Overview of Environmental and Health Effects Related to Glyphosate Usage
Abstract
:1. Introduction
2. Environmental Impact of Glyphosate
2.1. Behavior and Fate of Glyphosate
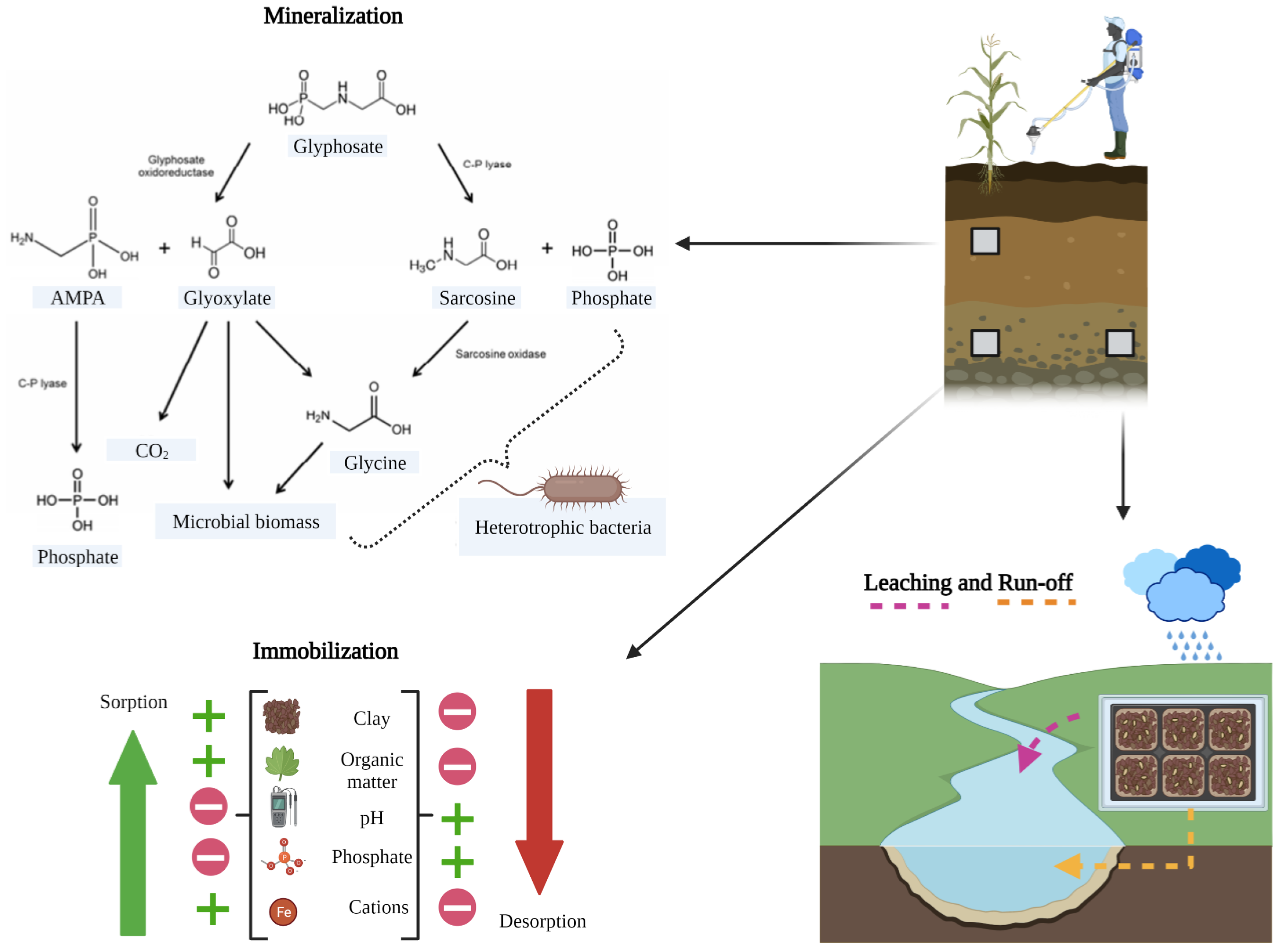
2.2. Glyphosate Residuality
3. Health Effects of Glyphosate
3.1. Human Health Effects
3.2. Health Effects on Other Organisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Benbrook, C.; Antoniou, M.N. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem. Toxicol. 2019, 128, 137–145. [Google Scholar] [CrossRef]
- Ren, Z.; Dong, Y.; Liu, Y. Enhanced glyphosate removal by montmorillonite in the presence of Fe(III). Ind. Eng. Chem. Res. 2014, 53, 14485–14492. [Google Scholar] [CrossRef]
- Martinez, D.A.; Loening, U.E.; Graham, M.C. Impacts of glyphosate-based herbicides on disease resistance and health of crops: A review. Environ. Sci. Eur. 2018, 30, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabalza, A.; Orcaray, L.; Fernández-Escalada, M.; Zulet-González, A.; Royuela, M. The pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pestic. Biochem. Physiol. 2017, 141, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaner, D.L.; Lindenmeyer, R.B.; Ostlie, M.H. What have the mechanisms of resistance to glyphosate taught us? Pest Manag. Sci. 2012, 68, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on plant mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J. Agric. Food Chem. 2012, 60, 10375–10397. [Google Scholar] [CrossRef]
- Shehata, A.A.; Schrödl, W.; Aldin, A.A.; Hafez, H.M.; Krüger, M. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr. Microbiol. 2013, 66, 350–358. [Google Scholar] [CrossRef]
- Villamar-Ayala, C.A.; Carrera-Cevallos, J.V.; Vasquez-Medrano, R.; Espinoza-Montero, P.J. Fate, eco-toxicological characteristics, and treatment processes applied to water polluted with glyphosate: A critical review. Crit. Rev. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Rolando, C.; Baillie, B.; Thompson, D.; Little, K. The Risks Associated with Glyphosate-Based Herbicide Use in Planted Forests. Forests 2017, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Clements, D.; Dugdale, T.M.; Butler, K.L.; Florentine, S.K.; Sillitoe, J. Herbicide efficacy for aquatic Alternanthera philoxeroides management in an early stage of invasion: Integrating above-ground biomass, below-ground biomass and viable stem fragmentation. Weed Res. 2017, 57, 257–266. [Google Scholar] [CrossRef]
- Maqueda, C.; Undabeytia, T.; Villaverde, J.; Morillo, E. Behaviour of glyphosate in a reservoir and the surrounding agricultural soils. Sci. Total Environ. 2017, 593–594, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, E.; Allinson, M.; Barral, M.P.; Clarke, B.; Allinson, G. Glyphosate and aminomethylphosphonic acid (AMPA) are commonly found in urban streams and wetlands of Melbourne, Australia. Water Res. 2020, 168, 115139. [Google Scholar] [CrossRef]
- Matozzo, V.; Fabrello, J.; Marin, M.G. The effects of glyphosate and its commercial formulations to marine invertebrates: A review. J. Mar. Sci. Eng. 2020, 8, 399. [Google Scholar] [CrossRef]
- Green, J.M. The rise and future of glyphosate and glyphosate-resistant crops. Pest Manag. Sci. 2018, 74, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Brookes, G.; Taheripour, F.; Tyner, W.E. The contribution of glyphosate to agriculture and potential impact of restrictions on use at the global level. GM Crop. Food 2017, 8, 216–228. [Google Scholar] [CrossRef] [Green Version]
- Hearon, S.E.; Wang, M.; McDonald, T.J.; Phillips, T.D. Decreased bioavailability of aminomethylphosphonic acid (AMPA) in genetically modified corn with activated carbon or calcium montmorillonite clay inclusion in soil. J. Environ. Sci. 2021, 100, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Bergström, L.; Börjesson, E.; Stenström, J. Laboratory and Lysimeter Studies of Glyphosate and Aminomethylphosphonic Acid in a Sand and a Clay Soil. J. Environ. Qual. 2011, 40, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Heap, I.; Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, H.; Jaisi, D.P. Degradation of glyphosate and bioavailability of phosphorus derived from glyphosate in a soil-water system. Water Res. 2019, 163, 114840. [Google Scholar] [CrossRef] [PubMed]
- Rainio, M.J.; Ruuskanen, S.; Helander, M.; Saikkonen, K.; Saloniemi, I.; Puigbò, P. Adaptation of bacteria to glyphosate: A microevolutionary perspective of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Environ. Microbiol. Rep. 2021, 13, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Delkash-Roudsari, S.; Chicas-Mosier, A.M.; Goldansaz, S.H.; Talebi-Jahromi, K.; Ashouri, A.; Abramson, C.I. Assessment of lethal and sublethal effects of imidacloprid, ethion, and glyphosate on aversive conditioning, motility, and lifespan in honey bees (Apis mellifera L.). Ecotoxicol. Environ. Saf. 2020, 204, 111108. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Herek, J.S.; Vargas, L.; Trindade, S.A.R.; Rutkoski, C.F.; Macagnan, N.; Hartmann, P.A.; Hartmann, M.T. Can environmental concentrations of glyphosate affect survival and cause malformation in amphibians? Effects from a glyphosate-based herbicide on Physalaemus cuvieri and P. gracilis (Anura: Leptodactylidae). Environ. Sci. Pollut. Res. 2020, 27, 22619–22630. [Google Scholar] [CrossRef]
- Faria, M.; Bedrossiantz, J.; Ramírez, J.R.R.; Mayol, M.; García, G.H.; Bellot, M.; Prats, E.; Garcia-Reyero, N.; Gómez-Canela, C.; Gómez-Oliván, L.M.; et al. Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environ. Int. 2021, 146, 106253. [Google Scholar] [CrossRef]
- Agostini, L.P.; Dettogni, R.S.; dos Reis, R.S.; Stur, E.; dos Santos, E.V.W.; Ventorim, D.P.; Garcia, F.M.; Cardoso, R.C.; Graceli, J.B.; Louro, I.D. Effects of glyphosate exposure on human health: Insights from epidemiological and in vitro studies. Sci. Total Environ. 2020, 705, 135808. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Autthority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 107. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; Straif, K.; Blair, A.; et al. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Evaluation of Five Organophosphate Insecticides and Herbicides. IARC Monographs Volume 112. Available online: https://www.iarc.who.int/news-events/iarc-monographs-volume-112-evaluation-of-five-organophosphate-insecticides-and-herbicides/ (accessed on 8 January 2021).
- Beckie, H.J.; Flower, K.C.; Ashworth, M.B. Farming without Glyphosate? Plants 2020, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudsk, P.; Mathiassen, S.K. Pesticide regulation in the European Union and the glyphosate controversy. Weed Sci. 2020, 68, 214–222. [Google Scholar] [CrossRef]
- Antier, C.; Kudsk, P.; Reboud, X.; Ulber, L.; Baret, P.V.; Messéan, A. Glyphosate Use in the European Agricultural Sector and a Framework for Its Further Monitoring. Sustainability 2020, 12, 5682. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [Green Version]
- Alcántara-de la Cruz, R.; Cruz-Hipolito, H.E.; Domínguez-Valenzuela, J.A.; De Prado, R. Glyphosate ban in Mexico: Potential impacts on agriculture and weed management. Pest Manag. Sci. 2021, 77, 3820–3831. [Google Scholar] [CrossRef]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Asaduzzaman, M.; Parven, A.; Megharaj, M. Controversies over human health and ecological impacts of glyphosate: Is it to be banned in modern agriculture? Environ. Pollut. 2020, 263, 114372. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Aardema, M.; Acquavella, J.; Berry, S.C.; Brusick, D.; Burns, M.M.; de Camargo, J.L.V.; Garabrant, D.; Greim, H.A.; Kier, L.D.; et al. A review of the carcinogenic potential of glyphosate by four independent expert panels and comparison to the IARC assessment. Crit. Rev. Toxicol. 2016, 46, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Klingelhöfer, D.; Braun, M.; Brüggmann, D.; Groneberg, D.A. Glyphosate: How do ongoing controversies, market characteristics, and funding influence the global research landscape? Sci. Total Environ. 2021, 765, 144271. [Google Scholar] [CrossRef]
- la Cecilia, D.; Maggi, F. Analysis of glyphosate degradation in a soil microcosm. Environ. Pollut. 2018, 233, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajab, A.J.; Hakami, O.M. Behavior of the non-selective herbicide glyphosate in agricultural soil. Am. J. Environ. Sci. 2014, 10, 94–101. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Luo, J.; Wu, Z.; Wang, L.; Li, B.; Wang, Y.; Sun, G. Degradation dynamics of glyphosate in different types of citrus orchard soils in China. Molecules 2015, 20, 1161–1175. [Google Scholar] [CrossRef] [Green Version]
- Mamy, L.; Barriuso, E.; Gabrielle, B. Environmental fate of herbicides trifluralin, metazachlor, metamitron and sulcotrione compared with that of glyphosate, a substitute broad spectrum herbicide for different glyphosate-resistant crops. Pest Manag. Sci. 2005, 61, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, S.H.; Hollister, E.B.; Senseman, S.A.; Gentry, T.J. Effects of repeated glyphosate applications on soil microbial community composition and the mineralization of glyphosate. Pest Manag. Sci. 2010, 66, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ololade, I.A.; Oladoja, N.A.; Oloye, F.F.; Alomaja, F.; Akerele, D.D.; Iwaye, J.; Aikpokpodion, P. Sorption of Glyphosate on Soil Components: The Roles of Metal Oxides and Organic Materials. Soil Sediment Contam. 2014, 23, 571–585. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Dörfler, U.; Welzl, G.; Munch, J.C.; Schroll, R.; Suhadolc, M. Large variation in glyphosate mineralization in 21 different agricultural soils explained by soil properties. Sci. Total Environ. 2018, 627, 544–552. [Google Scholar] [CrossRef]
- Muskus, A.M.; Krauss, M.; Miltner, A.; Hamer, U.; Nowak, K.M. Effect of temperature, pH and total organic carbon variations on microbial turnover of 13C315N-glyphosate in agricultural soil. Sci. Total Environ. 2019, 658, 697–707. [Google Scholar] [CrossRef]
- Laitinen, P.; Rämö, S.; Nikunen, U.; Jauhiainen, L.; Siimes, K.; Turtola, E. Glyphosate and phosphorus leaching and residues in boreal sandy soil. Plant Soil 2009, 323, 267–283. [Google Scholar] [CrossRef]
- Okada, E.; Costa, J.L.; Bedmar, F. Glyphosate Dissipation in Different Soils Under No-Till and Conventional Tillage. Pedosphere 2019, 29, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, L.; Fomsgaard, I.S.; Svensmark, B.; Spliid, N.H. Fate and availability of glyphosate and AMPA in agricultural soil. J. Environ. Sci. Health Part B 2008, 43, 365–375. [Google Scholar] [CrossRef]
- Carretta, L.; Cardinali, A.; Onofri, A.; Masin, R.; Zanin, G. Dynamics of Glyphosate and Aminomethylphosphonic Acid in Soil Under Conventional and Conservation Tillage. Int. J. Environ. Res. 2021, 15, 1037–1055. [Google Scholar] [CrossRef]
- Veiga, F.; Zapata, J.M.; Fernandez Marcos, M.L.; Alvarez, E. Dynamics of glyphosate and aminomethylphosphonic acid in a forest soil in Galicia, north-west Spain. Sci. Total Environ. 2001, 271, 135–144. [Google Scholar] [CrossRef]
- Gómez Ortiz, A.M.; Okada, E.; Bedmar, F.; Costa, J.L. Sorption and desorption of glyphosate in Mollisols and Ultisols soils of Argentina. Environ. Toxicol. Chem. 2017, 36, 2587–2592. [Google Scholar] [CrossRef]
- Sidoli, P.; Baran, N.; Angulo-Jaramillo, R. Glyphosate and AMPA adsorption in soils: Laboratory experiments and pedotransfer rules. Environ. Sci. Pollut. Res. 2016, 23, 5733–5742. [Google Scholar] [CrossRef]
- Padilla, J.T.; Selim, H.M. Interactions among Glyphosate and Phosphate in Soils: Laboratory Retention and Transport Studies. J. Environ. Qual. 2019, 48, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shushkova, T.V.; Vasilieva, G.K.; Ermakova, I.T.; Leontievsky, A.A. Sorption and microbial degradation of glyphosate in soil suspensions. Appl. Biochem. Microbiol. 2009, 45, 599–603. [Google Scholar] [CrossRef]
- Piccolo, A.; Celano, G.; Conte, P. Adsorption of Glyphosate by Humic Substances. J. Agric. Food Chem. 1996, 44, 2442–2446. [Google Scholar] [CrossRef]
- Grandcoin, A.; Piel, S.; Baurès, E. Amino Methyl Phosphonic acid (AMPA) in natural waters: Its sources, behavior and environmental fate. Water Res. 2017, 117, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, T.; Moldrup, P.; Ferré, T.P.A.; Olsen, P.; Rosenbom, A.E.; de Jonge, L.W. Leaching of Glyphosate and Aminomethylphosphonic Acid from an Agricultural Field over a Twelve-Year Period. Vadose Zo. J. 2014, 13, 1–18. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: A review. Pest Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef]
- Skeff, W.; Neumann, C.; Schulz-Bull, D.E. Glyphosate and AMPA in the estuaries of the Baltic Sea method optimization and field study. Mar. Pollut. Bull. 2015, 100, 577–585. [Google Scholar] [CrossRef]
- Van Stempvoort, D.R.; Roy, J.W.; Brown, S.J.; Bickerton, G. Residues of the herbicide glyphosate in riparian groundwater in urban catchments. Chemosphere 2014, 95, 455–463. [Google Scholar] [CrossRef]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Heal. 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagner, M.; Mikola, J.; Saloniemi, I.; Saikkonen, K.; Helander, M. Effects of a glyphosate-based herbicide on soil animal trophic groups and associated ecosystem functioning in a northern agricultural field. Sci. Rep. 2019, 9, 8540. [Google Scholar] [CrossRef] [PubMed]
- Gonze, D.; Coyte, K.Z.; Lahti, L.; Faust, K. Microbial communities as dynamical systems. Curr. Opin. Microbiol. 2018, 44, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ingham, R.E.; Trofymow, J.A.; Ingham, E.R.; Coleman, D.C. Interactions of Bacteria, Fungi, and their Nematode Grazers: Effects on Nutrient Cycling and Plant Growth. Ecol. Monogr. 1985, 55, 119–140. [Google Scholar] [CrossRef]
- Pulleman, M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Pérès, G.; Rutgers, M. Soil biodiversity, biological indicators and soil ecosystem services-an overview of European approaches. Curr. Opin. Environ. Sustain. 2012, 4, 529–538. [Google Scholar] [CrossRef]
- García-Pérez, J.A.; Alarcón-Gutiérrez, E.; Perroni, Y.; Barois, I. Earthworm communities and soil properties in shaded coffee plantations with and without application of glyphosate. Appl. Soil Ecol. 2014, 83, 230–237. [Google Scholar] [CrossRef]
- Wolmarans, K.; Swart, W.J. Influence of glyphosate, other herbicides and genetically modified herbicide-resistant crops on soil microbiota: A review. S. Afr. J. Plant Soil 2014, 31, 177–186. [Google Scholar] [CrossRef]
- Allegrini, M.; Zabaloy, M.C.; Gómez, E. del V. Ecotoxicological assessment of soil microbial community tolerance to glyphosate. Sci. Total Environ. 2015, 533, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Druille, M.; Cabello, M.N.; García Parisi, P.A.; Golluscio, R.A.; Omacini, M. Glyphosate vulnerability explains changes in root-symbionts propagules viability in pampean grasslands. Agric. Ecosyst. Environ. 2015, 202, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Riah, W.; Laval, K.; Laroche-Ajzenberg, E.; Mougin, C.; Latour, X.; Trinsoutrot-Gattin, I. Effects of pesticides on soil enzymes: A review. Environ. Chem. Lett. 2014, 12, 257–273. [Google Scholar] [CrossRef]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Botta, F.; Lavison, G.; Couturier, G.; Alliot, F.; Moreau-Guigon, E.; Fauchon, N.; Guery, B.; Chevreuil, M.; Blanchoud, H. Transfer of glyphosate and its degradate AMPA to surface waters through urban sewerage systems. Chemosphere 2009, 77, 133–139. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Wang, S.; Seiwert, B.; Kästner, M.; Miltner, A.; Schäffer, A.; Reemtsma, T.; Yang, Q.; Nowak, K.M. (Bio)degradation of glyphosate in water-sediment microcosms—A stable isotope co-labeling approach. Water Res. 2016, 99, 91–100. [Google Scholar] [CrossRef]
- Braz-Mota, S.; Sadauskas-Henrique, H.; Duarte, R.M.; Val, A.L.; Almeida-Val, V.M.F. Roundup® exposure promotes gills and liver impairments, DNA damage and inhibition of brain cholinergic activity in the Amazon teleost fish Colossoma macropomum. Chemosphere 2015, 135, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hued, A.C.; Oberhofer, S.; De Los Ángeles Bistoni, M. Exposure to a commercial glyphosate formulation (Roundup®) alters normal gill and liver histology and affects male sexual activity of Jenynsia multidentata (Anablepidae, cyprinodontiformes). Arch. Environ. Contam. Toxicol. 2012, 62, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.L.; von Mérey, G.; Minderhout, T.; Manson, P.; Sutton, P. Aminomethylphosphonic acid has low chronic toxicity to Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2015, 34, 1382–1389. [Google Scholar] [CrossRef]
- Struger, J.; Thompson, D.; Staznik, B.; Martin, P.; McDaniel, T.; Marvin, C. Occurrence of glyphosate in surface waters of southern Ontario. Bull. Environ. Contam. Toxicol. 2008, 80, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Von Osten, J.; Dzul-Caamal, R. Glyphosate residues in groundwater, drinking water and urine of subsistence farmers from intensive agriculture localities: A survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Rocha, D.C.; Moreira de Brito, J.C.; Tavares, D.S.; Marques, R.Z.; Soffiatti, P.; Sant’Anna-Santos, B.F. Emerging contaminants in water used for maize irrigation: Economic and food safety losses associated with ciprofloxacin and glyphosate. Ecotoxicol. Environ. Saf. 2020, 196, 110549. [Google Scholar] [CrossRef]
- Gunarathna, S.; Gunawardana, B.; Jayaweera, M.; Manatunge, J.; Zoysa, K. Glyphosate and AMPA of agricultural soil, surface water, groundwater and sediments in areas prevalent with chronic kidney disease of unknown etiology, Sri Lanka. J. Environ. Sci. Heal. Part B Pestic. Food Contam. Agric. Wastes 2018, 53, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, K.; Peterson, D. Soybean (Glycine max) Response to Simulated Drift from Selected Sulfonylurea Herbicides, Dicamba, Glyphosate, and Glufosinate. Weed Technol. 1999, 13, 264–270. [Google Scholar] [CrossRef]
- Kolberg, R.; Wiles, L. Effect of Steam Application on Cropland Weeds1. Weed Technol. 2002, 16, 43–49. [Google Scholar] [CrossRef]
- Reddy, K.N.; Rimando, A.M.; Duke, S.O. Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J. Agric. Food Chem. 2004, 52, 5139–5143. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Zentner, R.P.; Basnyat, P.; Gehl, D.; Selles, F.; Huber, D. Glyphosate associations with cereal diseases caused by Fusarium spp. in the Canadian Prairies. Eur. J. Agron. 2009, 31, 133–143. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Zentner, R.P.; DePauw, R.M.; Gehl, D.; Stevenson, F.C. Impacts of crop production factors on common root rot of barley in Eastern Saskatchewan. Crop Sci. 2007, 47, 1585–1595. [Google Scholar] [CrossRef]
- Kanissery, R.; Alferez, F.; Batuman, O. Glyphosate related fruit drop in citrus. EDIS 2018, 2018. [Google Scholar]
- Zoller, O.; Rhyn, P.; Rupp, H.; Zarn, J.A.; Geiser, C. Glyphosate residues in Swiss market foods: Monitoring and risk evaluation. Food Addit. Contam. Part B Surveill. 2018, 11, 83–91. [Google Scholar] [CrossRef]
- Saunders, L.E.; Pezeshki, R. Glyphosate in runoffwaters and in the root-zone: A review. Toxics 2015, 3, 462–480. [Google Scholar] [CrossRef] [Green Version]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef]
- Sesin, V.; Davy, C.M.; Stevens, K.J.; Hamp, R.; Freeland, J.R. Glyphosate Toxicity to Native Nontarget Macrophytes Following Three Different Routes of Incidental Exposure. Integr. Environ. Assess. Manag. 2021, 17, 597–613. [Google Scholar] [CrossRef] [PubMed]
- de Brito Rodrigues, L.; Gonçalves Costa, G.; Lundgren Thá, E.; da Silva, L.R.; de Oliveira, R.; Morais Leme, D.; Cestari, M.M.; Koppe Grisolia, C.; Campos Valadares, M.; de Oliveira, G.A.R. Impact of the glyphosate-based commercial herbicide, its components and its metabolite AMPA on non-target aquatic organisms. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 842, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Soukup, S.T.; Merz, B.; Bub, A.; Hoffmann, I.; Watzl, B.; Steinberg, P.; Kulling, S.E. Glyphosate and AMPA levels in human urine samples and their correlation with food consumption: Results of the cross-sectional KarMeN study in Germany. Arch. Toxicol. 2020, 94, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, M.; Schledorn, P.; Schrödl, W.; Hoppe, H.-W.; Lutz, W.; Shehata, A.A. Detection of Glyphosate Residues in Animals and Humans. J Env. Anal Toxicol 2014, 4, 2. [Google Scholar] [CrossRef]
- Niemann, L.; Sieke, C.; Pfeil, R.; Solecki, R. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J. Verbraucherschutz Leb. 2015, 10, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Torretta, V.; Katsoyiannis, I.A.; Viotti, P.; Rada, E.C. Critical review of the effects of glyphosate exposure to the environment and humans through the food supply chain. Sustainability 2018, 10, 950. [Google Scholar] [CrossRef] [Green Version]
- Gillezeau, C.; Van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health A Glob. Access Sci. Source 2019, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Vigfusson, N.V.; Vyse, E.R. The effect of the pesticides, dexon, captan and roundup, on sister-chromatid exchanges in human lymphocytes in vitro. Mutat. Res. Genet. Toxicol. 1980, 79, 53–57. [Google Scholar] [CrossRef]
- Alvarez-Moya, C.; Silva, M.R.; Valdez Ramírez, C.; Gallardo, D.G.; León Sánchez, R.; Aguirre, A.C.; Velasco, A.F. Comparison of the in vivo and in vitro genotoxicity of glyphosate isopropylamine salt in three different organisms. Genet. Mol. Biol. 2014, 37, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Santovito, A.; Ruberto, S.; Gendusa, C.; Cervella, P. In vitro evaluation of genomic damage induced by glyphosate on human lymphocytes. Environ. Sci. Pollut. Res. 2018, 25, 34693–34700. [Google Scholar] [CrossRef]
- Martínez, A.; Reyes, I.; Reyes, N. Citotoxicidad del glifosato en células mononucleares de sangre periférica humana. Biomédica 2007, 27, 594. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, E.; Sicińska, P.; Michałowicz, J.; Woźniak, K.; Reszka, E.; Huras, B.; Zakrzewski, J.; Bukowska, B. The mechanism of DNA damage induced by Roundup 360 PLUS, glyphosate and AMPA in human peripheral blood mononuclear cells—genotoxic risk assessement. Food Chem. Toxicol. 2018, 120, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Huras, B.; Bukowska, B. The effect of metabolites and impurities of glyphosate on human erythrocytes (in vitro). Pestic. Biochem. Physiol. 2014, 109, 34–43. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Nowacka-Krukowska, H.; Bukowska, B. The effect of glyphosate, its metabolites and impurities on erythrocyte acetylcholinesterase activity. Environ. Toxicol. Pharmacol. 2014, 37, 1101–1108. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Jarosiewicz, P.; Michałowicz, J.; Koter-Michalak, M.; Huras, B.; Bukowska, B. The impact of glyphosate, its metabolites and impurities on viability, ATP level and morphological changes in human peripheral blood mononuclear cells. PLoS ONE 2016, 11, e0156946. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Reszka, E.; Woźniak, K.; Jabłońska, E.; Michałowicz, J.; Bukowska, B. DNA damage and methylation induced by glyphosate in human peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2017, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Elie-Caille, C.; Heu, C.; Guyon, C.; Nicod, L. Morphological damages of a glyphosate-treated human keratinocyte cell line revealed by a micro-to nanoscale microscopic investigation. Cell Biol. Toxicol. 2010, 26, 331–339. [Google Scholar] [CrossRef]
- Heu, C.; Berquand, A.; Elie-Caille, C.; Nicod, L. Glyphosate-induced stiffening of HaCaT keratinocytes, a Peak Force Tapping study on living cells. J. Struct. Biol. 2012, 178, 1–7. [Google Scholar] [CrossRef]
- Mañas, F.; Peralta, L.; Raviolo, J.; Ovando, H.G.; Weyers, A.; Ugnia, L.; Cid, M.G.; Larripa, I.; Gorla, N. Genotoxicity of glyphosate assessed by the comet assay and cytogenetic tests. Environ. Toxicol. Pharmacol. 2009, 28, 37–41. [Google Scholar] [CrossRef]
- Mesnage, R.; Bernay, B.; Séralini, G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef]
- Benachour, N.; Séralini, G.E. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 2009, 22, 97–105. [Google Scholar] [CrossRef]
- Benachour, N.; Sipahutar, H.; Moslemi, S.; Gasnier, C.; Travert, C.; Séralini, G.E. Time- and dose-dependent effects of roundup on human embryonic and placental cells. Arch. Environ. Contam. Toxicol. 2007, 53, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Al-Ahmad, A.J. Effects of glyphosate and aminomethylphosphonic acid on an isogeneic model of the human blood-brain barrier. Toxicol. Lett. 2019, 304, 39–49. [Google Scholar] [CrossRef]
- Gao, H.; Chen, J.; Ding, F.; Chou, X.; Zhang, X.; Wan, Y.; Hu, J.; Wu, Q. Activation of the N-methyl-d-aspartate receptor is involved in glyphosate-induced renal proximal tubule cell apoptosis. J. Appl. Toxicol. 2019, 39, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Biserni, M.; Wozniak, E.; Xenakis, T.; Mein, C.A.; Antoniou, M.N. Comparison of transcriptome responses to glyphosate, isoxaflutole, quizalofop-p-ethyl and mesotrione in the HepaRG cell line. Toxicol. Rep. 2018, 5, 819–826. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Ni, H.; Gao, J.; Yang, Y.; Xu, W.; Tao, L. Evaluation of the cytotoxic effects of glyphosate herbicides in human liver, lung, and nerve. J. Environ. Sci. Heal. Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 737–744. [Google Scholar] [CrossRef]
- Kašuba, V.; Milić, M.; Rozgaj, R.; Kopjar, N.; Mladinić, M.; Žunec, S.; Vrdoljak, A.L.; Pavičić, I.; Čermak, A.M.M.; Pizent, A.; et al. Effects of low doses of glyphosate on DNA damage, cell proliferation and oxidative stress in the HepG2 cell line. Environ. Sci. Pollut. Res. 2017, 24, 19267–19281. [Google Scholar] [CrossRef]
- Stur, E.; Aristizabal-Pachon, A.F.; Peronni, K.C.; Agostini, L.P.; Waigel, S.; Chariker, J.; Miller, D.M.; Thomas, S.D.; Rezzoug, F.; Detogni, R.S.; et al. Glyphosate-based herbicides at low doses affect canonical pathways in estrogen positive and negative breast cancer cell lines. PLoS ONE 2019, 14, e0219610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnage, R.; Phedonos, A.; Biserni, M.; Arno, M.; Balu, S.; Corton, J.C.; Ugarte, R.; Antoniou, M.N. Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem. Toxicol. 2017, 108, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lambrechts, M.J.; Zhang, Q.; Liu, S.; Ge, D.; Yin, R.; Xi, M.; You, Z. Glyphosate and AMPA inhibit cancer cell growth through inhibiting intracellular glycine synthesis. Drug Des. Devel. Ther. 2013, 7, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Anifandis, G.; Katsanaki, K.; Lagodonti, G.; Messini, C.; Simopoulou, M.; Dafopoulos, K.; Daponte, A. The effect of glyphosate on human sperm motility and sperm DNA fragmentation. Int. J. Environ. Res. Public Health 2018, 15, 1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrigan, P.J.; Belpoggi, F. The need for independent research on the health effects of glyphosate-based herbicides. Environ. Health A Glob. Access Sci. Source 2018, 17, 51. [Google Scholar] [CrossRef]
- Leon, M.E.; Schinasi, L.H.; Lebailly, P.; Beane Freeman, L.E.; Nordby, K.C.; Ferro, G.; Monnereau, A.; Brouwer, M.; Tual, S.; Baldi, I.; et al. Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: A pooled analysis from the AGRICOH consortium. Int. J. Epidemiol. 2019, 48, 1519–1535. [Google Scholar] [CrossRef]
- Hoppin, J.A.; Umbach, D.M.; Long, S.; London, S.J.; Henneberger, P.K.; Blair, A.; Alavanja, M.; Freeman Beane, L.E.; Sandler, D.P. Pesticides are associated with allergic and non-allergic wheeze among male farmers. Environ. Health Perspect. 2017, 125, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.; Amiri, S.; Denney, J.T.; Monsivais, P.; Hystad, P.; Amram, O. Estimated residential exposure to agricultural chemicals and premature mortality by Parkinson’s disease in Washington state. Int. J. Environ. Res. Public Health 2018, 15, 2885. [Google Scholar] [CrossRef] [Green Version]
- Von Ehrenstein, O.S.; Ling, C.; Cui, X.; Cockburn, M.; Park, A.S.; Yu, F.; Wu, J.; Ritz, B. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: Population based case-control study. BMJ 2019, 364, 962. [Google Scholar] [CrossRef] [Green Version]
- Parvez, S.; Gerona, R.R.; Proctor, C.; Friesen, M.; Ashby, J.L.; Reiter, J.L.; Lui, Z.; Winchester, P.D. Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environ. Health A Glob. Access Sci. Source 2018, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Zaller, J.G.; Brühl, C.A. Editorial: Non-target Effects of Pesticides on Organisms Inhabiting Agroecosystems. Front. Environ. Sci. 2019, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar] [CrossRef]
- Howe, C.M.; Berrill, M.; Pauli, B.D.; Helbing, C.C.; Werry, K.; Veldhoen, N. Toxicity of glyphosate-based pesticides to four North American frog species. Environ. Toxicol. Chem. 2004, 23, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.J.; Fuentes, L.; Rodgers, J.H.; Bowerman, W.W.; Yarrow, G.K.; Chao, W.Y.; Bridges, W.C. Relative toxicity of the components of the original formulation of Roundup® to five North American anurans. Ecotoxicol. Environ. Saf. 2012, 78, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Brown, G.G.; Sautter, K.D.; De Oliveira, C.M.R.; De Vasconcelos, E.C.; Niva, C.C.; Bartz, M.L.C.; Bedano, J.C. Toxicity of AMPA to the earthworm Eisenia andrei Bouché, 1972 in tropical artificial soil. Sci. Rep. 2016, 6, 19731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.T.; Frans, R.E.; Talbet, R.E. Reactions of Euglena gracilis to fluometuron, MSMA, metribuzin, and glyphosate. Weed Sci. 1979, 619–624. [Google Scholar] [CrossRef]
- Newman, M.M.; Hoilett, N.; Lorenz, N.; Dick, R.P.; Liles, M.R.; Ramsier, C.; Kloepper, J.W. Glyphosate effects on soil rhizosphere-associated bacterial communities. Sci. Total Environ. 2016, 543, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Estok, D.; Freedman, B.; Boyle, D. Effects of the herbicides 2,4-D, glyphosate, hexazinone, and triclopyr on the growth of three species of ectomycorrhizal fungi. Bull. Environ. Contam. Toxicol. 1989, 42, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Paul Kittle, R.; McDermid, K.J. Glyphosate herbicide toxicity to native Hawaiian macroalgal and seagrass species. J. Appl. Phycol. 2016, 28, 2597–2604. [Google Scholar] [CrossRef]
- Whiles, M.R.; Charlton, R.E. The ecological significance of tallgrass prairie arthropods. Annu. Rev. Entomol. 2006, 51, 387–412. [Google Scholar] [CrossRef] [Green Version]
- Alberdi, J.L.; Sàenz, M.E.; Di Marzio, W.D.; Tortorelli, M.C. Comparative acute toxicity of two herbicides, paraquat and glyphosate, to Daphnia magna and D. spinulata. Bull. Environ. Contam. Toxicol. 1996, 57, 229–235. [Google Scholar] [CrossRef]
- Frontera, J.L.; Vatnick, I.; Chaulet, A.; Rodríguez, E.M. Effects of glyphosate and polyoxyethylenamine on growth and energetic reserves in the freshwater crayfish Cherax quadricarinatus (Decapoda, Parastacidae). Arch. Environ. Contam. Toxicol. 2011, 61, 590–598. [Google Scholar] [CrossRef]
- Balbuena, M.S.; Tison, L.; Hahn, M.L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef] [Green Version]
- Bueno, A. de F.; Bueno, R.C.O. de F.; Parra, J.R.P.; Vieira, S.S. Efeitos dos agroquimicos utilizados na cultura da soja ao parasitoide de ovos Trichogramma pretiosum. Cienc. Rural 2008, 38, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- Tate, T.M.; Spurlock, J.O.; Christian, F.A. Effect of glyphosate on the development of Pseudosuccinea columella snails. Arch. Environ. Contam. Toxicol. 1997, 33, 286–289. [Google Scholar] [CrossRef]
- Druart, C.; Millet, M.; Scheifler, R.; Delhomme, O.; de Vaufleury, A. Glyphosate and glufosinate-based herbicides: Fate in soil, transfer to, and effects on land snails. J. Soils Sediments 2011, 11, 1373–1384. [Google Scholar] [CrossRef]
- Murussi, C.R.; Costa, M.D.; Leitemperger, J.W.; Guerra, L.; Rodrigues, C.C.R.; Menezes, C.C.; Severo, E.S.; Flores-Lopes, F.; Salbego, J.; Loro, V.L. Exposure to different glyphosate formulations on the oxidative and histological status of Rhamdia quelen. Fish Physiol. Biochem. 2016, 42, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Bach, N.C.; Natale, G.S.; Somoza, G.M.; Ronco, A.E. Effect on the growth and development and induction of abnormalities by a glyphosate commercial formulation and its active ingredient during two developmental stages of the South-American Creole frog, Leptodactylus latrans. Environ. Sci. Pollut. Res. 2016, 23, 23959–23971. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Rainio, M.J.; Uusitalo, M.; Saikkonen, K.; Helander, M. Effects of parental exposure to glyphosate-based herbicides on embryonic development and oxidative status: A long-term experiment in a bird model. Sci. Rep. 2020, 10, 6349. [Google Scholar] [CrossRef] [Green Version]

| Glyphosate Dose (kg ha−1) | Half-Life (d) | Soil Properties | Location | Reference |
|---|---|---|---|---|
| 1.54 | 98 (Gly), 51 (AMPA) | Clay and sandy | Sweden | [20] |
| 5 | 42 (Gly) | Loamy | China | [43] |
| 1 | 613 (Gly and AMPA) | Boreal sandy | Finland | [49] |
| 2.1 | 60 (Gly and AMPA) | Silty loam | Argentina | [50] |
| 0.25 | 9 (Gly) and 32 (AMPA) | Sandy | Denmark | [51] |
| 1.8 | 18 (Gly) and 250 (AMPA) | Sandy | Italy | [52] |
| 8 | 31 (Gly and AMPA) | Sandy loam | Spain | [53] |
| Food Source | Glyphosate (mg kg−1) | AMPA (mg kg−1) |
|---|---|---|
| Beer | <0.0005 | <0.001 |
| Wine | 0.0031 | <0.0007 |
| Mineral water | <0.0006 | <0.0005 |
| Milk | <0.0006 | <0.0025 |
| Fruit juice | 0.0016 | <0.0006 |
| Baby food | <0.001 | <0.0025 |
| Potatoes and vegetables | <0.001 | <0.0025 |
| Honey | 0.0030 | <0.0025 |
| Eggs | <0.001 | <0.0025 |
| Meat and fish | <0.001 | <0.0025 |
| Pulses | 0.0012 | <0.0025 |
| Oilseeds and vegetable oil | <0.001 | <0.0025 |
| Pseudo cereals | <0.001 | <0.0025 |
| Breakfast cereals | 0.0036 | <0.0025 |
| Durum wheat | 0.139 | 0.0107 |
| Pastry and snacks | <0.001 | <0.0025 |
| Bread | 0.0019 | <0.0025 |
| Flour and baking mixtures | <0.001 | <0.0025 |
| Other cereal products | <0.001 | <0.0025 |
| Human Cell Type | Dose of Glyphosate (µg mL−1) | Exposure Time (h) | Evaluated Effects | References |
|---|---|---|---|---|
| Blood | 0.500 | 52 | Mutagenicity, Cytotoxicity, DNA damage, Hemolysis, Acetyl cholinesterase activity | [101,102,103,104,105,106,107,108,109] |
| Epithelial | 0.300 | 18 | Oxidative stress, Cell damage, Genotoxicity | [110,111,112] |
| Embryonic | 0.450 | 24 | Cell damage, Toxicity, Endocrine disruption | [113,114,115] |
| Pluripotent stem | 0.100 | 48 | Blood–brain barrier | [116] |
| Renal | 0.600 | 24 | Cell viability, Apoptosis, Cell viability | [117] |
| Hepatic | 0.540 | 24 | Transcriptomic changes, Genotoxicity, Oxidative stress, DNA damage, | [112,118,119,120] |
| Breast | 0.100 | 48 | Endocrine disruption, Toxicity, DNA damage, | [121,122] |
| Ovarian | 0.500 | 72 | Abnormal growth, | [123] |
| Pulmonary | 0.540 | 24 | Cell viability | [119] |
| Neuronal | 0.540 | 24 | Toxicity, DNA damage | [119] |
| Sperm | 0.36 | 1 | Cell viability, DNA fragmentation | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Garcia, T.; Espinosa-Calderón, A.; Hernández-Vázquez, B.; Schwentesius-Rindermann, R. Overview of Environmental and Health Effects Related to Glyphosate Usage. Sustainability 2022, 14, 6868. https://doi.org/10.3390/su14116868
Rivas-Garcia T, Espinosa-Calderón A, Hernández-Vázquez B, Schwentesius-Rindermann R. Overview of Environmental and Health Effects Related to Glyphosate Usage. Sustainability. 2022; 14(11):6868. https://doi.org/10.3390/su14116868
Chicago/Turabian StyleRivas-Garcia, Tomas, Alejandro Espinosa-Calderón, Benjamin Hernández-Vázquez, and Rita Schwentesius-Rindermann. 2022. "Overview of Environmental and Health Effects Related to Glyphosate Usage" Sustainability 14, no. 11: 6868. https://doi.org/10.3390/su14116868
APA StyleRivas-Garcia, T., Espinosa-Calderón, A., Hernández-Vázquez, B., & Schwentesius-Rindermann, R. (2022). Overview of Environmental and Health Effects Related to Glyphosate Usage. Sustainability, 14(11), 6868. https://doi.org/10.3390/su14116868






