollisols) area of northeast China. Sustainability 2020, 12, 3552. [Google Scholar] [CrossRef]
- Liu, R.; Pan, Y.; Bao, H.; Liang, S.; Jiang, Y.; Tu, H.; Nong, J.; Huang, W. Variations in Soil Physico-Chemical Properties along Slope Position Gradient in Secondary Vegetation of the Hilly Region, Guilin, Southwest China. Sustainability 2020, 12, 1303. [Google Scholar] [CrossRef] [Green Version]
- Moeslund, J.E.; Arge, L.; Bøcher, P.K.; Dalgaard, T.; Odgaard, M.V.; Nygaard, B.; Svenning, J.-C. Topographically controlled soil moisture is the primary driver of local vegetation patterns across a lowland region. Ecosphere 2013, 4, 1–26. [Google Scholar] [CrossRef]
- Sun, F.; Lü, Y.; Fu, B.; Ma, Z.; Yao, X. Spatial explicit soil moisture analysis: Pattern and its stability at small catchment scale in the loess hilly region of China. Hydrol. Processes 2014, 28, 4091–4109. [Google Scholar] [CrossRef]
- Yang, J.Z.; Zhang, Z.M.; Shen, Z.H.; Ou, X.K.; Geng, Y.P.; Yang, M.Y. Review of research on the vegetation and environment of dry-hot valleys in Yunnan. Biodivers. Sci. 2016, 24, 462–474. [Google Scholar] [CrossRef]
- Pei, J.; Yang, W.; Cai, Y.; Yi, Y.; Li, X. Relationship between vegetation and environment in an arid-hot valley in Southwestern China. Sustainability 2018, 10, 4774. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.; Tischer, J.; Roscher, C.; Eisenhauer, N.; Ravenek, J.; Gleixner, G.; Attinger, S.; Jensen, B.; de Kroon, H.; Mommer, L. Plant species diversity affects infiltration capacity in an experimental grassland through changes in soil properties. Plant Soil 2015, 397, 1–16. [Google Scholar] [CrossRef]
- Guo, W.; Song, Y.-B.; Yu, F.-H. Heterogeneous light supply affects growth and biomass allocation of the understory fern Diplopterygium glaucum at high patch contrast. PLoS ONE 2011, 6, e27998. [Google Scholar] [CrossRef] [Green Version]
- Titlyanova, A.; Romanova, I.; Kosykh, N.; Mironycheva-Tokareva, N. Pattern and process in above-ground and below-ground components of grassland ecosystems. J. Veg. Sci. 1999, 10, 307–320. [Google Scholar] [CrossRef]
- Berhongaray, G.; Cotrufo, F.M.; Janssens, I.A.; Ceulemans, R. Below-ground carbon inputs contribute more than above-ground inputs to soil carbon accrual in a bioenergy poplar plantation. Plant Soil 2019, 434, 363–378. [Google Scholar] [CrossRef] [Green Version]
- Kätterer, T.; Bolinder, M.A.; Andrén, O.; Kirchmann, H.; Menichetti, L. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric. Ecosyst. Environ. 2011, 141, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Okin, G.S.; Heras, M.M.-D.L.; Saco, P.M.; Throop, H.L.; Vivoni, E.R.; Parsons, A.J.; Wainwright, J.; Peters, D.P. Connectivity in dryland landscapes: Shifting concepts of spatial interactions. Front. Ecol. Environ. 2015, 13, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Rietkerk, M.; Dekker, S.C.; de Ruiter, P.C.; van de Koppel, J. Self-organized patchiness and catastrophic shifts in ecosystems. Science 2004, 305, 1926–1929. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Holmgren, M.; Van Nes, E.H.; Maestre, F.T.; Soliveres, S.; Berdugo, M.; Kéfi, S.; Marquet, P.A.; Abades, S.; Scheffer, M. Can we infer plant facilitation from remote sensing? A test across global drylands. Ecol. Appl. 2015, 25, 1456–1462. [Google Scholar] [CrossRef] [Green Version]
- Berdugo, M.; Kéfi, S.; Soliveres, S.; Maestre, F.T. Plant spatial patterns identify alternative ecosystem multifunctionality states in global drylands. Nat. Ecol. Evol. 2017, 1, 3. [Google Scholar] [CrossRef]
- Abades, S.R.; Gaxiola, A.; Marquet, P.A. Fire, percolation thresholds and the savanna forest transition: A neutral model approach. J. Ecol. 2014, 102, 1386–1393. [Google Scholar] [CrossRef]
- Berdugo, M.; Maestre, F.T.; Kéfi, S.; Gross, N.; Le Bagousse-Pinguet, Y.; Soliveres, S. Aridity preferences alter the relative importance of abiotic and biotic drivers on plant species abundance in global drylands. J. Ecol. 2019, 107, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Deblauwe, V.; Barbier, N.; Couteron, P.; Lejeune, O.; Bogaert, J. The global biogeography of semi-arid periodic vegetation patterns. Glob. Ecol. Biogeogr. 2008, 17, 715–723. [Google Scholar] [CrossRef]
- von Hardenberg, J.; Kletter, A.Y.; Yizhaq, H.; Nathan, J.; Meron, E. Periodic versus scale-free patterns in dryland vegetation. Proc. R. Soc. B Biol. Sci. 2010, 277, 1771–1776. [Google Scholar] [CrossRef] [Green Version]
- Borthagaray, A.I.; Arim, M.; Marquet, P.A. Connecting landscape structure and patterns in body size distributions. Oikos 2012, 121, 697–710. [Google Scholar] [CrossRef]
- Cartenì, F.; Marasco, A.; Bonanomi, G.; Mazzoleni, S.; Rietkerk, M.; Giannino, F. Negative plant soil feedback explaining ring formation in clonal plants. J. Theor. Biol. 2012, 313, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Almenar, J.B.; Bolowich, A.; Elliot, T.; Geneletti, D.; Sonnemann, G.; Rugani, B. Assessing habitat loss, fragmentation and ecological connectivity in Luxembourg to support spatial planning. Landsc. Urban Plan 2019, 189, 335–351. [Google Scholar] [CrossRef]
- McGarigal, K. Landscape pattern metrics. In Encyclopedia Environ; John Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Mairota, P.; Cafarelli, B.; Boccaccio, L.; Leronni, V.; Labadessa, R.; Kosmidou, V.; Nagendra, H. Using landscape structure to develop quantitative baselines for protected area monitoring. Ecol. Indic. 2013, 33, 82–95. [Google Scholar] [CrossRef]
- Pueyo, Y.; Kéfi, S.; Alados, C.; Rietkerk, M. Dispersal strategies and spatial organization of vegetation in arid ecosystems. Oikos 2008, 117, 1522–1532. [Google Scholar] [CrossRef]
- Alados, C.L.; El Aich, A.; Komac, B.; Pueyo, Y.; García-Gonzalez, R. Self-organized spatial patterns of vegetation in alpine grasslands. Ecol. Model. 2007, 201, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Hook, P.B.; Burke, I.C. Biogeochemistry in a shortgrass landscape: Control by topography, soil texture, and microclimate. Ecology 2000, 81, 2686–2703. [Google Scholar] [CrossRef]
- Wan, Q.; Zhu, G.; Guo, H.; Zhang, Y.; Pan, H.; Yong, L.; Ma, H. Influence of vegetation coverage and climate environment on soil organic carbon in the Qilian Mountains. Sci. Rep. 2019, 9, 17623. [Google Scholar] [CrossRef] [PubMed]
- Dyer, C.L.; Kopittke, P.M.; Sheldon, A.R.; Menzies, N.W. Influence of soil moisture content on soil solution composition. Soil Sci. Soc. Am. J. 2008, 72, 355–361. [Google Scholar] [CrossRef]
- Yan, Y.; Xin, X.; Xu, X.; Wang, X.; Yan, R.; Murray, P.J. Vegetation patches increase wind-blown litter accumulation in a semi-arid steppe of northern China. Environ. Res. Lett. 2016, 11, 124008. [Google Scholar] [CrossRef] [Green Version]
- Vereecken, H.; Huisman, J.-A.; Hendricks Franssen, H.-J.; Brüggemann, N.; Bogena, H.R.; Kollet, S.; Javaux, M.; van der Kruk, J.; Vanderborght, J. Soil hydrology: Recent methodological advances, challenges, and perspectives. Water Resour. Res. 2015, 51, 2616–2633. [Google Scholar] [CrossRef]
- Wu, J. Change in soil microbial biomass and regulating factors in an alpine meadow site on the Qinghai-Tibetan Plateau. Soil Sci. Plant Nutr. 2020, 66, 177–194. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Soil microbial community response to drying and rewetting stress: Does historical precipitation regime matter? Biogeochemistry 2012, 109, 101–116. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Tu, C.; Hoyt, G.D.; DeForest, J.L.; Hu, S. Long-term no-tillage and organic input management enhanced the diversity and stability of soil microbial community. Sci. Total Environ. 2017, 609, 341–347. [Google Scholar] [CrossRef]



| Patch | Species | Family | Important Value |
|---|---|---|---|
| BS | Blysmus sinocompressus | Cyperaceae | 1 |
| Pedicularis kansuenis | Scrophulariaceae | 0.03 | |
| Potentilla anserina | Rosaceae | 0.13 | |
| Puccinia chinensis | Pucciniaceae | 0.19 | |
| Rheum pumilum | Polygonaceae | 0.06 | |
| ES | Elymus nutans | Poaceae | 1 |
| Medicago ruthenica | Fabaceae | 0.25 | |
| Artemisia sphaerocephala | Asteraceae | 0.23 | |
| Oxytropis ochrocephala | Fabaceae | 0.11 | |
| Polygala tenuifolia | Polygalaceae | 0.1 | |
| Silene gallica | Caryophyllaceae | 0.14 | |
| Poa araratica | Poaceae | 0.28 | |
| Koeleria pers | Poaceae | 0.38 | |
| Kobresia humulis | Cyperaceae | 0.2 | |
| IL | Iris lactea | Iridaceae | 1 |
| Elymus nutans | Poaceae | 0.41 | |
| Polygonum viviparum | Polygonaceae | 0.23 | |
| Thalictrum var. sibricum | Ranunculaceae | 0.077 | |
| Leontopodium nanum | Asteraceae | 0.15 | |
| Sphallerocarpus gracilis | Apiaceae | 0.09 | |
| Gentianopsis paludosa | Gentianaceae | 0.14 | |
| Ranunculus tanguticus | Ranunculaceae | 0.1 | |
| Rheum pumilum | Polygonaceae | 0.14 | |
| Potentilla anserina | Rosaceae | 0.11 | |
| Saussurea japonica | Asteraceae | 0.058 | |
| KH | Kobresia humilis | Cyperaceae | 1 |
| Medicago ruthenica | Fabaceae | 0.3 | |
| Plantago asiatica | Plantaginaceae | 0.27 | |
| Elymus nutans | Poaceae | 0.45 | |
| Taraxacum mongolicum | Asteraceae | 0.11 | |
| Potentilla multifida | Rosaceae | 0.099 | |
| Leontopodium nanum | Asteraceae | 0.098 | |
| Aconitum carmichaelii | Ranunculaceae | 0.074 | |
| Astragalus licentianus | Fabaceae | 0.09 | |
| Potentilla discolor | Rosaceae | 0.027 |
| Species | Score |
|---|---|
| Pedicularis kansuenis | −0.8768 |
| Saussurea japonica | −0.1569 |
| Rheum pumilum | −0.1534 |
| Thalictrum var. sibricum | 0.2365 |
| Aconitum carmichaelii | 0.2856 |
| Sphallerocarpus gracilis | 0.7895 |
| Astragalus licentianus | 0.8257 |
| Leontopodium nanum | 0.9854 |
| Potentilla multifida | 1.2980 |
| Polygala tenuifolia | 1.4216 |
| Ranunculus tanguticus | 1.5465 |
| Oxytropis ochrocephala | 1.5980 |
| Taraxacum mongolicum | 1.6870 |
| Potentilla anserina | 1.8452 |
| Silene gallica | 1.9673 |
| Gentianopsis paludosa | 2.1093 |
| Artemisia frigida | 2.2451 |
| Puccina chinensis | 2.3852 |
| Stipa aliena | 2.4170 |
| Poa pova | 2.4862 |
| Polygonum viviparum | 2.6214 |
| Artemisia sphaerocephala | 2.7452 |
| Medicago ruthenica | 3.0289 |
| Poa araratica | 3.4538 |
| Potentilla discolar | 3.6832 |
| Plantago asiatica | 3.8945 |
| Aster alpinus | 4.1268 |
| Kobresia pygmea | 4.4320 |
| Poa annua | 4.5021 |
| Potentilla bifurca | 4.7358 |
| Iris lactea | 6.2138 |
| Blysmus sinocompressus | 6.4350 |
| Elymus nutans | 6.5842 |
| Kobresia humilis | 6.6085 |
| Site | Index | Min | Max | Mean | Std | Coefficient of Variation (%) |
|---|---|---|---|---|---|---|
| Wetlands | MPS | 0.98 | 2.50 | 1.74 | 0.17 | 8.62 |
| PARA_MN | 24.68 | 148.09 | 84.29 | 20.01 | 23.74 | |
| SHAPE_MN | 1.34 | 2.94 | 1.67 | 0.1 | 5.98 | |
| PAFRAC | 1.32 | 1.75 | 1.53 | 0.08 | 5.23 | |
| Drylands | MPS | 0.26 | 1.27 | 0.76 | 0.13 | 17.11 |
| PARA_MN | 28.15 | 182.95 | 105.55 | 25.06 | 23.74 | |
| SHAPE_MN | 1.33 | 2.95 | 1.69 | 0.27 | 15.98 | |
| PAFRAC | 1.45 | 3.78 | 2.63 | 0.15 | 5.70 |
| Site | P | Height | Coverage | Density | BGB | AGB | Shannon | Simpson | Evenness | Richness |
|---|---|---|---|---|---|---|---|---|---|---|
| Wet land | KH | 20.40 ± 4.20 de | 94.66 1.20 b | 775.33 ± 82.10 b | 82.00 ± 4.92 a | 167.57 ± 25.22 a | 2.65 ± 0.22 ab | 0.92 ± 0.17 a | 0.83 ± 0.05 a | 9.11 ± 2.66 a |
| Wet land | BS | 14.23 ± 0.31 f | 96.16 ± 1.45 b | 1495.66 ± 95.71 a | 70.29 ± 4.80 b | 93.68 ± 2.01 c | 1.98 ± 0.22 c | 0.85 ± 0.03 ab | 0.63 ± 0.02 b | 7.33 ± 2.35 bc |
| Wetland | IL | 62.41 ± 0.45 b | 97.22 ± 0.38 b | 99.56 ± 0.74 d | 44.63 ± 0.48 c | 150.12 ± 0.89 ab | 2.71 ± 0.12 ab | 0.93 ± 0.04 a | 0.84 ± 0.07 a | 9.0 ± 2.76 a |
| Dryland | BS | 11.21 ± 0.67 f | 81.34 ± 0.75 c | 98.18 ± 0.59 d | 50.67 ± 0.84 bc | 79.82 ± 0.84 d | 1.81 ± 0.14 c | 0.78 ± 0.06 c | 0.66 ± 0.08 ab | 7.34 ± 2.45 bc |
| Dry land | IL | 51.36 ± 0.54 c | 95.66 ± 1.85 b | 99.00 ± 9.45 d | 38.77 ± 0.90 d | 133.11 ± 11.30 b | 2.60 ± 0.18 ab | 0.92 ± 0.13 a | 0.85 ± 0.07 a | 9.22 ± 1.86 a |
| Dry land | KH | 26.38 ± 2.09 d | 75.66 ± 2.90 d | 56.66 ± 8.87 e | 28.41 ± 0.96 e | 90.37 ± 24.68 c | 3.12 ± 0.01 a | 0.95 ± 0.10 a | 0.82 ± 0.08 a | 9.05 ± 2.65 a |
| Shady slope | EN | 76.93 ± 2.06 a | 100.00 ± 9.7.00 a | 125.33 ± 11.46 c | 24.53 ± 0.42 e | 150.81 ± 3.50 ab | 2.44 ± 0.12 ab | 0.91 ± 0.01 a | 0.76 ± 0.06 ab | 7.55 ± 1.24 bc |
| Sunny slope | EN | 74.35 ± 2.31 a | 89.00 ± 6.24 bc | 99.00 ± 9.45 d | 23.29 ± 0.81 e | 133.11 ± 0.30 ab | 2.45 ± 0.16 ab | 0.91 ± 0.01 a | 0.79 ± 0.89 ab | 7.68 ± 1.35 bc |
| Name | Explains % | Pseudo-F | p |
|---|---|---|---|
| MC | 33.4 | 8 | 0.002 |
| BGB | 32.7 | 7.8 | 0.002 |
| MBC | 30.1 | 6.9 | 0.002 |
| pH | 25 | 5.3 | 0.002 |
| TEMP | 24.7 | 5.3 | 0.004 |
| TP | 24.4 | 5.2 | 0.002 |
| AK | 23.1 | 4.8 | 0.004 |
| NO3-N | 22.8 | 4.7 | 0.004 |
| MBN | 21 | 4.3 | 0.008 |
| EC | 19.3 | 3.8 | 0.006 |
| SOC | 16.6 | 3.2 | 0.008 |
| BD | 11.5 | 2.1 | 0.082 |
| POROSITY | 11.5 | 2.1 | 0.08 |
| NH4-N | 10.3 | 1.8 | 0.126 |
| TK | 10.1 | 1.8 | 0.132 |
| TN | 8.4 | 1.5 | 0.226 |
| AP | 5.5 | 0.9 | 0.438 |
| Depth (cm) | Site | P | pH | SOC (g/kg) | NH4-N (mg/kg) | TN (g/kg) | NO-3N (mg/kg) | TP (g/kg) | TK (g/kg) | AP (mg/kg) | AK (mg/kg) | MBN (mg/kg) | MBC (mg/kg) | EC(dS/m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 | Wet land | KH | 7.93 ± 0.09 b | 103.87 ± 0.93 c | 35.42 ± 0.11 e | 5.65 ± 0.06 a | 16.53 ± 0.03 a | 0.55 ± 0.01 a | 12.7 ± 0.07 d | 24.43 ± 0.11 e | 127.48 ± 1.09 c | 12.52 ± 0.13 c | 259.79 ± 0.71 a | 211.67 ± 24.45 a |
| Wet land | BS | 7.39 ± 0.05 a | 95.26 ± 0.20 b | 21.37 ± 0.04 a | 4.77 ± 0.09 b | 21.28 ± 0.06 d | 0.52 ± 0.01 a | 8.03 ± 0.08 b | 15.48 ± 0.11 b | 118.24 ± 0.50 b | 12.42 ± 0.02 c | 312.91 ± 0.41 b | 175.33 ± 35.84 a | |
| Dry land | IL | 8.09 ± 0.00 b | 77.02 ± 0.55 a | 26.67 ± 0.09 b | 4.06 ± 0.03 bc | 16.84 ± 0.03 a | 0.55 ± 0.01 a | 6.94 ± 0.11 a | 14.73 ± 0.08 a | 101.96 ± 1.02 a | 10.89 ± 0.06 a | 348.52 ± 0.96 d | 173 ± 26.35 b | |
| Dry land | KH | 8.00 ± 0.01 b | 76.53 ± 0.77 a | 32.4 ± 0.12 d | 4.54 ± 0.03 cd | 19.12 ± 0.05 b | 0.57 ± 0.01 a | 7.82 ± 0.02 b | 20.34 ± 0.09 c | 243.44 ± 1.81 e | 11.89 ± 0.06 b | 321.06 ± 0.81 c | 120.67 ± 6.98 a | |
| Shady slope | EN | 8.05 ± 0.01 b | 110.56 ± 0.24 e | 35.26 ± 0.11 e | 5.14 ± 0.04 d | 23.67 ± 0.13 d | 0.74 ± 0.03 b | 12.68 ± 0.14 d | 23.57 ± 0.04 d | 252.99 ± 2.69 f | 18.48 ± 0.10 e | 505.69 ± 2.08 e | 154.33 ± 1.86 a | |
| Sunny slope | EN | 8.03 ± 0.02 b | 90.31 ± 0.14 b | 30.48 ± 0.12 c | 4.93 ± 0.02 e | 24.12 ± 0.07 e | 0.78 ± 0.02 b | 10.68 ± 0.07 c | 23.88 ± 0.01 d | 234.46 ± 1.52 d | 17.78 ± 0.09 d | 510.83 ± 1.03 e | 119.67 ± 2.91 a | |
| 10–20 | Wet land | KH | 7.94 ± 0.07 b | 83.82 ± 1.39 d | 26.71 ± 0.07 c | 4.68 ± 0.01 b | 15.08 ± 0.06 a | 0.38 ± 0.01 a | 11.49 ± 0.08 d | 17.31 ± 0.18 c | 102.74 ± 1.10 c | 9.83 ± 0.05 c | 204.58 ± 1.67 a | 195.67 ± 12.71 c |
| Wet land | BS | 7.49 ± 0.03 a | 82.41 ± 0.04 cd | 18.51 ± 0.13 a | 3.97 ± 0.07 ab | 18.28 ± 0.09 c | 0.38 ± 0.00 a | 8.5 ± 0.13 b | 11.3 ± 0.12 b | 90.98 ± 0.17 b | 9.39 ± 0.09 c | 287.2 ± 0.98 c | 99.33 ± 10.47 a | |
| Dry land | IL | 8.1 ± 0.01 b | 60.03 ± 0.09 a | 23.65 ± 0.11 b | 3.81 ± 0.02 a | 15.42 ± 0.06 a | 0.38 ± 0.01 a | 6.95 ± 0.08 a | 9.89 ± 0.04 a | 86.73 ± 1.06 a | 7.69 ± 0.11 a | 256.83 ± 1.98 b | 164.67 ± 16.34 bc | |
| Dry land | KH | 7.99 ± 0.00 b | 68.22 ± 0.24 b | 29.44 ± 0.02 d | 3.90 ± 0.01 ab | 16.63 ± 0.13 b | 0.38 ± 0.00 a | 7.3 ± 0.29 a | 17.26 ± 0.04 c | 190.27 ± 0.17 e | 8.80 ± 0.07 b | 258.97 ± 1.16 b | 146.33 ± 11.05 abc | |
| Shady slope | EN | 8.05 ± 0.02 b | 90.41 ± 0.10 e | 26.60 ± 0.10 c | 4.49 ± 0.06 c | 18.58 ± 0.26 c | 0.45 ± 0.02 b | 12.55 ± 0.03 e | 17.5 ± 0.06 cd | 199.46 ± 0.23 f | 14.56 ± 0.13 e | 417.55 ± 8.81 d | 145.67 ± 4.09 abc | |
| Sunny slope | EN | 8.04 ± 0.01 b | 80.34 ± 0.07 c | 26.50 ± 0.15 c | 4.09 ± 0.02 b | 19.91 ± 0.07 d | 0.49 ± 0.00 b | 10.69 ± 0.05 c | 17.71 ± 0.13 d | 180.95 ± 0.13 d | 13.45 ± 0.09 d | 400.82 ± 1.04 d | 130.33 ± 7.05 ab | |
| 20–30 | Wet land | KH | 7.89 ± 0.09 b | 46.57 ± 1.51 b | 25.33 ± 0.14 a | 3.56 ± 0.08 b | 13.07 ± 0.02 b | 0.30 ± 0.00 a | 10.74 ± 0.12 e | 10.12 ± 0.04 c | 95.24 ± 0.73 b | 5.38 ± 0.11 bc | 188.90 ± 0.23 a | 217.33 ± 9.68 d |
| Wet land | BS | 7.47 ± 0.03 a | 56.54 ± 0.26 d | 16.72 ± 0.15 a | 3.22 ± 0.04 a | 15.33 ± 0.06 e | 0.31 ± 0.01 a | 7.34 ± 0.13 c | 9.39 ± 0.07 e | 74.85 ± 0.41 a | 5.23 ± 0.04 bc | 203.90 ± 1.20 c | 106 ± 8.54 a | |
| Dry land | IL | 8.06 ± 0.02 b | 40.02 ± 0.17 a | 15.14 ± 0.07 a | 3.17 ± 0.03 a | 12.84 ± 0.01 a | 0.31 ± 0.00 a | 6.13 ± 0.04 a | 8.73 ± 0.01 a | 74.67 ± 0.95 a | 4.86 0.04 a | 200.27 ± 0.24 b | 198 ± 12.52 cd | |
| Dry land | KH | 8.02 ± 0.00 b | 45.07 ± 0.30 b | 27.61 ± 0.14 a | 3.18 ± 0.03 a | 13.55 ± 0.02 c | 0.31 ± 0.01 a | 6.76 ± 0.18 b | 10.31 ± 0.10 c | 100.26 ± 0.19 c | 5.28 ± 0.03 bc | 200.74 ± 0.30 bc | 159.33 ± 15.49 bc | |
| Shady slope | EN | 8.09 ± 0.01 b | 53.05 ± 1.39 cd | 22.18 ± 0.07 ab | 3.45 ± 0.01 b | 14.12 ± 0.04 d | 0.34 ± 0.02 ab | 11.88 ± 0.01 f | 11.26 ± 0.05 d | 120.08 ± 0.07 e | 5.49 ± 0.11 c | 298.94 ± 0.97 d | 129.66 ± 2.33 ab | |
| Sunny slope | EN | 8.06 ± 0.02 b | 50.76 ± 0.13 c | 24.53 ± 0.21 ab | 3.93 ± 0.01 c | 15.92 ± 0.01 f | 0.37 ± 0.00 b | 9.80 ± 0.04 d | 11.57 ± 0.10 d | 117.08 ± 0.70 d | 5.31 ± 0.05 bc | 300.43 ± 0.43 d | 135.00 ± 2.64 ab |
| Depth (cm) | Site | P | TEMP (°C) | MC (%) | BD (g/cm3) | Porosity (%) |
|---|---|---|---|---|---|---|
| 0–10 | Wet land | KH | 14.33 ± 1.65 a | 65.7 ± 1.70 c | 1.29 ± 0.06 b | 51.19 ± 2.31 b |
| Wet land | BS | 15.9 ± 0.88 a | 64.67 ± 0.33 c | 1.27 ± 0.04 b | 51.95 ± 1.40 b | |
| Dry land | IL | 16.67 ± 0.82 ab | 20.24 ± 1.36 a | 1.77 ± 0.03 c | 33.33 ± 1.20 a | |
| Dry land | KH | 20.23 ± 0.26 bc | 29.93 ± 1.67 b | 0.97 ± 0.05 a | 63.14 ± 1.83 c | |
| Shady slope | EN | 18.37 ± 0.41 ab | 29.7 ± 1.58 b | 1.23 ± 0.06 b | 53.71 ± 2.18 b | |
| Sunny slope | EN | 24.47 ± 0.75 c | 19.54 ± 1.18 a | 1.13 ± 0.04 a | 57.36 ± 1.52 bc | |
| 10–20 | Wet land | KH | 13.3 ± 0.50 a | 66.06 ± 0.90 c | 1.38 ± 0.12 ab | 47.93 ± 4.47 ab |
| Wet land | BS | 13.8 ± 0.40 a | 68.67 ± 0.33 c | 1.35 ± 0.05 ab | 48.91 ± 1.74 ab | |
| Dry land | IL | 14.53 ± 0.12 ab | 19.27 ± 1.03 a | 1.49 ± 0.13 b | 43.77 ± 4.95 a | |
| Dry land | KH | 17.20 ± 0.60 bc | 29.55 ± 1.67 b | 1.04 ± 0.08 a | 60.38 ± 2.72 b | |
| Shady slope | EN | 18.53 ± 0.59 c | 21.91 ± 1.62 a | 1.28 ± 0.04 ab | 51.7 ± 1.52 ab | |
| Sunny slope | EN | 19.10 ± 0.87 c | 19.56 ± 1.65 a | 1.25 ± 0.03 ab | 52.83 ± 0.99 ab | |
| 20–30 | Wet land | KH | 12.56 ± 0.34 a | 60.83 ± 2.33 c | 1.48 ± 0.14 b | 44.15 ± 5.30 a |
| Wet land | BS | 12.93 ± 0.20 a | 67.00 ± 0.00 d | 1.32 ± 0.06 ab | 50.06 ± 2.50 ab | |
| Dry land | IL | 12.96 ± 0.20 ab | 13.65 ± 0.89 a | 1.40 ± 0.05 ab | 48.30 ± 3.35 ab | |
| Dry land | KH | 15.56 ± 0.56 bc | 27.19 ± 1.63 b | 1.08 ± 0.03 a | 59.24 ± 1.43 b | |
| Shady slope | EN | 19.93 ± 0.91 d | 18.86 ± 0.64 a | 1.34 ± 0.06 ab | 49.43 ± 2.56 ab | |
| Sunny slope | EN | 16.23 ± 0.69 c | 15.28 ± 1.22 a | 1.23 ± 0.03 ab | 56.85 ± 2.13 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abalori, T.A.; Cao, W.; Weobong, C.A.-A.; Li, W.; Wang, S.; Deng, X. Spatial Vegetation Patch Patterns and Their Relation to Environmental Factors in the Alpine Grasslands of the Qilian Mountains. Sustainability 2022, 14, 6738. https://doi.org/10.3390/su14116738
Abalori TA, Cao W, Weobong CA-A, Li W, Wang S, Deng X. Spatial Vegetation Patch Patterns and Their Relation to Environmental Factors in the Alpine Grasslands of the Qilian Mountains. Sustainability. 2022; 14(11):6738. https://doi.org/10.3390/su14116738
Chicago/Turabian StyleAbalori, Theophilus Atio, Wenxia Cao, Conrad Atogi-Akwoa Weobong, Wen Li, Shilin Wang, and Xiuxia Deng. 2022. "Spatial Vegetation Patch Patterns and Their Relation to Environmental Factors in the Alpine Grasslands of the Qilian Mountains" Sustainability 14, no. 11: 6738. https://doi.org/10.3390/su14116738
APA StyleAbalori, T. A., Cao, W., Weobong, C. A.-A., Li, W., Wang, S., & Deng, X. (2022). Spatial Vegetation Patch Patterns and Their Relation to Environmental Factors in the Alpine Grasslands of the Qilian Mountains. Sustainability, 14(11), 6738. https://doi.org/10.3390/su14116738





