Abstract
Environmental pollution caused by conventional petro-diesel initiates at time of crude oil extraction and continues until its consumption. The resulting emission of poisonous gases during the combustion of petroleum-based fuel has worsened the greenhouse effect and global warming. Moreover, exhaustion of finite fossil fuels due to extensive exploitation has made the search for renewable resources indispensable. In light of this, biodiesel is a best possible substitute for the regular petro-diesel as it is eco-friendly, renewable, and economically viable. For effective biodiesel synthesis, the selection of potential feedstock and choice of efficient catalyst is the most important criteria. The main objective of this bibliographical review is to highlight vital role of different catalytic systems acting on variable feedstock and diverse methods for catalysis of biodiesel synthesis reactions. This paper further explores the effects of optimized reaction parameters, modification in chemical compositions, reaction operating parameters, mechanism and methodologies for catalysts preparation, stability enhancement, recovery, and reusability with the maximum optimum activity of catalysts. In future, the development of well-planned incentive structures is necessary for systematic progression of biodiesel process. Besides this, the selection of accessible and amended approaches for synthesis and utilization of specific potential catalysts will ensure the sustainability of eco-green biodiesel.
1. Introduction
A specific portion of economic development of a country can be represented by its fuel consumption, since fuel plays a role in many commercial sectors such as the transport sector, where Petro-diesel is in particularly high demand. In addition, the emission of poisonous gases during fuel combustion has also become a matter of parallel concern because these gases are directly contributing to global warming as the greenhouse effect is becoming progressively worse. In this developing era, extensive research has been conducted with the objective of producing renewable and sustainable substitutes as a backup for non-renewable and exhausting fossil fuel either partially or completely. In this scenario, biodiesel is considered as successor for regular diesel produced by petroleum fractionation. It causes less greenhouse gases emissions due to its renewable nature; hence, it is non-toxic and environmentally friendly. In addition to environmental benefits, the production of biodiesel also offers socioeconomic advantages as it provides job opportunities in various economic sectors.
Biodiesel is defined as alkyl esters synthesized from triglycerides present in vegetable oil or animal’s fat in the presence of a suitable catalyst under optimum reaction conditions [1,2]. In a chemical reaction, triglycerides are first converted into diglyceride and then diglycerides are converted into monoglyceride; the end products are glycerol and biodiesel. This reaction is governed by a specific catalyst, used to enhance the rate of reaction and minimize the reaction time. For biodiesel synthesis, catalysts of chemical or biological nature are used and their selection is essentially determined by Free Fatty Acid (FFA) content of feedstock oil.
- Chemical-based catalysts can be either homogeneous or heterogeneous depending on their solubility in reaction media;
- Recently, Nanotechnology has attracted researchers to synthesize nano-sized catalysts with large catalytic surface and meet the demands of industrial applications;
- Biological catalysts comprised of enzymatic systems such as free lipases [3].
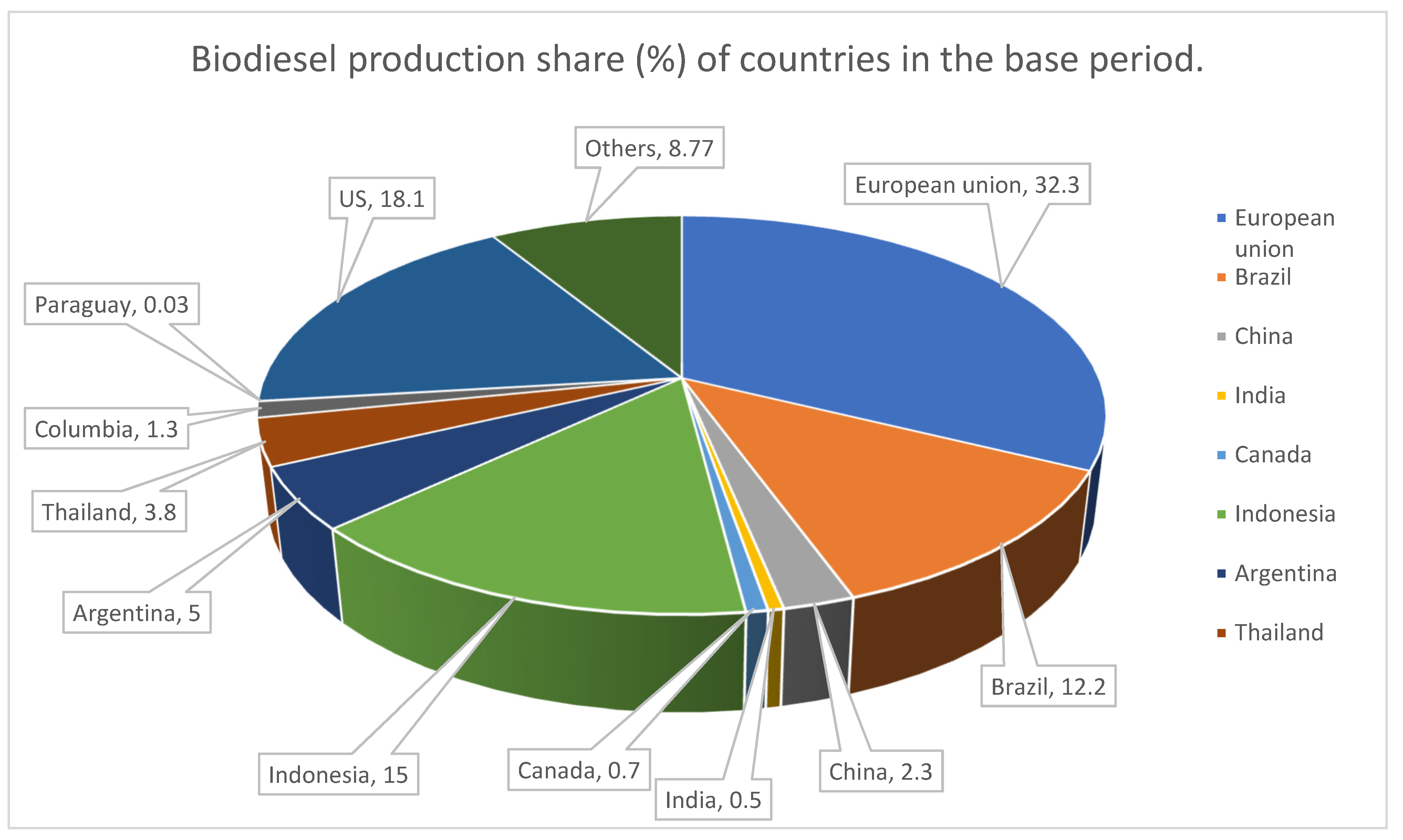
With the increasing demand for biodiesel, research is being focused on proposing new routes for biodiesel production. These routes will lead to high conversion yields without posing damage to climate and at the same time viable economically. The production capacity of biodiesel from animal fats and vegetable oil has 8.3 billion gallon per annum and is increasing day by day. Figure 1 depicts production share (%) of countries in the base period. It is expected that biofuel will account up to 27% of liquid fuel supply across the globe. Agricultural countries that cultivate different oil yielding crops have favorable climatic conditions and enormous fertile lands, have made biofuel synthesis largely conducive compared to other feedstocks.
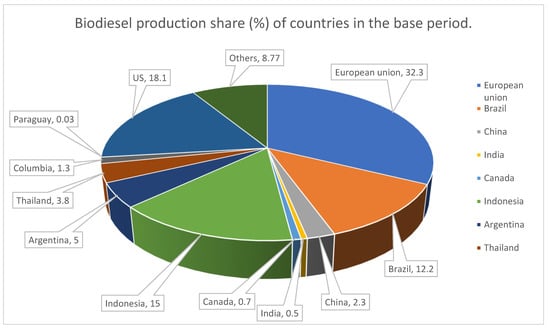
Figure 1.
World biodiesel production, 2021 (Percentage refers to production share of countries in the base period) Source: OECD/FAO. (2021). OECD-FAO agricultural outlook 2021–2030.
Biodiesel is utilized in the form of blended fuel with petroleum diesel fuel known as BXX, where XX denotes the percentage of biodiesel in the blended fuel. Many countries have already applied different portions of biodiesel in the blended fuel [4]. Worldwide, biodiesel is used either in pure form (100%) represented by B100 or mixed with petro-diesel (biodiesel-petrodiesel blending) such as 5% (B5), 20% (B20) and 80% (B80) [5].
Our current study is focused on a comprehensive review of various techniques to synthesize catalysts used for sustainability of biodiesel synthesis processes with special emphasis on transesterification. The novelty angle of this article is to survey the works of researchers on the advancement of distinctive types of catalysts, for example, homogeneous, heterogeneous, nano sized catalysts (especially clay derived nanocatalysts and Plant and animal based nanocatalysts) and biocatalysts utilized for biodiesel generation to date. This article compares the reasonableness and associated challenges within the transesterification with an accentuation on the catalysts and their reusability. Moreover, this review also compares different methodologies to synthesize catalysts that are in turn used for sustainable production eco-green biodiesel at large scale. This analysis will offer an insight into catalyst design and the most optimal combination of three main factors in biodiesel synthesis process: catalyst type, oil type and reaction conditions in order to enhance the biodiesel yield, eventually.
Highlights
- Vital role of diverse catalytic systems for producing biodiesel has been discussed.
- Synthesis and application of potential catalysts during transesterification have been elaborated systematically.
- Advanced techniques for synthesizing cost effective and highly efficient catalyst synthesis have been reviewed critically.
- The development of an efficient, promising, cost-effective catalyst can resolve issues in erstwhile catalysis.
2. Biodiesel Synthesis Processes
Biodiesel is an outstanding substitute for petroleum based diesel fuel that can be directly used in compression ignition engine with little or no modifications thus securing cleaner emissions.
Biodiesel from potential feedstock is produced by using various techniques, for example, pyrolysis, micro-emulsification, dilution and transesterification using both catalytic and superfluid method (Table 1). Moreover, for the process intensification, microwave technology, ultrasonication assisted methods are used.
- Pyrolysis or catalytic cracking is the breakdown of vegetable oil or animal fats in the absence of air using a catalyst. The required catalyst enhances the thermal decomposition of biodiesel feedstock, as a result long chain hydrocarbons are broken down into short chain hydrocarbons. The biofuel obtained from pyrolysis process is acidic in nature and encompass different hydrocarbons and moisture contents. Therefore, a pretreatment process is required to remove moisture content and use it as alternative fuel;
- In micro-emulsification, crude oil is mixed with emulsifying agent, this can be any type of alcohol to make emulsions. These emulsions cause accumulation of carbon in the engine and inappropriate burning;
- In the dilution process, a required amount of crude oil and diesel are mixed together. Dilution is achieved by enhancing the solvent and lowering the solute concentration resulting in decrement in viscosity and density. Conventionally, diesel and ethanol are used as solvents. The dilution process is easy, however, due to high FFA content, viscosity and acid value makes this fuel unsuitable to be used directly in diesel engine yet some problems associated to it, for example, incomplete burning and carbon deposition.
- Transesterification is the most utilized technology in biodiesel synthesis as it is favorable from economic point of view at industrial scale production. It involves the reaction between oil/fat obtained from a feedstock and alcohol in presence of suitable catalyst.
Intensification strategies such as microwave assisted technology and ultrasonic cavitation are used to promote mass transport effect in overcoming the drawback of the high immiscibility of the solid catalyst with the oil and the alcohol liquid phases in the normal mixing system of the transesterification reaction. Process intensification through the use of active catalyst, pressure reactor, high temperature and high stirring rate often subjects to drawbacks with respect to energy consumption, product quality and reactants cost [6]. Process intensification is achieved by using microwave and ultrasonication technologies.
Microwave technology offers a fast rate of reaction and less heat loss. However, after the process catalyst is removed and conversion process is influenced by catalyst activity.
Ultrasonication or acoustic cavitation is considered as a promising, ecofriendly, and efficient method for biodiesel production. It involves utilization of cavity collapse to generate highly disruptive forces that rupture the layer between alcohol and oil and reactant get uniformly distributed due to the microlevel intermixing hence the reaction yield and rate is enhanced [7].

Table 1.
Merits and demerits of few biodiesel Synthesis processes [8].
Table 1.
Merits and demerits of few biodiesel Synthesis processes [8].
| Biodiesel Methods | Merits | Demerits |
|---|---|---|
| Direct use of oil and blends | Liquid based-portability, renewability, readily available, Heat content Retains total power without/little modification to diesel based engine No major operational difficulty | Low cetane, high viscosity, natural gum in oils, low flash point, low volatility Reaction of unsaturated chains Gumming and Plugging of, lines, filters and injectors Engine knocking and carbon deposits on engine, |
| Micro emulsion | Isotropic fluid, stable and clear with aqueous phase, an oil phase and surfactant. | Extensive deposits in exhaust valve of engine. Amass of carbon all over the orifice of injector outlet Partial combustion in 200 h lab screening endurance test. Irregular needle sticking of injector |
| Pyrolysis | Pyrolyzed oil has satisfactory sulphur amount, sediment, water and copper corrosion value. Undesirable carbon residue amounts, ash, pour point. Short term engine tests. | Equipment is expensive Low moisture is required Products are similar to petroleum diesel fuel. Deoxygenation during pyrolysis removes eco-friendly aspects. Production of low graded materials and less diesel as compared to gasoline. |
| Dilution | Low viscosity of oil makes it efficient for short term use. | Not recommended for long term usage Severe sticking and coking of injector nozzle Heavy carbon deposits on intake valve and appearance of top ring wear. |
| Transesterification | Fuel properties almost similar to petroleum diesel Low cost High conversion yield efficiency Suitable for large scale production of biodiesel | Low water and FFA content of oil is required (Base catalyzed transesterification) Washing of biodiesel is required that produces pollutants Accompanied by side products |
| Adequate reaction time Efficient conversion and easy to perform Inexpensive | Catalyst recovery not possible Saponification Low quality glycerol is produced |
| High biodiesel yield Eco-friendly as washing is not required No saponification and hydrolysis | Expensive as compared to Homogeneous catalyzed transesterification Longer reaction time |
| Tolerance to water content in oil No FFA saponification Low energy consumption/input | Very Expensive Enzymes Inhibition via MeOH Much Longer reaction time Enzymes absorb glycerol on its surface |
| No catalyst required Easier product purification Fast reaction time No effect of high FFA and Water | Need of High reaction temperature and pressure Large amount of methanol High energy consumption High capita cost |
When transesterification is used for biodiesel production, one of two routes are followed: supercritical methanol transesterification or catalytic transesterification.
- Supercritical fluids (SCF) are used for biodiesel synthesis in the absence of catalysts. It results in fast rate of reaction, high efficiency of conversion; there is no requirement of a catalyst but it requires energy and has a high installation cost. The supercritical transesterification involves single phase mixture, produced at 340 °C temperature and 43 MPa pressure with reduction in dielectric constant of alcohol. The reaction time is 2–4 min at supercritical stage. Though product purification is comparatively easy due to the absence of a catalyst, the production cost is high.
- Catalytic transesterification involves interchange of alkyl groups (R) of esters with alkyl groups of alcohols. It results in production of new ester (biodiesel) and alcohol (glycerol) [1].
Following two basic steps are involved in transesterification process:
- Alcohol and the catalyst react to form alkoxide ion;
- A reaction between an alkoxide ion and triglycerides in oil to produce fatty acid alkyl esters [9]
In cases where methanol is used as a solvent, the first step includes the formation of a methoxide ion. Hydrogen ions are liberated from methanol and at the same time hydroxides are donated by the base, for example NaOH/KOH. The combination of proton (from alcohol) and hydroxyl (from alkali) results in the formation of water. Soap is formed when metal ions (released from base) react with free fatty acids. This unwanted reaction of saponification is due to excess water in the reaction mixture. To avoid saponification, a water-free medium is required.
The second step is the synthesis of fatty esters and glycerol when there is an excess of alcohol, for example, three moles or above react with one mole of triglycerides in oil. In order to attain 100 lbs. of biodiesel, 100 lb. of fat, 1 lb. of catalyst and 20 lbs. of alcohol are required [10].
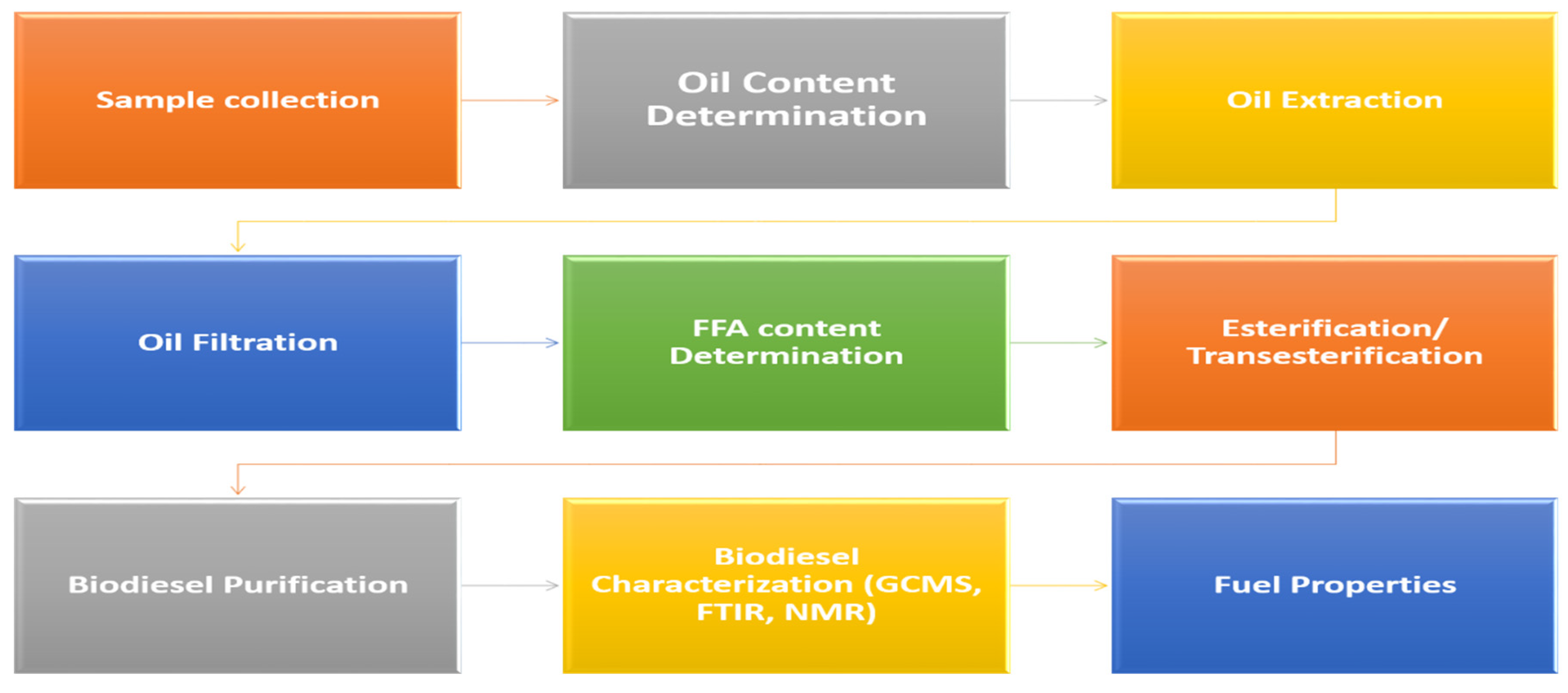
Basic schematic steps required for biodiesel production via alcoholysis followed by its characterization by advanced analytical techniques are presented in Figure 2.
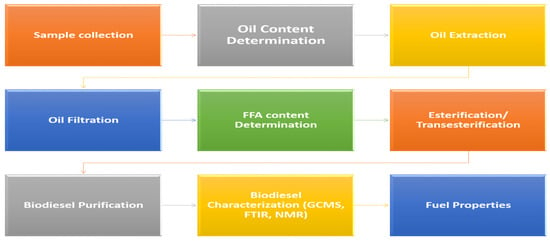
Figure 2.
Schematic steps involved in biodiesel production and characterization.
The yield of biodiesel is directly influenced by optimization parameters, for instance feedstock oil, amount and type of catalyst, time, and temperature, oil to methanol ratio and agitation rate.
The brief discussion on criteria to select feedstock, alcohol (solvent/cosolvant) and catalyst selection for esterification and transesterification is given below:
2.1. Criteria for Feedstock Selection
Two requirements should be fulfilled by feedstock for biodiesel: large production scale and low production cost. Vegetable oil contains carboxylic acid and/or triglycerides which take part in chemical reaction of biodiesel synthesis. In addition to this, animal fat can also be used as raw material that reacts with the methanol or ethanol and this reaction is supported by a catalyst. Table 2 summarizes various raw materials for biodiesel synthesis [11].

Table 2.
Feedstock for biodiesel production.
Biodiesel is synthesized from rapeseed oil globally. In addition to edible and waste vegetable oil, non-edible seed oil has also been explored among which Jatropha has shown a promising potential for biodiesel production. Other feedstocks include bovine tallow, pork fat, fish oil and other animal fats. Bovine tallow is by-product of meat industry and is mostly used to produce biodiesel. It contains a saturated chain of fatty acids with chemical structure just similar to the oil obtained from plant seeds. Amid all types of raw materials, vegetable oil extracted from seeds is the main feedstock for biodiesel production, for instance, is of utmost importance.
Oil yielding crops have been reconnoitered for biodiesel production and more than 350 oil producing seed plants have been identified as biodiesel feedstock [12]. While current pursues are being performed to explore novel potential sources that will be promising, natural, and sustainable with respect to food security and environment protection thus favoring the production of high-quality biodiesel. Among plant-based oils, almost 95% biodiesel is prepared from vegetative edible oil. However, it is in competition with the supply of food, and this has led to price hike of both edible oil as well as biodiesel. Since, non-edible seed oils have gained the distinctive attention of researchers.
Nonedible oil yielding crops are reasonable in terms of cost for cultivation, can grow best in waste land without exceptional care. Among biodiesel feedstocks, besides vegetable oil, animal fats are also used. These are saturated at room temperature because they have high amount of saturated fatty acids, therefore creating problems for the synthesis of alkyl esters. Moreover, it is more expensive than vegetable oil. Bovine tallow biodiesel can produce cold filter clogging at temperature about 19 °C. It is a factor for analysis of biodiesel quality and in order to reduce clogging point, it is mixed with vegetable oil. Therefore, the depression in the clogging point occurs at 10 °C.
The financial expenses depend on geographical area, price of crude petroleum, base stock, and seasonal variations in crop production. The high expenditure associated with biodiesel production is due to the high cost of feedstock. Non-edible feedstock does not compete with food supply and hence, the crop failure is not at risk and has a lower financial cost. The high budget needed for raw material is also one of the prominent contributing factors in overall price-hike of biodiesel. Therefore, a financially affordable substitute is the use of residual oil or fat as feedstock for biodiesel synthesis process [13].
The collection of residual oil is usually made at large (industrial) as well as small scale (domestic) etc. The advantage of using waste or residual oil is its less aggressiveness to environment via correct disposal. However, it demands high production expense and low production scale.
Algae, sewerage, and non-edible seed oil are less expensive and are more readily accessible compared to refined oil. Moreover, the percentage of feedstock oil and its yield per hector are vital considerations for the selection of a source for biodiesel production. In this regard, algae can be considered as the best option because it grows widely, even in tropical climates and saline water. The productivity of algal oil is high; thus, this leads to a high yield of biodiesel at a low cost. It has been estimated by researchers that the yield of algal oil per acre is >200 times as compared to the best performing oil of plant-based origin.
2.2. Criteria for Alcohol Selection
While dealing with high FFA content, double step acid-base catalyzed method is used. Esterification of FFA by acid followed by transesterification. Conventionally, in biodiesel production, esterified products are separated from water produced and then further proceeded [14].
Transesterification is recognized as alcoholysis in which three moles of alcohol reacts with triglycerides in oil to produce biodiesel as main product and glycerin as byproduct and reaction rate is supported by catalyst [15]
- Alcohol such as methanol, ethanol, propanol, butanol, pentanol and even isopropanol can be used. Higher alcohols are avoided because they are more sensitive to the contamination of water and the reaction is inhibited. Hence, methanol and ethanol are frequently; used.
- When compared, methanol is more effective than ethanol and it provides a high biodiesel yield in less time duration. It helps to purify the product efficiently by separating the glycerin, which is byproduct of a biodiesel process. A most relevant factor for choosing methanol is its low cost. It was found that 6:1 alcohol to oil ratio is the basic requirement for single step reaction completion. More than 1.6 times excess of alcohol is necessary to complete the transesterification [16]. Nevertheless, methanol usage is exposed to few drawbacks, for example it is produced from natural gas (CH4) that increases its toxicity. Therefore, the production of one of the reactants of non-renewable origin makes the perception of green fuel ambiguous. A pressure vessel is mandatory when methanol is used as solvent in order to tolerate the boiling temperature, especially at 65 °C temperature for 1–2 h of reaction time. Reaction temperature directly influences the rate of reaction, product viscosity, reaction equilibrium, kinetics and mass transfer limitations.
On the other hand, ethanol is produced from vegetable feedstock for example beet, potato, cassava, and sugar cane. Therefore, ethanol is non-toxic and could be the best alternate for methanol in biodiesel synthesis reactions. However, ethanol is not preferred due to several drawbacks such as it is required in excess with less reactivity, long reaction duration/time and high optimization temperature and high operating cost. Moreover, product purification is also a challenging task due to emulsion formation. These issues can be resolved by conducting the reaction in various steps. Hence, the amount of alcohol needed to complete the process can be reduced. In each step, alcohol and the catalyst are added, and glycerol is removed by water washing. By increasing the amount of reactant and removing the product, the reaction will proceed in forward direction at a high speed with maximum yield [17].
- c.
- In the case of high moisture content in alcohol, acids such as hydrochloric acid and sulfuric acids (0.87–2.5 M) are preferable for transesterification.
- d.
- Diglycerides, in reaction with alcohol, disperse in the oil phase and result in yield reduction. Therefore, a co-solvent can be used that limits this mass transfer. To enhance oil and alcohol miscibility, Tetrahydrofuran and Methyl tert-butyl ether have proven effective; tetrahydrofuran is best co-solvent as compared to Methyl tert-butyl ether. Various investigations have been conducted for methanol with co-solvent, for example, di-ethyl ether, acetone, di-isopropyl ether, dichlorobenzene with 1:0.5 to 1:2 mole/mole ratios.
- e.
- A vacuum-based heat exchanger is used at the last stage to remove excess water from biodiesel. Both biodiesel and glycerin are present in the reaction mixture in the form of layers and physical separation required to recover them. Glycerol, being the unwanted byproduct, is removed from the main product (biodiesel). The glycerol regeneration occurs when it is neutralized by the acid addition. It is further used for various purposes after purification [16,18].
2.3. Criteria for Catalyst Selection
The rate of a chemical reaction is enhanced by a catalyst that functions by lowering the activation energy without itself being consumed. The catalyst does not appear in products as it is supposed to be unchanged at variance with stoichiometric reactions.
A chemical equilibrium is quickly attained whereas the reaction thermodynamics remain unaffected during the action of catalyst; this process is known as catalysis that involves a little proportion of it. Similarly to all chemical reactions, the rate of transesterification is also improved by acidic or basic catalyst.
Acid catalysts cause slower the speed of reaction and causes the equipment corrosion, therefore they are not really applicable at a large scale. On the other hand, base catalysts are not only cost effective, but also readily available and much more efficient. A major issue associated with base catalyzed reaction is that free fatty acids (FFA) should be 0.5% or less to hinder saponification.
Catalysts used for transesterification are either homogeneous (acid/base), heterogeneous (acid/base) or enzymatic (lipases) in nature.
- Homogeneous catalysts are required in less concentration to provide rapid results but are difficult to recover and reuse, for example, a high yield of biodiesel is produced in the presence of sodium alkoxide at low concentration in short reaction time but is difficult to be recovered;
- Conversely, heterogeneous catalysts are recoverable and reusable but less reactive due to leaching. A selection of highly efficient and active catalysts is the main focus of researchers for production of high yield of biodiesel. In most cases, base catalysts are in the form of sodium and potassium carbonates that form hydroxides and alkoxides and the rate of reaction is faster as compared to acid catalyzed reactions;
- A catalyst with large surface area of a catalyst can resolve different issues concerned with catalytic types and synthesis of nano-sized catalyst fulfils the above-mentioned criteria, hence, it can be the best option for producing high quality biodiesel with maximum yield in reasonable budget in less time;
- Enzyme or biocatalysts produce highly purified product, easily separable, mild reaction but highly expensive.
Table 3 represents various types of catalysts used to enhance rate of reaction [19].

Table 3.
Types of catalysts used for biodiesel synthesis [20].
3. Types of Catalysis in Biodiesel Production
Biodiesel is an ester that is produced when carboxylic acid or triglyceride chemically reacts with alcohol. The chemical reactions involved in this process are categorized as esterification and/or transesterification and both reactions use catalysts such as homogeneous (same phase as reagents) and heterogeneous (not in same phase as reagents). During the course of the reaction, the catalysts modify the selectivity and kinetics by enhancing the rate of reaction and remain unconsumed by the end of reaction [21].
3.1. Esterification
Esters are substances which form as a result of elimination of water molecule during a slow reaction at 25 °C between carboxylic acid and an alcohol. Generally, esters are slow melting solids or liquids (all methyl and alkyl esters) and are lighter than water. These have lower boiling points compared to the alcohols or acids (both having H-bond) of relative molecular weight because hydrogen bonding is not possible in esters. hese are usually liquids even though parent acid is a solid and all these above-mentioned physical properties are attributed to loss of hydrogen bonding in esters. All esters are in soluble in water and soluble in organic solvents such as ethyl acetate. Esters have a wide range of applications such as in medicines, flavoring agents, paint removers and part of our daily diet as found naturally in oils and fats [22]. Esters are prepared via direct “esterification” of carboxylic acid or their derivatives with alcohol or phenol [23]. It involves the refluxing of mixture of above-mentioned compounds in the presence of catalyst that is a mineral acid such as dry HCl and H2SO4, as shown in Equation (1):
The treatment of feedstock is achieved via this reaction for the reduction in concentration of free fatty acids in oils and fats that results in the production of ester: biodiesel and H2O.
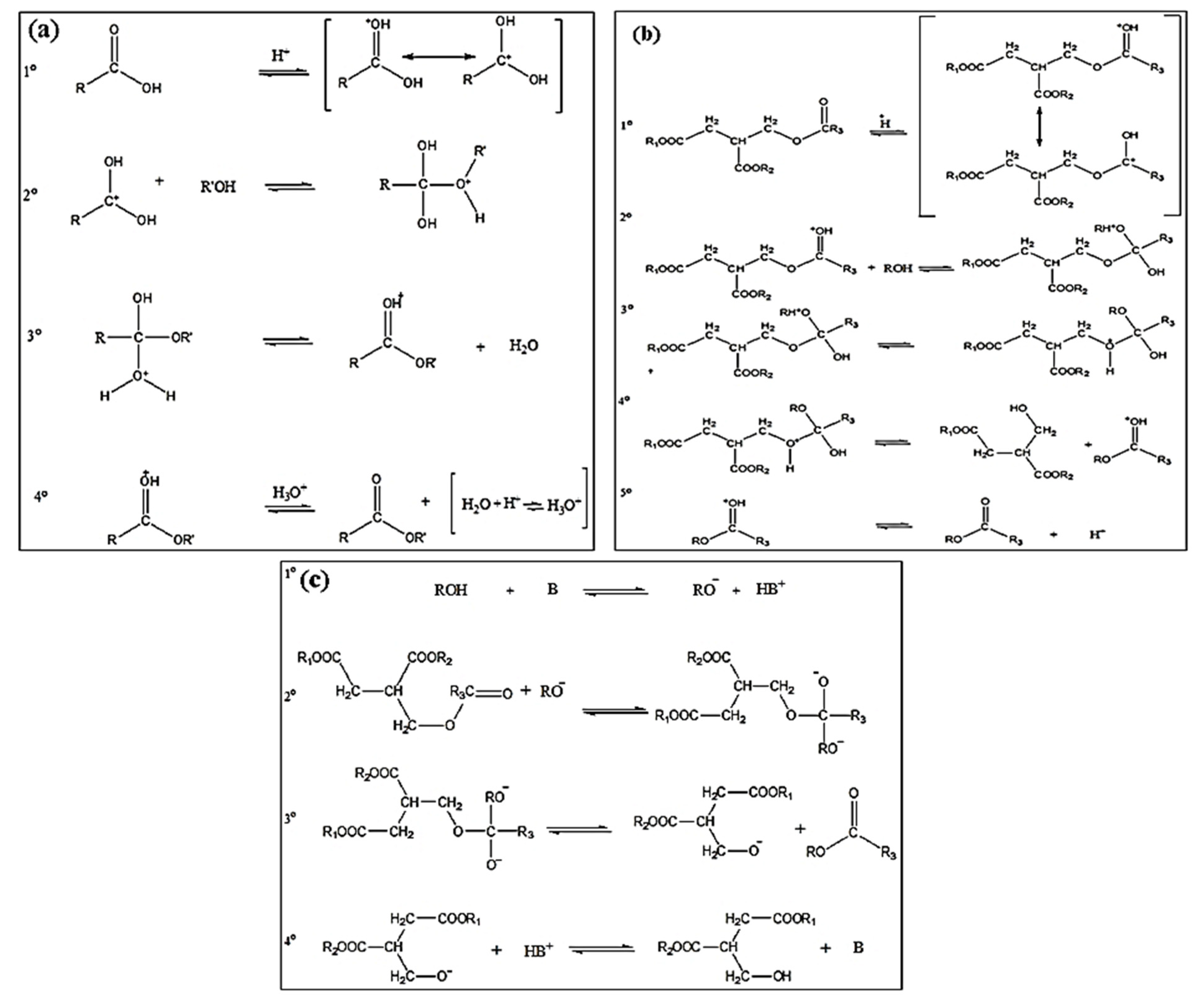
This is the first order reaction and factors affecting the rate of reaction include reaction temperature, oil to alcohol molar ratio, catalyst concentration and reaction time. The mechanism of acid esterification involves s typical acid- catalyzed nucleophilic substitution at acyl carbon atom as shown in Figure 3a.
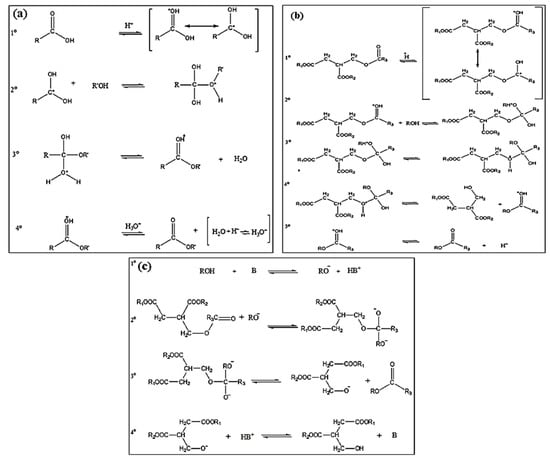
Figure 3.
Mechanisms of catalysis of biodiesel synthesis process (a) Mechanism of acid esterification (b) Mechanism of acid catalyzed transesterification (c) Mechanism of alkaline transesterification reaction [23].
The protonation of fatty acid followed by nucleophilic attack results in the formation of tetrahedral intermediate. It undergoes molecular rearrangement and ester (biodiesel) is formed after elimination of water molecule. The alkaline esterification mechanism has not been presented in this review because the alkali-based catalysts form soap when they react with fatty acid and alcohol.
3.2. Transesterification
Transesterification reaction is also known as alcoholysis, involving the transformation of one ester into another therefore known as transesterification. In the case of a biodiesel synthesis process, a triglyceride (formed by a combination of fatty acids with glycerol) facilitated by a catalyst (strong acid/alkoxide) reacts with alcohol that results in production of biodiesel and glycerin. Base catalyzed transesterification is frequently used for biodiesel production. Equation (2) shows transesterification reaction.
Both acid catalyzed and base catalyzed transesterification reactions are used to produce esters (biodiesel). Transesterification involves the generation of a highly potent electrophile when a proton is donated to carbonyl group in presence of a strong acid catalyst. When a proton is removed from alcohol, a nucleophile is produced, and the reaction rate is enhanced by strong base catalyst. Almost 96% Fatty acid methyl esters (FAMEs) and fatty acid ethyl esters (FAEEs) are produced in reaction mixture depending on the alcohol used [10]. In base catalyzed transesterification, an alkoxide ion is formed in the first step then a carbonyl group of triglyceride act as electrophile as a result a tetrahedral intermediate is formed followed by synthesis of an alkyl ester. At the final step, the regeneration of alkali occurs with H+ attached to anionic diglyceride [24]. However, same reaction pattern is followed by acid catalysts except formation of carboxylic acid due to nucleophilic attack. These catalysts are highly reactive and have potential to provide a 100% transesterification reaction.
3.2.1. Acid Catalyzed Transesterification
In acid catalyzed transesterification reaction, one carbonyl is activated that favors alcohol’s nucleophilic attack followed by molecular rearrangement, the diglyceride is eliminated and deprotonation of carbonyl produces the ester. Figure 3b depicts the acid catalyzed transesterification mechanism.
3.2.2. Alkaline Transesterification
The alkaline transesterification reaction involves the reaction of alkali and alcohol that produces an alkoxide and a protonated catalyst. The alkoxide acts as a nucleophile and attacks a carbonyl of triglyceride and tetrahedral intermediated forms; that then produces diglyceride anion and ester. The deprotonation of catalyst results in synthesis of diglyceride and a new catalytic cycle. Figure 3c shows this mechanism.
4. Types of Catalysts Used in Transesterification
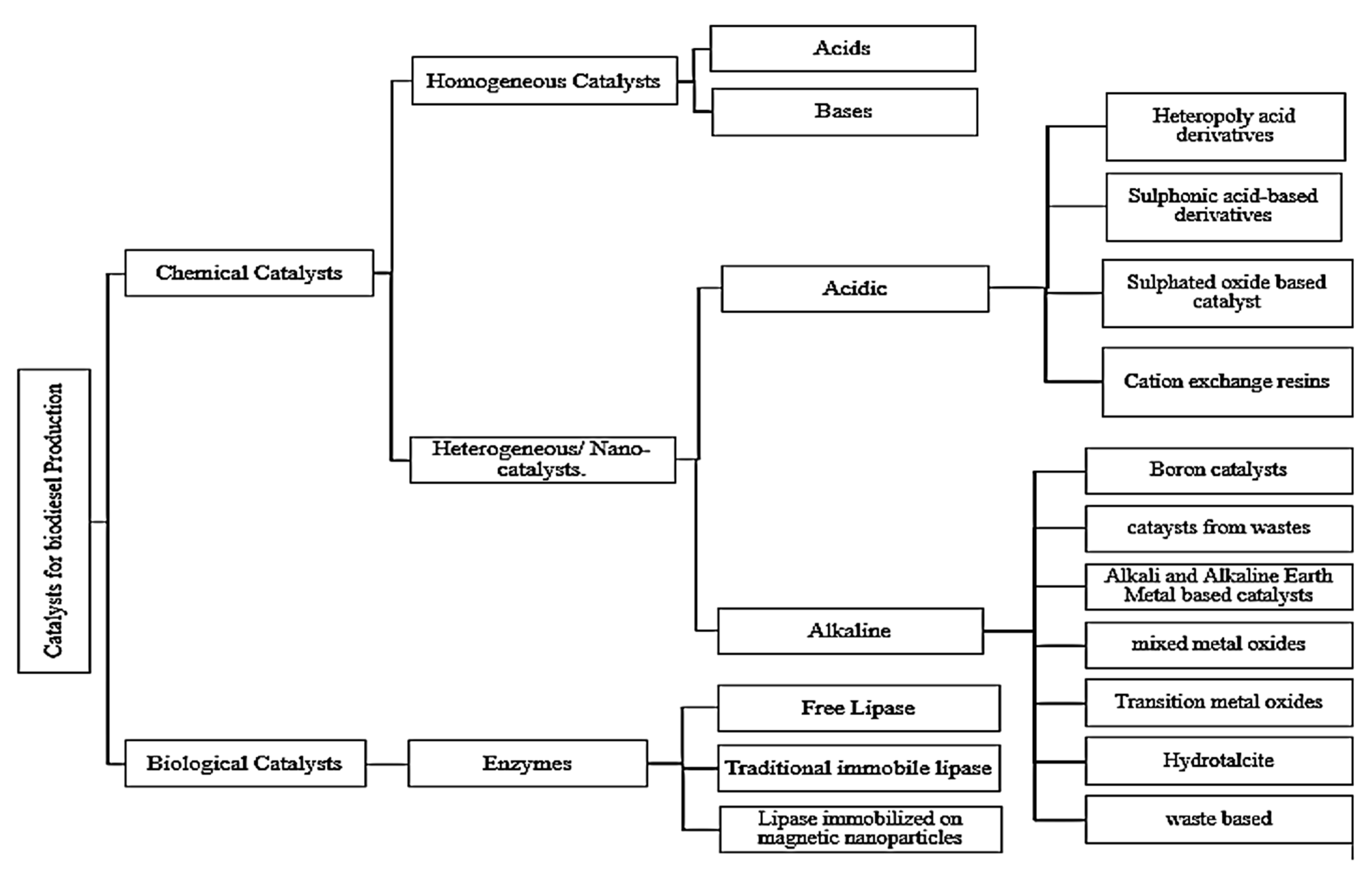
A catalyst is a substance used to increase the rate of a thermodynamically feasible chemical reaction and is not consumed during the reaction because it is used in one stage but regenerates during the next stage. The process continues until the reaction is completed and no permanent change is imposed on the composition and properties of catalyst. Generally, transesterification is achieved via three types of catalysts-homogeneous, heterogeneous, and enzymatic catalysts. Figure 4 depicts the schematic categorizations of catalysts.
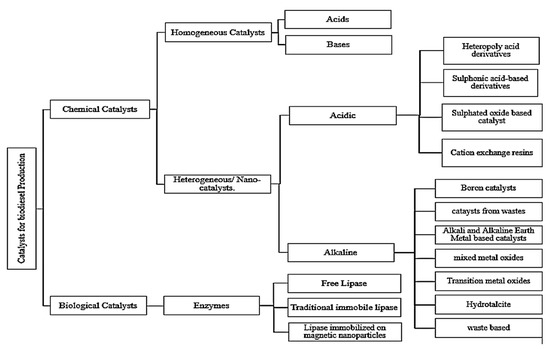
Figure 4.
Classification of Catalysts for transesterification.
4.1. Homogeneous Catalysts
These catalysts are in similar phase with reactants and products. During the synthesis of the methoxide ion, these are soluble in alcohol. They are categorized into alkaline catalysts and acid catalysts. Under optimum conditions of moderate temperature and pressure, these catalysts accelerate the transesterification of oil and fats. However, the disadvantage is that the catalyst cannot be recovered at the end of reaction, forming soap instead of biodiesel by reacting with free fatty acids in oil. Table 4 shows the efficiency of homogeneous base and acid catalysts by optimization of reaction conditions. It shows that >99% performance of biodiesel is feasible by a slight change in the catalyst concentration and type [25].

Table 4.
Summary of homogeneous base and acid catalysts used for biodiesel synthesis. [25].
4.1.1. Homogeneous Alkaline Catalysts
These catalysts are suitable for oils having <2% free fatty acid (FFA) content for transesterification reaction and oils should be pre-treated when FFA content is >2%. FFA content of animal fat is 5–30% and 2–7% in cooking oil; Trap grease is a low quality raw material has FFA content up to 100%. When homogeneous alkaline catalysts are added to such feedstock oils, soap and water is produced as a result of reaction between free fatty acids and catalyst (Equation (3))
Notable homogeneous alkaline catalysts used for transesterification involve NaOC4H9, KOH, KOCH3, NaOC2H5, NaOH, Na2O2, NaOCH3. The selection of catalysts is a key factor in determining the fuel performance. Potassium hydroxide and sodium hydroxide lead to the formation of water that reduces efficiency of biodiesel, but when transesterification is achieved by converting them into potassium methoxide and sodium methoxide increases the performance of biodiesel as water is not formed. Biodiesel synthesized from two feedstocks corn oil and soybean oil. The alkali-based homogeneous catalysts were NaOH and KOH and a binary mixture of both (NaOH/KOH). The process of biodiesel synthesis was performed by using an of oil/alcohol molar ratio (1:6), catalyst concentration (0.5% of oil taken), reaction time (2 h), reaction temperature (65–70 °C) with constant stirring. The results depict higher conversion yield of soybean oil compared to maize oil where a lower yield was the result of lesser linolenic acid formation. Binary mixture of catalysts showed maximum yield and thus authors concluded that medium contains high concentration of ethoxide ion that gives high biodiesel yield [25].
4.1.2. Homogeneous Acid Catalysts
These catalysts are more tolerant to the moisture content in reaction mixture. In order to decrease the FFA content of oil and fats, the homogeneous acid catalysts are used before transesterification with alkaline catalyst. The reactions catalyzed by these catalysts require high alcohol to oil ratio and high temperature, hence they are undesirable over alkali catalysts. The most common homogeneous acid catalysts are sulfonic acid (HSO3R), sulfuric acid (H2SO4) and hydrochloric acid (HCl). These catalysts reduce high FFA content of cooking oil and animal fat, and they require specialized but expensive processing equipment is required due to the corrosive nature of these catalysts. When the FFA content is above 5%, the separation of methyl esters and glycerol is inhibited due to soap and then emulsion formation. In order to overcome this issue, an acid catalyst such as H2SO4 is used in process called esterification, as shown by Equation (4).
This pretreatment process lowers FFA content in feedstock, which are thus converted into methyl esters. Palm oil contains 51% free fatty acid (FFA) and it was treated with Sulfuric acid H2SO4 0.75% w/w of oil and methanol CH3 was mixed with oil under optimum conditions of 70 °C temperature and agitation at 600 rpm for 1 h and then sodium methoxide ion (1.25%) was added. At 50 °C, the mixture was stirred again for an hour. This procedure led to over a 97% yield and an incomplete reaction occurred due to water content and its accumulation stops the reaction before completion. These acid catalysts used in the process of esterification can synthesize biodiesel from products of refining industry that are in fact low grade raw material, for example soap stock. It is dried and esterified with alcohol by a catalyst; in this reaction, an inorganic acid. A sufficient amount of homogeneous acid catalysts, methanol and long duration are required for the reaction to take place; reaction time can be helpful in achieving desirable yield and good performance of biodiesel through homogeneous acid catalyst. Reactions were optimized at the methanol to oil molar ratio from 3:1 to 30:1, reaction temperature (25 and 60 °C), acid (H2SO4 from 1–3.5 weight percent), 48–96 h of reaction time, from 1 to 5 wt. % water content and FFA content from 2 to 30 wt. % was investigated. Even in case of FFA ~5, the ester yield was approximately 90%.
4.2. Heterogeneous Catalysts
Reactants and catalysts are in dissimilar phases and the reaction occurs at the surface of catalyst. Compared to homogeneous catalyst, the heterogeneous catalysts turn out to be solids and act antagonistically and differently in liquid reaction mixtures. However, similarly to homogeneous catalysts, they efficiently speed up the rate of reaction of fatty acid methyl ester production.
They are also divided into alkaline and acid catalysts. Heterogeneous catalyst such as bases (Alkali metals and Alkaline earth metal-based catalyst, mixed metal-based, transition metal-based, Hydrotalcite-based, waste-based catalysts) and acids (Cation exchange resins, heteropolyacid derivatives, sulphated oxide based and sulphonic acid-based catalysts), Acid/Base catalyst (Zirconia, zeolite based etc.). Such types of catalysts are quite easier to be recovered from the final products and can easily be recycled for reuse after a completed reaction; this pathway of biodiesel synthesis appears to be cost effective and considered to be environmentally friendly green process because there is null production of pollutants. These catalysts are able to tolerate high moisture and FFA content. Moreover, they can withstand in water treatment steps. With a high temperature and aqueous treatment steps, the water consumption and energy saving are possible due to elimination of side process such minimization of biodiesel filtration procedure. These are heterogeneous catalysts are integral in high pressure and temperature reaction conditions. Such catalysts are submissive to modifications in order to get high selectivity, activity and long lifetime. However, Table 5 depicts that under conditions of long reaction time and high temperature, heterogeneous base and acid catalysts perform their function adeptly in order to achieve high product yield [19,25,26,27].

Table 5.
Heterogeneous base and acid catalyst used for biodiesel production [15,20,22,23].
4.2.1. Heterogeneous Base Catalyst
Heterogeneous base catalysts overcome limitations associated with homogeneous base catalysts, for example hindrance in glycerol separation from biodiesel layer due to saponification. Metal oxides are known as the best heterogeneous base catalyst, these include Li/CaO, CaO, MgO, metal oxides (Mg/Zr and Mg/Al), CaO/ZnO as well as hydrotalcite; each of these have shown excellent catalysis performances in transesterification reactions. Heterogeneous alkaline catalysts also include MgZnAlO, CaO/Fe3O4, CuO/Al2O3, Li/MgO, K2CO3 Sodium silicate, Cr/Al mixed oxide catalyst. Heterogeneous alkaline catalysts are designed in order to graft and lock in active molecules inside the pores and entrap on the surface of solid support such as alumina, ceria and silica [19,25,26]. Alkali-based heterogeneous catalysts have a high potential for catalysis for biodiesel production and overcome some of the drawbacks and limitations such as soap formation due to homogeneous catalysts. Soap formation makes the separation of by-product (glycerol) from fatty acid methyl ester layer. In contrast to homogeneous catalysts, these catalysts are easy to separate and easy to reuse because of their low solubility and their active sites during reaction are destroyed. However, they are also reconstructed in an easy way. The only disadvantage of heterogeneous catalysts that they absorb water when stored. Many metallic oxides such as alkalis, alkaline earth and transition metals have appeared as potential candidate for oils transesterification. As metal oxides have both bronsted acid (cations) and bronsted base characters (anions). Sometimes, composites are formed to be used as catalysts [26].
Alkali and Alkaline Earth Metallic Catalysts
Among frequently exploited heterogeneous catalysts, metal-based oxides are common catalyst for transesterification. Fattah et al. (2020) demonstrated that the amount of basic site/unit wt. corresponds to the surface area approx. and sequence CaO > MgO > SrO > BaO: oxides of strong basic sites enhance the reaction rate more efficiently [25]. Among these oxides of metals, CaO is most widely used catalyst, owing to its comparatively high basic strength, long life, low solubility in methanol, high activity and working under moderate conditions. Previous studies reported basic strength by FTIR analysis as: MgO-KOH > MgO-NaOH > MgO-CeO2. Alkaline fluoride is homogeneous strong base catalyst, and it is mixed with other compounds and composites which are used for transesterification of oil or fats, and biodiesel is synthesized. These catalysts are KF/ZnO, KF/CaO and KF/CaO and other catalysts are supported. Catalysts are synthesized from calcium carbonate (CaCO3) present in organic based materials such as agricultural shells, bones, and marine shells. Through calcination at 800–1000 °C CaCO3 is converted into CaO and CO2. Gupta and Rathod (2018) treated the CaO catalyst with methanol and glycerol and its activity of was enhanced after this process. The resultant catalyst derived from CaO was denoted by CADG (Calcium diglyceroxide) that presents high catalytic activity compared to CaO when used for transesterification of waste cooking oil. The optimization parameters for CaO were 12:1 methanol to oil molar ratio, 65 °C temperature, 3% catalyst amount, 400 rpm agitation and 90 min reaction duration and a 93% yield was obtained. However, CADG gave a 96% biodiesel yield under optimized conditions such as 10:1 methanol to oil molar ratio, 60 °C temperature, 1.5% catalyst amount, 300 rpm agitation and 50 min reaction time. Hence, it was concluded that CADG was more efficient catalyst than CaO [26].
A heterogeneous base catalyst appears to be superior to homogeneous catalyst as it is easy to separate and purify reaction products, has less sensitivity to moisture and free fatty acids, is less corrosive, and is ecofriendly, recyclable active at less reaction temperature. Additionally, these catalysts are able to catalyze transesterification of low-quality oils with high content of free fatty acids in it. Rizwanul Fattah et al. (2020) reported limitations of heterogeneous base catalysts such as synthesis cost and reaction rate. Hence, such facts are needed to be focused for the application of heterogeneous alkaline catalysts for utilization at a large scale. If the reusability potential is regarded as a point of consideration, then the shortcoming of synthesis budget can be eliminated. However, further investigations are prerequisite for raw material and reaction rate for catalysts [25]. Roschat et al. (2018) used strontium oxide (SrO) to produce biodiesel from palm oil via ethanolysis under optimized conditions of 1:12 oil/ethanol molar ratio, 5 wt. % catalyst loading at 80 °C for 3h and 98.2% yield was achieved and reused for 5 times with >90% yield. Dai et al. (2018) prepared LiFe5O8-LiFeO2 and this catalyst was prepared by mixing Li2CO3 (aq. soln.) and Fe2O3 followed by calcination (800 °C) and then cooling. This catalyst was used with 8 wt. % loading, 1:36 oil to methanol molar ratio at 65 °C for 2 h and 96.5% yield was obtained. Furthermore, it was reused 5 times with a 94% yield [27].
Mixed Metal-Based Catalysts
Based on the catalytic mixture, mixed metal oxides have been used as a base catalyst and this nature is tuned by changing their structure, synthesis procedure, activation temperature, and chemical composition that strongly influences the final basicity. Salina’s et al. (2018) reported on La2O3 in ZrO2 by sol-gel method followed by calcination at 600 °C and used it to produce biodiesel from canola oil via transesterification as basicity was enhanced [28]. A catalytic activity of heterogeneous catalyst, CaMgZn, mixed oxides (3:1:1) prepared via coprecipitation method in presence of precipitant, such as Na2CO3 (0.75 mol/L) and CaMg(CO3)2 and CaZn(CO3)2 with 1.0 metal ion ratio to synthesize biodiesel from palm kernel oil and 97.5 wt. % and 6 wt. % catalyst loading, 20:1 methanol to oil ratio and 60 °C Na2ZrO3 as heterogeneous basic catalyst (3% loading) to prepare biodiesel (98.3%) at 65 °C for 3 h [29].
Transition Metal Catalyst
Among the most used materials for biodiesel synthesis are titanium oxide (TiO2) and zinc oxide (ZnO). Tungsten (W) supported TiO2/SiO2 catalyst synthesized by Kaur et al. (2018) via sol-gel method and waste cotton seed oil was converted in to respective methyl esters with 5 wt. % catalyst loading, 30:1 M methanol/oil ratio at 65 °C for 4 h. Mixed oxides TiO2-ZnO (200 mg) on palm oil with 1:6 M oil to methanol ratio at 60 °C for 5 h and 83% yield was achieved [30,31].
Boron Group Catalyst
Al2O3 is widely used to support alloys, halides, metal oxides and nitrates. For methanolysis of coconut oil and palm oil kernel, Al2O3 supported nitrates of alkali and alkaline earth metals were prepared via impregnation method and 450–850 °C calcination. These included NaNO3/Al2O3, LiNO3/Al2O3, KNO3/Al2O3, Mg (NO3)2/Al2O3), Ca(NO3)2/Al2O3. and effect of calcination temperature was thoroughly observed and recommended transesterification conditions with 15–20 wt. % Ca(NO3)2/Al2O3 for coconut oil and 10 wt. % for palm kernel oil with 1:65 oil/methanol molar ratio at 60 °C for 3 h [32,33].
Hydrotalcite-Based Catalyst
These are anionic basic clays that are used as heterogeneous catalyst due to their tunable strength through a change in Mg/Al and intrinsic better morphology in comparison with alkaline earth metal oxides. The only demerit associated with their usability is their sensitiveness to FFA and non-homogeneity. Rizwanul Fattah (2020) studied Zr-droped Mg/Al hydrotalcite and varied Zr/Mg molar ratios for biodiesel synthesis from refined rapeseed oil and tetravalent cation Zr4+ improved the catalytic activity and 99.9% optimum conversion for (Zr/Mg/Al = 0.45:2.55:1 M) hydrotalcite, 4.8–5 atm pressure and 373–393 k temp for 6 h time [25]. Navajas et al. (2018) produced biodiesel from sunflower oil and Mg-Al hydrotalcite (1.5–5 M) ratios by co-precipitation and used 1:48 M oil: Methanol, 2 wt. % catalyst and 1 atm pressure in 24 h and achieved a 96% optimum conversion [34].
Waste Based Catalyst
The creation of cost-effective base catalyst is aided by waste produced from the environment surrounding the industries and more sustainable and eco-friendlier biodiesel is obtained. Calcium-rich wastes such as mussel, snail and oyster shells, animal bones and ash based catalysts are inexpensive and readily available [35]. Calcium derived from such wastes are transformed to CaO, the most versatile catalyst. Yasar (2019) used CaO from waste egg shell and compared with pure CaO. Biodiesel synthesis was optimized with at 1:9 M oil/methanol, 60 °C for 1h and the yield was 95.12% for waste eggshell and 96.81% for CaO.
4.2.2. Heterogeneous Acidic Catalysts
These catalysts include heteropoly acids, zeolite, oxides, silica, alumina, molybdenum, and zirconium. Such catalysts are more eco-friendly and more stable compared to their homogeneous counterparts. Such catalysts should have many active sites, water repellency, moderate acidity and porosity for minimizing the diffusion problems. Advantages include efficient conversion rates, easy product filtration due to quick separation as well as reusability. Their usage will enable a reduction in corrosion phenomena by excluding separation steps and their reusability. A suitable solid acid catalyst is of great interest for conducting esterification of free fatty acids in feedstock oil and transesterification of triglycerides simultaneously. Furthermore, these catalysts allow simple and easy incorporation into continuous packed bed operation in flow reactors and reduce waste formation [36]. In this scenario, different solid acids have ion exchange resins, H-form zeolites, sulfonated catalysts (carbon based), sulfated metal oxides, silica materials, acidic ionic liquids, heteropolyacids have been studied as best catalysts. In the meantime, to facilitate the mass transport process of biodiesel synthesis catalyzed by such catalysts and to improve their catalytic stability in reactions they catalyze, strong and novel inorganic-organic hybrids having distinctive characteristics such as typical mesostructure, Lewis and bronsted acid properties and improved surface hydrophobicity have been positively designed. Moreover, solid acid catalysts are more preferable compared to liquid acid catalysts because they possess multiple sites with diversity in strength of Lewis or Bronsted acidity [37]. Even low-cost feedstock with high FFA can undergo esterification and transesterification simultaneously. When compared to bronsted acid, more activity is shown by the Lewis acid site, but there is a possibility of poisoning from free fatty acids and/or water. Different reaction mechanisms pertain to little activity of acid catalysts when compared with basic catalysts. Encouraging results can be achieved under moderate conditions and a slow reaction as compared to solid base catalysts. Moreover, high temperature, more catalyst loading, and long reaction time are, respectively, required for employing this catalyst. The possible adversarial side reactions and low reaction rate are basic reasons behind limited efforts for their practice for transesterification of oils and fats [38].
Cation-Exchange Resins
Cation exchange resins have been used at a small scale in research labs for biodiesel production. They are macroporous with several acidic sites that have the capability of producing biodiesel by preventing saponification as they can catalyze the FFA. Sulfonated polystyrene-divinyl benzene (ST-DVB-SO3H) was studied for esterification of free fatty acids as catalytic macroporous resins with 10 wt. % loading, 1:15 M methaonol/oil ratio, 100 °C for 3 h and 97.8% yield was achieved [39]. Waste fried oil (36 mg KOH/g) was esterified, and 98% conversion yield was achieved. The reaction was conducted in in fixed bed reactor via commercial resin (NKC-9) with 0.62 mL/min flowrate, 1:2.8 mass ratio of oleic acid/methanol, 65 °C in 500 h run, 44 cm catalyst bed high. Interesterfication by ion-exchange resin was performed by [40] for biodiesel production in packed bed reactor.
Heteropoly Acid (HPAs)
HPAs with Keggin structure are preferred due to easy synthesis process and their thermal stability but they have less surface area; therefore. a suitable supporting material can overcome this disadvantage. HPAs with carriers have super acidity and structural mobility; hence, they can be utilized to produce biodiesel. Kurhade and Dalai (2018) produced biodiesel (94.9 ± 2.3%) with 1:17.5 oil/methanol ratio, 10 wt. % 12-tungstophosphoric acid (TPA) (H3PW12O40·H2O) on γ-Al2O3, 4 MPa pressure, at 200 °C in 10 h whereas the second turn result in achievement of a 90.3 ± 4.3 biodiesel yield [41].
Sulfated Oxide Catalysts
In esterification reactions, sulfated metal oxide function as acidic heterogeneous catalysts. Titania with sulfuric acid and ammonium sulfate [TiO2/SO42−-H2SO4] and [TiO2/SO42−-(NH4)2SO4] Hammet indicators determined their strong acidity. FTIR analysis showed both Lewis type and Lewis and Bronsted type acidic properties, respectively. A high activity in oleic acid (79%) was observed in fatty acid esterification and at 80 °C 3 h reaction 82.2% yield was attained. In another study, Ce/ZrO2-TiO2/SO42− as catalyst and its activity is due to its bronsted acid sites depending on Ce conc. Vegetable oil esterification was achieved with 1:6 M Oil/methanol ratio, 5 wt. % catalyst loading, at 65 °C for an hour. Its catalytic activity was retained without a significant loss until 5th cycle [42].
Sulfonic Acid Catalyst
Sulfonated cross-linked polystyrene are ecofriendly and less corrosive. They are grouped as a sulfonic acid group and their activity is enhanced by fatty acid and alcohol tails attraction by polymer support. Furthermore, a site’s acidity is increased due to the attachment of sulfonic acid group to polymers chains. Hypercrosslinked polystyrene (D5082) supported sulfonic acid has been used. Despite having a less acidic site and a lower concentration, D5082 depicted high catalytic activity because of easy access to acidic sites [43].
4.3. Acid-Base Catalyst
These catalysts have both acidic and conjugated basic sites that are involved in catalyzing both esterification and transesterification of carboxylic acid and triglycerides, respectively. Such heterogenic catalytic systems, by working either as acid, base or bifunctional, are potentially efficient in biodiesel production form oils with high FFA content [44,45,46]. Jitputti and coworkers studied acid-base catalysts such as ZnO, ZrO2, Zeolite, SO4−/ZrO2, SO4−/SnO2 etc. for crude coconut oil and palm kernel oil transesterification with methanol [47]. ZnO and SO4−/ZrO2 showed high activity using palm kernel oil and crude coconut oil. Macario and coworkers reported biodiesel production by homogeneous/heterogeneous catalytic system of acid–base type and a biodiesel yield of 80% was achieved. However, the recovered catalyst gave a decreased yield due to catalytic leaching [48].
4.3.1. Zeolite-Based Catalysts
Both natural as well as synthetic zeolites are promising catalysts. Naturally, zeolites are found as aluminosilicates that are crystalline, interlinked by Oxygen and microporous and their versatile catalytic properties are related to their pore size (equal molecular pores or channels), ion-exchange characteristics and chemical composition. Negative ions are produced within the catalytic structure and, as a result, a basic catalyst emerges. As electropositivity of exchange cation increases, the base strength is increased. Their electro-neutrality maintenance by cation-polar interaction and shape selectivity makes them unique catalysts. Small pore diameter and weak basic strength are demerits associated with basic zeolites [38]. The castor oil biodiesel was synthesized by NaY zeolite-supported La2O3 reported by Du et al., 2018. S-La2O3/NaY-800 an optimized catalyst produced by mixing of La2O3, Kaolin, Sodiumcarboxymethyl cellulose (CMC) and zeolite NaY and the mass ratio was 70:20:10:2.5 for NaY/kaolin/La2O3,/CMC and 4 wt. % surfactant was added and calcination was conducted at 800 °C. FAEE yield (84.6%) was achieved with 10 wt. % catalyst loading, 1:15 M oil to ethanol ratio at 70 °C for 50 min [49]. Li et al. (2019) reported FAEE from castor oil (98.6 %) using 3 wt. % Li/NaY zeolite as catalyst. Catalyst, with 1:1 M (Li2O3:NaY), synthesized via co-precipitation method followed by calcination at 750 °C for 4 h from fly ash. The optimized conditions were 1:18 M oil/ethanol ratio, 3 wt. % catalyst in 2 h.
4.3.2. Zirconium-Based Catalysts
Zirconia (Zirconium dioxide (ZrO2)) is primarily acidic in nature due to strong surface acidity and used as both acid/base heterogeneous catalyst. Other derivatives include metal supported zirconia, metal oxides having ZrO2, sulfated ZrO2 etc. Tungsted Zirconia (WO3/ZrO2) showed properties of Bronsted and Lewis acid sites in biodiesel production with 94.58% yield having 15 wt. % catalyst 1:12 M oil/methanol ratio, at 100 °C in 3 h [50]. ZrO2 with Al2O3, SiO2 and TiO2 were studied by Ibrahim et al. (2019) using sol-gel auto-combustion. ZrO2/SiO2 provided a 48.6% yield using 0.1 mass % conc., 1:120 M acid/alcohol, 120 °C for 3 h and was reusable for up to 5 turns [51].
4.4. Homogeneous versus Heterogeneous Catalysts: A Comparison
The major difference between homogeneous and heterogeneous catalysts is being in the same phase and the different phase, respectively, as the reactants of the reaction medium. Hence, heterogeneous catalysts are in distinct solid phase while homogeneous catalysts are in liquid phase. Homogeneous catalysts provide a fast conversion, and kinetics are favored by base catalysts. Homogeneous catalysts provide a high yield, whereas heterogeneous catalysts provide a medium conversion with more severe reaction optimizing parameters. Heterogeneous acid catalysts are insensitive to the presence of water and fatty acid as compared to homogeneous ones, whereas heterogeneous base catalysts show little sensitivity to FFA and water content. Recycling and reusing heterogeneous catalysts is possible as they are facile to regenerate readily but it is problematic and almost impossible in the case of homogeneous catalysts. Heterogeneous catalysts have mass transfer resistance as three phases catalyst/oil/alcohol are present in reaction mixture. Summary of merits and demerits of utilization of base homogeneous, acidic homogeneous, base heterogeneous and acidic heterogeneous catalysts are shown in Table 6.

Table 6.
Merits and demerits of Homogeneous and Heterogeneous Catalysts.
Comparatively, homogeneous catalysts are more active with well-defined active sites, moderate reaction time, difficult to recover/separate, are not diffusion controlled and less stability whereas heterogeneous catalysts are less active (acid catalysts are less active than base catalysts) with poorly defined active sites, more reaction time, recoverable/separate easily, can be diffusion controlled and more stable. Homogeneous catalysts have a variable but usually short life span compared to heterogeneous catalysts. Homogeneous catalysts need extensive and expensive purification steps as large quantities of water are required for neutralization and purification of biodiesel, while heterogeneous catalysts have less and simpler purification steps and require a smaller quantity of process units. Homogeneous catalysts are more soluble compared to the heterogeneous catalysts. The reaction mechanism of homogeneous catalyst is reasonably well understood but poorly understood in case of a heterogeneous catalyst; the still research gap is there. Hence, the modification of a homogeneous catalyst is easy, whereas a heterogeneous catalyst modification is difficult. Homogeneous catalysts are less thermostable while heterogeneous catalysts have high thermal stability [52,53].
4.5. Nano-Catalysts
Homogeneous and heterogeneous catalysts have their own merits and demerits, and a synergist framework of new catalytic system is urgently and critically needed. These catalysts should be dynamic and active as homogeneous catalysts are, and effective as well as easily recoverable in the way that heterogeneous catalysts are. Nano catalysts have combined point of interest and joint advantages of both homogeneous and heterogeneous catalysts. Nano catalysts permit selective and quick chemical transformation with easiness of recovery and catalyst separation with a tremendously high product yield [54].
The contact between catalyst and reactant is incremental due to the nano-size that exhibits large surface area, similarly to homogeneous catalysts. Nano-catalysts are insoluble in the way that heterogeneous catalysts are. Therefore, nano-catalysts are also easy to recover and separate without difficulty. The most technological and scientific challenge linked to the utilization of nanoparticles is to understand the atomic scale structure and composition of nano-catalysts that gives the best catalytic activity. Nano-catalysts are particles with size ranging from 1 nm to 100 nm. The nano size of catalyst affects the biodiesel yield because such catalysts offer a large surface area, thus efficiently enhancing the rate of reaction [55,56].
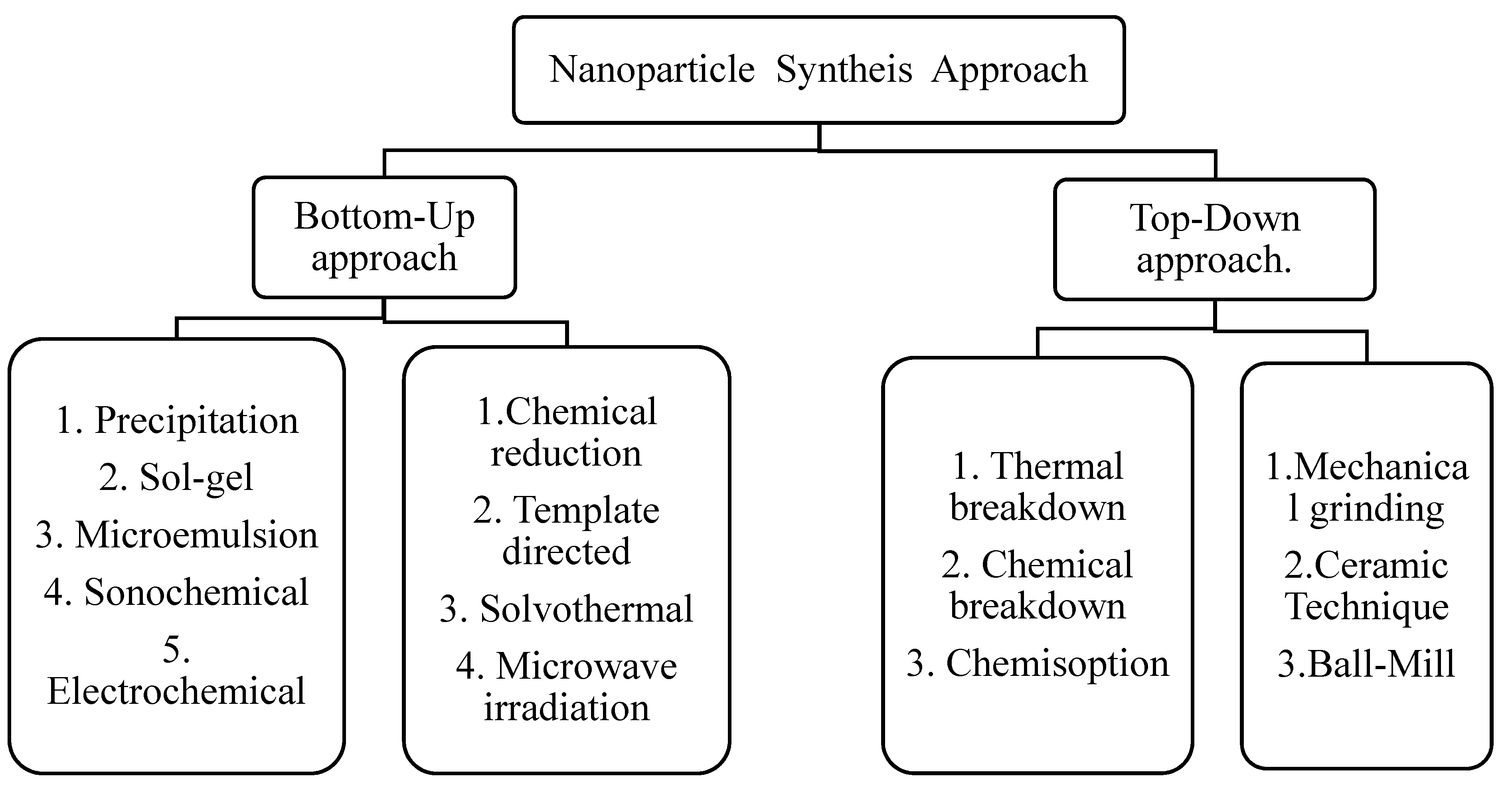
Their synthesis is the main task in the field of nano-catalysis. Top-down and bottom-up technologies are two basic categories of methods for preparation of nano-catalysts. The conventional methods include solid-state synthesis, combustion, sol-gel, co-precipitation, thermal decomposition and hydrothermal etc. However, unconventional methods include spray pyrolysis, microemulsion, pyrolysis (spray and laser), sonochemical, reverse micelle, drying and biosynthesis. Figure 5 shows scheme of methods for synthesis of nanoparticles.
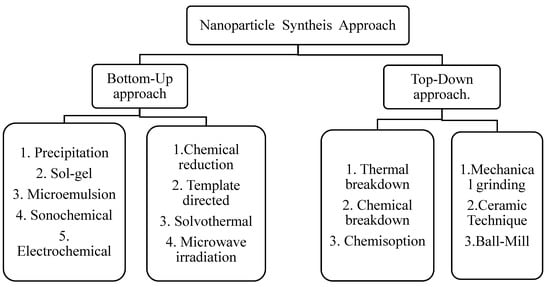
Figure 5.
Methods for synthesis of nanoparticles.
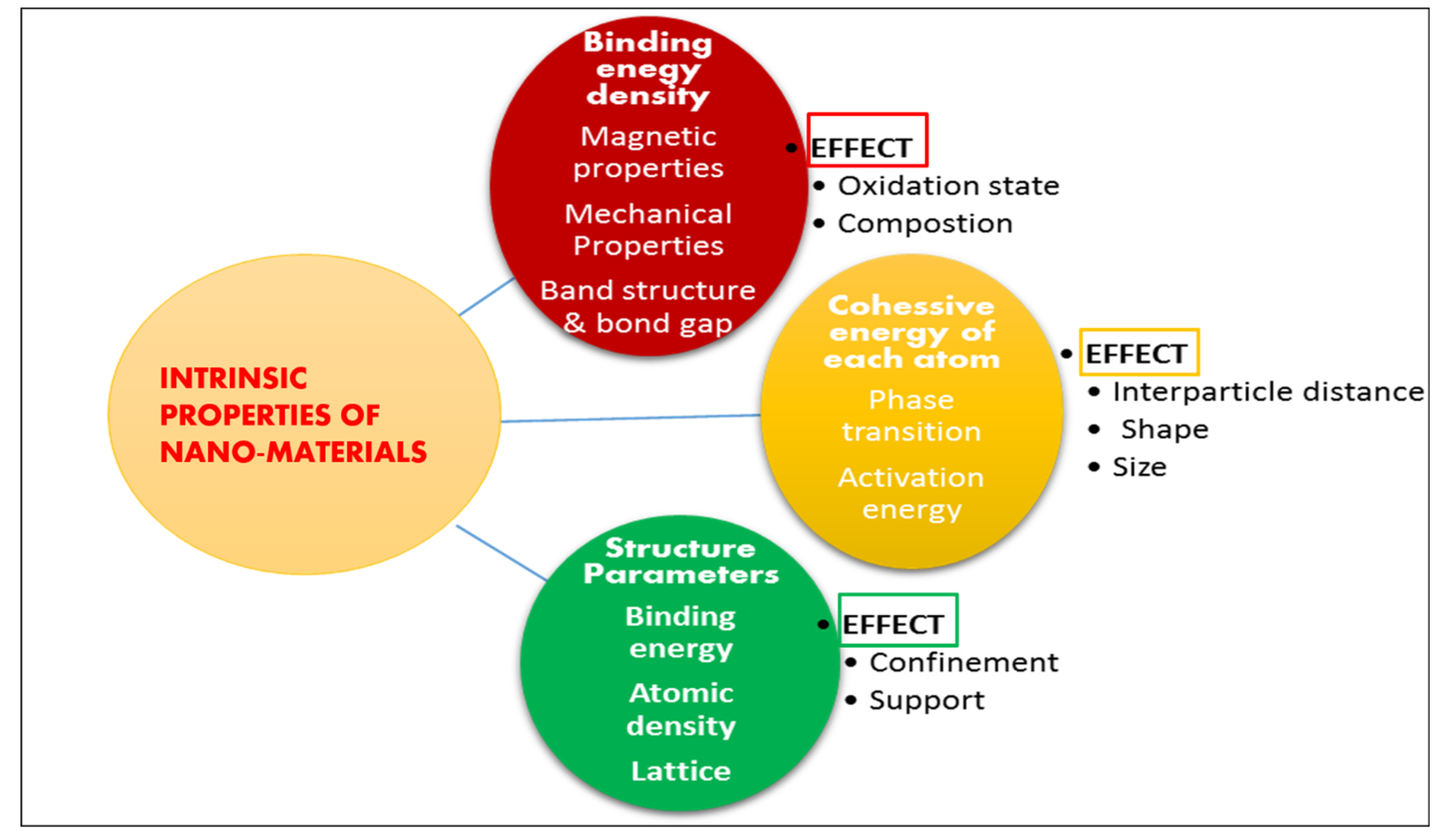
Nanoparticle science depends on two factors: first is the union of nanoparticles to manage size and shape, second is a subatomic mechanism in order to search particular utilization of nanoparticles, especially nano-catalysis. Synthesis of nano-catalysts with controlled size is primarily achieved by employing a range of stabilizing operators, for instance polymers, ligands and surfactants etc. The selection and reaction of nano-catalysts is considerably significant because it has high impact on progress of reaction that necessarily depends on the surface area of nanoparticles as well as relevant factors, for example controlling surface morphology, structure and composition. Recyclability is the property that acts as a bottleneck for their application at large scale. Additionally, such catalyst also possesses efficient volume and surface ratio, high stability, much resistance to saponification. Nano-catalysts can be synthesized using various methods such as impregnation (CaO-Au, Li-CaO, ZrO2/C4H4O6HK, Cs-Ca/SiO2-TiO2 and zeolite), wet impregnation Li/MgO, precipitation (MgO), deposition-precipitation (nano-sized MgO on Titanium), ball and mill method (CaO-Al2O3)glycerol nitrate combustion (TiO2-ZnO), co-precipitation (Fe/ZnO, KF/CaO-MgO), impregnation and sol-gel method (CsH2PW12O40/Fe-SiO2) Impregnation and Co-precipitation (KF/Ca-Fe3O4), sol-gel method (CaO-MgO, Sr3Al2O6, Sr-Ti nanocomposite), Supercritical sol-gel method Cs-MgO. With a maximum control over structure and composition, the preparation of these particles is the second challenge [55]. Current nano-technological methods for synthesis and characterization of catalytic nanoparticles are highly efficient. Figure 6 shows effect of intrinsic properties of nano-sized solids on catalysis.
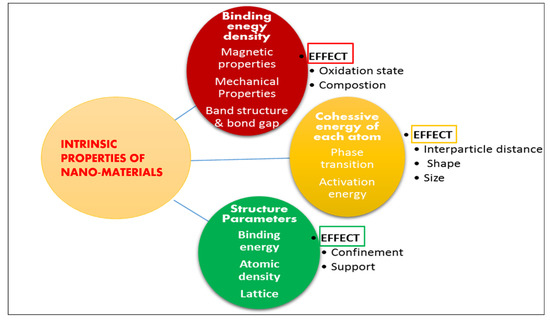
Figure 6.
Effect of Intrinsic properties of nano-sized solids on catalysis.
The intrinsic properties of nano-sized materials have a considerable impact on catalysis and catalytic activity [57]. These are:
- Quantities such as mean lattice constant, binding energy, and atomic density are directly related to bond length. Surface relaxation and densification are induced by lattice contraction in solids having nano-sized particles;
- Quantities such as thermal stability, activation energy, and critical temperature, coulomb blockade, self-organization growth are influenced by cohesive energy;
- Properties determining the complete band structure, for example Hamiltonian. Such characteristics change with binding energy density and other associated properties, for example photoemission, band gap and photoabsorption etc;
- Properties determined by combined effect of atomic cohesive energy and binding energy density, for example surface stress, magnetic performance (ferromagnets), extensibility, Young’s modulus, surface energy and compressibility.
Predominantly, the performance of atomic clusters or solid materials is different from that of isolated confined atom due to insertion of interatomic interface. The change in the relative number of atoms at surface that are under-coordinated and these under composed atoms mainly offer an additional chance that consents one to tune characteristics of nano-sized catalysts as compared to its counterpart [58]. As a result, participation of interatomic interface and under-coordinated atoms may be able to commence contemplation to sort out various complications in physical and chemical performance and these issues are between bulk solid and its counterpart: the isolated atom. The impact of the reduction in atomic coordination is remarkable and it combines the performance of a nano-catalyst, amorphous solid and surface steadily related to bond relaxation (unwinding) and its effects on bond energy. The irregular conduct and unpredictable behavior of nano-solid catalysts and a surface has been consistently followed and systematically comprehended because, with diminution in atomic coordination as well as its sub ordinates/derivatives (size support and reliance) on electron phonon coupling, crystal binding intensity, atomic trapping have potential. By precisely adjusting the surface composition, shape, spatial position, size, electronic structure, synthetic and thermal reliability of discrete nano-composites, it can be broadly used in field of catalysis with innovative features and activity [36,59].
There are potential benefits of nano-catalysts that make them a point of attention for researchers and these advantages can ensue through their utilization. Recently, advanced research has been conducted on novel and cheap nanomaterials for various purposes, including porous nano-materials, nano-fibers, nano-wires, nano-clays, nano-particles and nano-tubes. Size, form and shape are factors that affect the properties of nano- sized catalyst distribution of nano-particles and nano-particle processing bed [10].
Features of nanoparticles include maximum active surface (more the active surface available, better is efficiency and higher is the reaction rate), controlled size and shape (simulation and computational calculations for determination of active and stable nanoparticle), recoverability from reaction mixture (insolubility and large size than atoms and molecules), high selectivity (high percentage of main product with little unreacted material), clogging susceptibility (sticking together due to unstable structural state), chemical modifiability with high degree variation (nano-composites and linkage formation brings variability in their performance).
The advantages of nanomaterials include water remediation, improving the economy, reducing global warming, energy efficiency, reagents and super catalyst, minimum chemical wastes, variable and modifiable.
Nano-catalysts have been a subject of considerable commercial and academic explorations due to their potential merits that may be gathered by their utilization.
- Nano-sized crystalline particles can be synthesized by gas condensation. This involves the utilization of metals and alloys and is regulated by process such as evaporation and condensation. This procedure includes the evaporation of particles and their collision under inert conditions with gaseous molecules that results in reduction in total kinetic energy producing nanoparticles in vacuum. These particles are collected in a collector attached to the evaporator that has been kept at low temperature via N (liq.) and for combination of power, a compaction part is also existing. The main limitation of the process is the choice of temperature, difference in evaporation rate, incompatibility between source and antecedent, long process duration;
- Another procedure for preparing nano-catalyst is vacuum deposition, that involves the application of temperature for evaporation of various constituents such as alloys. The pressure of less than 0.1 Pascal is adjusted, the substrate molecule is heated and the optimum deposition rate is attained at 1.3 Pascal [60];
- Chemical vapor deposition (CVD) is a technique that involves material deposition on substrate surface via chemical reaction either with plasma (300–700 °C) or high temperature (900 °C) in plasma activated CVD and temperature induced CVD, respectively. There are merits of this procedure such as utilization of suitable deposition coating deprived of high vacuum and range of precursors. Nevertheless, its by-products pose the health risk, it is very expensive and it is utilized in the electronic industry;
- Chemical precipitation is a method to synthesize the nano-catalysts in which a dopant is added to the main solution before precipitation and with the aid of surfactant, the nanoparticles are separated and cleaned via a covering surfactant for nanocluster surface polymerization under UV light. A biofuel catalyst has been prepared in a number of researches; for example, MgO was synthesized via chemical precipitation and applied for rapeseed biodiesel production [61];
- The colloidal particles are produced via sol gel method that involves hydrolysis reactions. It is a liquid phase technique in which a specific amount of substrate is used to achieve the precipitation of nanoparticles. The merit of this method includes the requirement of low temperature, and much less effort is needed for shaping and inserting, in turn making the process flexible. Sol-gel method has been successfully applied to synthesize nano-catalyst such as MgO and CaO [61];
- Impregnation method is the method for preparation of nano-catalyst in which solid and liquid (having component) comes in contact and suitable constituent adsorbed on solid and the adsorption of various components happens at various rates [62]. Hydrogen bonding and van der Waals. For instance, the impregnation method has been used to prepare KOH/CaAl2O3. A known amount of KOH (aq. soln.) was mixed with CaOAl2O3 and alumina then refluxed for 2 h. at 80 °C for conversion in to gel. Later on, this gel is oven dried overnight at 100 °C and calcined for 5 h. at 700 °C. The merits include it being cost effective method which is simple and well-studied; however, the particle size is difficult to control [63];
- An economical method to control particle size is electrochemical deposition that does not need a high concentration or temperature yet results in a one dimensional nanoparticle. Synthesis parameters, for example, reducing agent, conc. and calcination temperature are adjusted and in this way the characters of nano-catalysts can be improved.
Table 7 shows selected nano-catalysts along with their synthesis techniques; such catalysts have been employed in biodiesel synthesis process on different feedstock oils to produce high biodiesel yield.

Table 7.
Nano-catalysts employed in biodiesel synthesis process.
4.5.1. Clay Derived Nano-Catalysts
Nowadays, nano-sized clay catalysts are being considered by researchers. A clay catalyst can efficiently catalyze hydrogenation, acylation, esterification, transesterification, epoxidation, alkylation and cyclic condensation. Clay and its minerals (Phyllosilicates) vary in composition and have different particle sizes. Inorganic clay as a support catalyst is gaining more significance, owning to its ecofriendly nature along with its abundance and economic feasibility. Phyllosilicates, (Al2-xMgx) Si4O10(OH)2(M.nH2O) having layered structure with tetrahedral and octahedral sheets (2:1) and form via volcanic ashes or weathering of soil and rocks [90]. The cation exchange capacity of clay enhances, owing to isomorphous substitution in sheets’ interlayers. Hence, this make them promising and potential candiadates for heterogeneous nano-catalysis in the biodiesel synthesis process [90,91]. They are modified by various techniques such as intercalated catalysts, pillared interlayer clays, hybrids and composites, clay supported catalysts, ion exchanged clays, hierarchically structured clays, acid activated clays and layered double hydroxides. These techniques have been implemented in order to obtain a synergetic effect: basic-acidic in catalyst to efficiently convert non-edible feedstock to biofuel [90]. Similarly to montmorillonite, sepiolite, kaolinite and bentonite are the best candidates to be used as catalysts, since they have a high cation exchange capacity and, hence, more adsorption ability beholden to large surface area and great porosity. Munir et al. (2019) achieved an 85% biodiesel yield from non-edible seed oil of Prunus cerasoides by Cd-Mn modified montmorillonite clay catalysts [92]. Similarly, wild non-edible seed oil of Celastrus paniculatus has been converted into biodiesel under optimized conditions (1:12 methanol:oil, 120 °C temp., 3.5% catalyst loading and 3 h reaction time) and 89.42% biodiesel yield was achieved by novel trimetallic montmorillonite clay catalyst [93]. There are studies that cover utilization of clay catalysts for transesterification reaction are discussed in Table 8.

Table 8.
Clay derived heterogeneous catalyst for sustainable biodiesel production.
4.5.2. Plant and Animal Origin Green Nanocatalysts
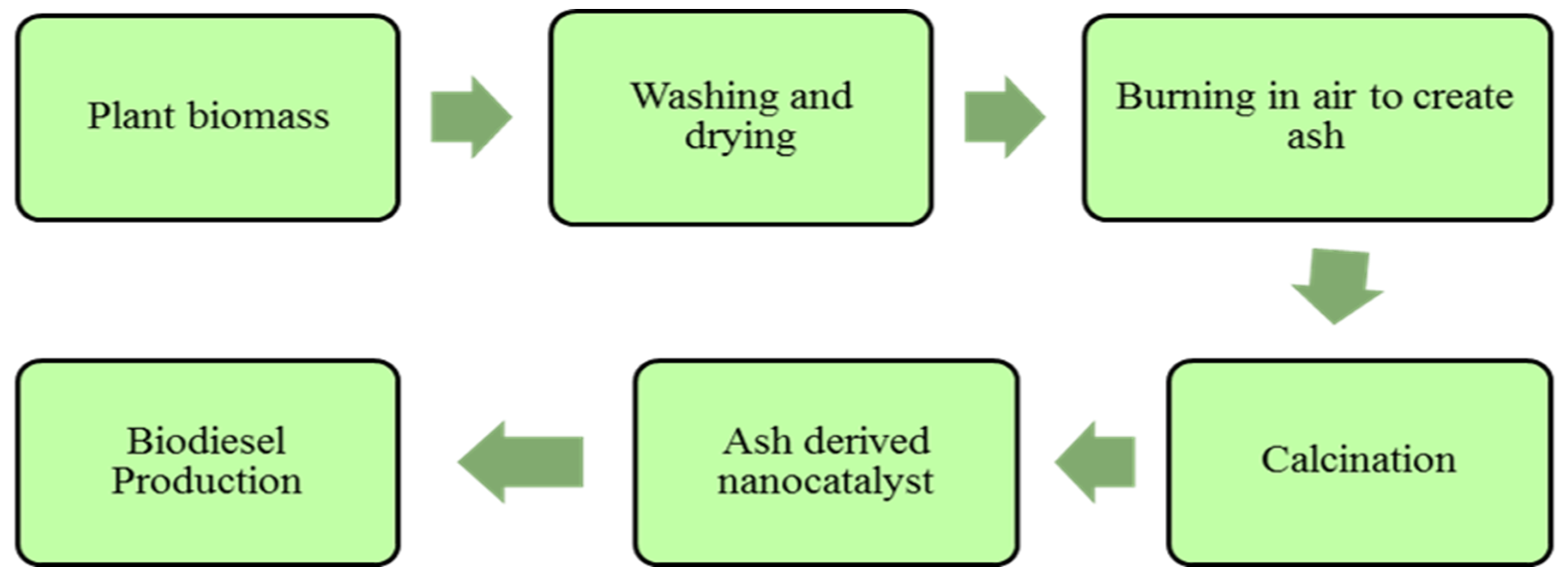
Plants and animal based heterogeneous nano-catalysts include rice husk, CaO based shell of palm kernel, snail shells etc. CaO based catalysts of animal origin are specifically obtained from shells and bones and shells are much preferable for synthesis of heterogeneous base catalysts. The by-product CO2 is required to be removed during reaction. Carbonates become dissociated in the case of an increase in temperate from outer towards inner surface, the conversion is initiated and there is increase in surface porosity during this reaction and as a result CaO catalyst (active) is produced. The newly synthesized CaO catalyst enhance the rate of transesterification reaction and oil is converted into biodiesel because of its active sites. Solid catalysts that are based on organic waste have been successfully used, such as sugarcane bagasse, coconut shell, palm trunk, peel, wood and ash [115]. Gohain et al. (2017) produced plant based catalyst by oven dried peel at 80 °C temperature for 48 h and calcined at 700 °C for 4 h [116]. Later on, it was used for two step transesterification of neem oil. Synthesis process of plant ash derived nano-catalyst is shown in Figure 7.
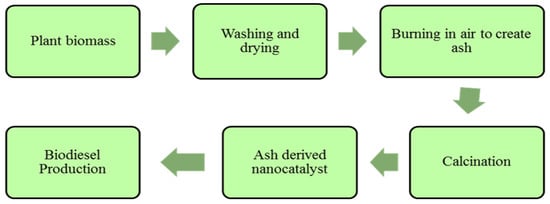
Figure 7.
Synthesis of ash derived nano-catalyst for biodiesel production.
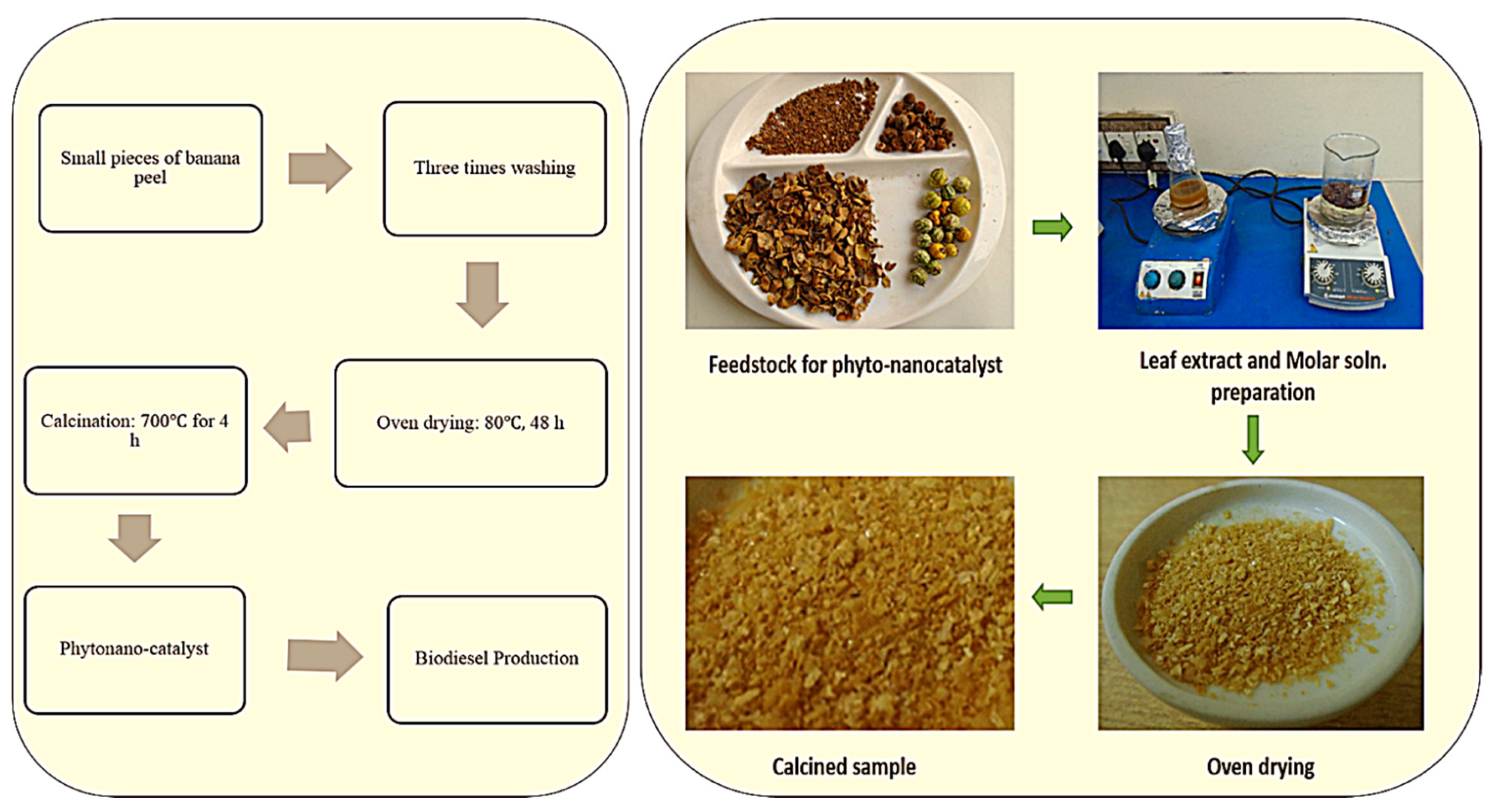
Tucuma palm peels showed high catalytic activity as they contained a considerable amount of potassium, phosphorus, calcium and magnesium ions [117]. Solid base catalysts have been successfully synthesized from waste pine apple leaves and this phytonanocatalyst used for biodiesel production from soybean oil and 98.92% yield was achieved [118]. However, sulfonated Sargassum horneri carbon (10 wt. %) used as a phytonanocatalyst (acid solid catalyst) for oleic acid esterification in the presence of 1:15 M oleic acid/methanol ratio, 70 °C for 3 h resulting in a 96.4% biodiesel yield [119]. Rubber deoiled cake was used as waste derived heterogeneous acid catalyst, synthesized from Sulfuric acid and chlorosulfuric acid, and a 91.2% FAME yield was achieved from mixed non-edible oil [120]. Previous studies show the use of chicken bones to prepare economical solid base catalyst with same method (calcination at 900 °C for 4 h). A conversion yield of 89.3% FAME was achieved from soybean seed oil via 5% wt. catalyst loading, 1:15 oil to methanol molar ratio, reaction temperature (65 °C) and 4 h of reaction time in transesterification reaction. This catalyst was used four times for improvement of process efficiency. So, the reported catalyst appeared to be effective and a prospective substitute to homogeneous catalysts [121]. Animal bones modified by Nisar et al. (2017 (with potassium hydroxide to check its efficiency on transesterification of Jatropha under optimized conditions. Methanol to oil ratio was 9:1, 6% wt./wt. of oil taken, 3h reaction time at 70 °C and it resulted in a high conversion yield (96.1%) [122]. Hebbar et al. (2018) synthesized CaO nanoparticles by using Bombax ceiba seed extract to synthesize biodiesel from Bombax ceiba seed oil and a 96.2% yield was achieved. The green nano-catalyst was reusable for up to 5 cycles [123]. Figure 8 presents the steps involved in synthesis of phytonanocatalyst by utilizing banana peel contains oxides of sodium and potassium.
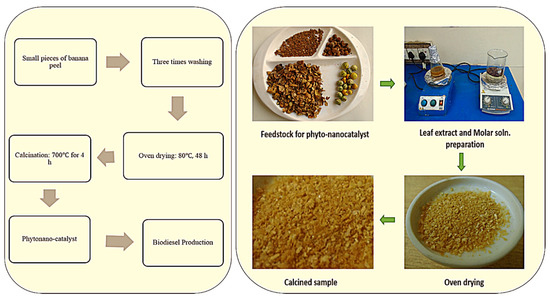
Figure 8.
Synthesis of Phyto-nanocatalyst for biodiesel synthesis.
Ali et al. (2018) used goat bone for transesterification of waste cooking oil and catalytically improved the bone by calcination with CaO and at 1:9 Oil: Met ratio at 65 °C for 5 h and a 84% yield was achieved with 3 reusability cycles [124]. In a study by Kara et al. (2019), eggshell was used as catalyst up to 8 times and it was prepared from CaO under air static conditions for 2 h at 850 °C. The vegetable oil was converted to biodiesel with 1:9 oil to methanol molar ratio, 60 °C, 3 h time duration and 3% catalyst loading, and a 96% conversion efficiency was achieved. Thus, environmentally friendly and economical biodiesel was produced [125]. Many studies have been conducted that involved CaO catalyzed transesterification of oil and fat for biodiesel synthesis. Similarly, in addition to chemical basis, a number of experiments have been performed to synthesize alkaline heterogeneous nano catalysts of animal and plant origin that offer high conversion yield, as shown in Table 9.

Table 9.
Plants and Animals based green catalysts for biodiesel production.
So, it can be concluded that the nano-size of catalyst affects the biodiesel yield, owing to their large surface area for catalytic reaction to takes place thus enhancing the rate of reaction efficiently.
4.6. Biocatalysts
The practice of biological systems or their parts can be used in order to speed up the rate of chemical reactions. Biocatalysts, owing to their renewability and natural origin, have emerged as an essential substitute to chemical catalysts and employed to process various reactions and succeeded to sort out various issues of human and environmental concerns. An ideal biocatalyst is one that is readily available, highly selective, cost-effective and reusable as such parameters have direct impact on overall expense of process. Most of the preferable biocatalysts are proteins regarded as enzymes and studies on enzymatic biodiesel used as lipases in powdered or liquid forms [135,136]. These enzymes, that are in free form, are considerably cheaper as compared to immobilized lipases but they have few drawbacks such as single time usability as get inactivated after utilization once and are less stable in terms of operation therefore regarded as less economical. Nanoparticles, nanofibers, nanotubes, nanoporous media, nanocomposites, and graphene are employed for immobilization because they have large surface areas that boost biocatalytic effectiveness for industrial applications by enhancing enzyme loading and improving reaction kinetics [137]. Moreover, powdered preparation makes aggregates, thus resulting in the reduction in available active sites. Moreover, health concerns are associated with the utilization of powder, as it has allergenic potential when inhaled. Due to the problems of utilizing free lipases, currently commercial immobilized lipases are being focused, as they depict high catalytic activity, more stability, easy recoverability and active reusability [138]. The lipase B of Canadida antarctica (CALB) named as Novozym 435 is used most frequently as it has been immobilized in macroporous resins [139]. Lipozyme RM IM and Lipozyme TL IM are also among commercial immobilized preparations, these are R. miehei lipase and T. lanuginosus lipase immobilized on anionic resin and granulated silica gel. However, a high budget is required for such preparations is actual hurdle in their utilization in large scale commodities production, for example, biodiesel synthesis. Hence, the focus of many researchers is on making it more economical and feasible for biodiesel synthesis [140,141]. Their use for biodiesel synthesis have solved many problems such as no soap formation even with low graded feedstock (waste cooking oil) with highly pure biodiesel, an antagonistic behavior to those of alkaline catalysts. Free fatty acids (FFA) and water in feedstock has caused serious issues with conventional ways of transesterification and such problem can be solved by enzymatic process. This is best suited for feedstock with high FFA levels such as pork, beef and waste cooking oils. FFA combine with them, and alkyl esters are produced: biodiesel. Generally, there are two types of enzymatic catalysts are there: intracellular and extracellular lipases. Various factors such as selectivity, efficiency, stability and recyclability make them best choice to carry out transesterification. The inactivation of biocatalysts due to methanol, acyl acceptor for transesterification that limits the mass of alcohol during the reaction. This irreversible inactivation is due to aggregation as solvent molecules generally bind to protein. Non-covalent binding of solvent molecules to the enzyme result in its competitive inhibition [136]. Hence, the mechanism must be understood in order to design various strategies to solve these problems. A way to sort out this issue is the immobilization of enzymes.
4.6.1. Immobilization of Lipases
When enzymes are immobilized in solid carriers that enhance their thermal as well as chemical stability, they are protected from denaturation. Moreover, physical enclosure and retention within the definite space while holding catalytic properties along with continuous reusability. So far, the immobilization is performed via modern techniques such as entrapment, crosslinking, physical adsorption and cross linking [142].
Physical Adsorption
Physical adsorption on a support is one of most frequently used method for immobilization of proteins via H-bonding and weak van der Wall forces in external surface of a support. A factor analysis of waste cooking oil for biodiesel synthesis via Rhizomucor miehei lipase from waste cooking oil [143]. An irregular shaped macroporous ion exchange hydrophilic resin was used to immobilize the enzyme, bulk density of 0.4 g/cm3, dimensions from 0.06 and 0.02 cm and 0.52 ± 0.02 of porosity. These model predictions coincide with experimental data and 90% yield for 8 h of reaction. Enzymatic transesterification of Jatropha was performed via Pseudomonas cepacia immobilized on celite under optimum reaction conditions such as 4–5% (w/w) water at 50 °C in 8 h. The catalyst was recycled four times without considerable loss of activity. Molecular sieve MCM-41has been prepared in order to immobilize the Candida rugosa lipase with maximum point (250 mg/g) at pH 6 was used to synthesize cottonseed oil biodiesel. Methanol was used as solvent and fatty acid methyl esters (FAME) were produced under optimum conditions such as temperature, pH and methanol to oil ratio. The yield (98.3%) was achieved but enzyme leaching was the major limit in its proper use. The possible solution could be implementing the entrapment for enzyme restriction within the support [19,144].
Entrapment
Entrapment is employed in order to immobilize the enzymes physically in which lipase is trapped in polymeric network, for example microcapsules that allows substrate to pass through still holding the lipase. Generally, entrapment of lipase is performed by placing it in monomeric phase solution in situ and polymerization results in entrapment. As compared to physical adsorption, the entrapment method of immobilization give more stability and protection from surroundings such as gas bubbles, hydrophobic solvent and mechanical stress [145].
Pseudomonas cepacia lipase was immobilized in phyllosilicate sol-gel matrix and enzyme catalyzed production of alkyl esterified from grease and tallow was investigated, >95% ethyl esters were produced and possessed 5-time reusability without considerable loss of activity was noticed. Nine lipases were tested in enzymatic transesterification of soybean oil with both methanol and ethanol. Pseudomonas cepacia was immobilized on sol-gel support resulted in highest yield of alkyl esters. After an hour, the yield of fatty acid methyl esters (FAME) was 67% mol, while fatty acid ethyl esters (FAEE) yield was 65% mol. Biodiesel from palm oil was synthesized via enzymatic catalysis by using methanol as well as ethanol. Candida rugosa lipase entrapped in activated carbon support and four-time reusability test results highest yield of fatty acid alkyl esters. An hour after the reaction, FAME and FAEE yield was 70% and 85% mol, correspondingly. Pseudomonas cepacia lipase was immobilized by entrapping it in polyallylamine-mediated biometric silica and used to synthesize biodiesel from soybean and waste cooking oil as feedstock. The soybean oil was trans-esterified under optimum conditions were 75% n-hexane, 43.6 °C, substrate molar ratio 4.3% and 79% predicted value and 76% experimental yield of biodiesel was achieved. Optimum conditions for transesterification of waste cooking oil were 38% n-hexane, 43.3 °C, substrate molar ratio 5% and 68% predicted value and 67% experimental yield of biodiesel was attained. The entrapped lipase stored for a month at 4 °C and efficiency of the catalyst remained the same. In contrast to covalent bonding, a comparatively simple technique is employed while maintaining the stability and catalytic activity of enzyme [136].
Covalent Bonding
When lipase is irreversibly bonded to solid support matrix by covalent bonds such enzymatic immobilization occurs when amino acid residue is present outside of enzymatic catalytic site towards support matrix. The strong interaction between the enzyme and support makes the leaching of lipase to reaction moiety rare because the stability and activity of the enzyme are linked to support characteristics such as binding affinity, pore size and chemical stability. When thermal and operational stability of enzymes is considered, multipoint covalent bonding appears to be more effective technique of lipase immobilization [136].
Sn-1, 3 regioselective lipase has been used for biofuel synthesis from Jatropha oil. Rhizopus oryzae lipase was covalently bonded to various supports for transesterification catalysis at 30 °C on stepwise additions of methanol. After 4 hours of reaction, 51–65% FAME efficiency was exhibited by biocatalysts [146]. Tang et al. (2019) immobilized Candida cylindracea via covalent bond to Ca-bentonite (alkaline) having glutamic acid modification. Lipase after immobilization showed a high catalytic activity and high yield (99%) was attained in transesterification reaction; however, when the lipase was free, a 52.8% yield was achieved. In the reusability test, the immobilized lipase showed a 56.2% conversion efficiency even after reusing it 5 times whereas free lipase became inactive after two cycles of reusability [147].
Cross Linking
This technique of enzymatic immobilization is characterized by cross linkages formed by cross linker (bisdiazobenzidine and gluteraldehyde) between the molecules. This support free technique involves enzymatic aggregation to attain 3-D structure. The enzymatic aggregate is treated with acetone and precipitation begins the process of cross linking via a cross linker and more robust structure is obtained. It can be considered as an upgradation in covalent bonding because of cross linker that cross linkages the enzyme to support. An ion exchange resin is used in previous step and then a schiff base is formed, using the buffered solution of the cross linker [148].
Biodiesel was synthesized from Medhuca indica oil by immobilization of Pseudomonas cepacia lipase and high yield obtained on use of cross-linked enzyme aggregates (CLEA). On the other hand, 98% conversion yield was attained in 6 h by using free lipase powder. Pseudomonas cepacia gave 92% yield in 2.5 h and almost same amount of lipase was used [136,149]. Another immobilization technique was used for Termomyces lanuginosus lipase. Polygluturaldehyde used as cross linking in hydrophilic polyurethane foam, followed by use of biocatalyst for transesterification of canola oil. Various optimization parameters were measured, and 90% FAME yield recorded with lipase activity and stability retention even after 10 batches. The loss in little activity noticed after repeated usage. Photobacterium lipolyticum lipase M37 has been immobilized by CLEA technique and olive oil was converted into biodiesel using alcohols such as methanol and ethanol via this biocatalyst that could also be used for organic synthesis. Burkholderia cepacia lipase that was immobilized by cross linking with glutaraldehyde and then entrapped on matrix having carrageenan and alginate in equal proportions, followed by application on Jatropha oil for biodiesel synthesis using ethanol. A total conversion of FAEE could be attained for optimum conditions and after 6 cycles of reusability and its transesterification activity was retained up to 73% [144,150]. A summary of methods of lipase immobilization is given below in tabular form. Immobilization technique, immobilized biocatalyst, respective name, feedstock, reaction optimization parameters and biodiesel yield are presented in Table 10.

Table 10.
Lipase mobilization techniques for biodiesel synthesis [136].
5. Benefits and Drawbacks of Different Catalysts: A Summary
The chemical reactions involved in this process are categorized as esterification and/or transesterification and both reactions use catalysts such as homogeneous (same phase as reagents) and heterogeneous (not in same phase as reagents). Basically, two types of catalytic systems were included: chemical and biological. Homogeneous catalysts are efficient but produce unreacted chemicals and water treatment are main drawbacks associated with their utilization. However, heterogeneous catalysts involve external surface and porous solid support, but leaching affects the reaction adversely. Nanotechnology by the synthesis of nano-catalysts helps to solve issues associated with homogeneous and heterogeneous catalysts. Nano-catalysts such as ash derived, clay derived, animal and plant origin based solid acid and solid base catalysts are highly efficient compared to homogeneous, heterogeneous and nano catalysts. Among biological systems, free enzymes are highly beneficial but costly and lack reusability, except for immobilized enzymes. Therefore, they are preferred. Immobilization via covalent binding prevents leaching. Hence, the proper selection of most optimal combination of the type of catalysts, the type of oils, and the process conditions is important to obtain the maximum biodiesel yield.
6. Challenges, Future Perspectives and Recommendations
Challenges in utilization of homogeneous catalysts have been elaborately addressed in the literature as extensive and exhaustive research has already been conducted in this aspect. However, due to significant ongoing studies, heterogeneous nanocatalysis appears to be an advanced and emerging field. The main challenges associated with heterogeneous catalysts include: instability due to short life span along with low reaction rate, sensitivity to FFA and water resulting in saponification (solid base catalysts), leaching and product contamination (solid acid catalysts). For high performance of nano-catalysts require high energy and severe conditions to operate in ordinary reaction time because under mild reaction conditions, these catalysts take relatively more reaction time. In case of biocatalysts, the methanol presence causes inhibition of lipase (enzymatic transesterification).
In future work, the following aspects need to be addressed:
- Tremendous efforts should be made in order to exploit the ways to enhance kinetic rate of heterogeneous nano-catalysts, especially CaO because it has high catalytic activity, long life span and mild operating conditions;
- Biomass or waste derived catalysts should be synthesized in order to utilize and reuse the superfluous matter. Highly selective, promising and economic catalysts are needed to be developed at commercial scale;
- To improve the sustainability of catalysts, preparation and treatment methodologies (especially to deal with high FFA and water) should be upgraded;
- Further investigations are required to ensure the effective recovery of nano-catalysts via energy efficient and cost-effective routes for reusability;
- At a large scale, enzymatic biodiesel production can be a viable option in future (as an environmentally benign method), therefore the process should be potentially sound and low-cost that will also ensure the production of high-quality biodiesel in favorable competition with regular petroleum-based diesel.
7. Conclusions
Biodiesel is produced via transesterification that is an auspicious method among existing technologies being applied that is primarily governed by catalytic systems: chemical and biological. The FFA content of fat or oil determines the specific catalyst to be used for transesterification. Catalysts are either chemical or biological in nature. When considering the chemical-based catalysts, homogeneous catalysts are operative; however, the high energy consumption and water wastage during purification are disadvantages of the process. For heterogeneous catalysts, a porous solid support is used and just its outer surface-active species participates but leaching has adverse impact on the reaction. Recent synthetic protocols for nano-catalysts have assisted the researchers in the modification of catalytic surface to solve the problems of conventional catalysts and to meet the demands of applications. Nano-catalysts behave as a linkage between homogeneous and heterogeneous catalysts that can produce promising solid acid or base catalysts because they possess the merits of both high contact surface in the way that homogeneous catalysts can and are readily separable in the way that heterogeneous catalysts are. However, biological catalysts include enzymatic systems such as free lipases that are beneficial over chemical catalysts, yet the production cost is high due to expensiveness as reusing the enzymes is not possible. Therefore, enzymes are immobilized to make them reusable and favorable to catalyze the biodiesel production process. Among immobilization techniques, covalent bonding is considered as the best option, specifically in the case of solids as the carrier. The consideration of cost is substantial factor that affects the economical production of biodiesel leading to the generation of highly efficient and priced fuel. Concerning the research conducted, it is claimed that nano-sized catalysts, owing to their highly active surface and outstanding selectivity, offer more suitable conditions for catalysis by enhancing their efficiency and eventually increase the reaction rate. In short, the synthesis time and low budget, animal, and plant based-biomass nano-catalysts can be assumed as the best option for producing biodiesel via transesterification. Hence, an insight into the catalyst design along with prudent selection of a suitable catalyst for efficient transesterification will mark a milestone in the biofuel industry.
Author Contributions
Writing—original draft and Data curation, M.A. (Maria Ameen); Methodology and Conceptualization, M.A. (Mushtaq Ahmad); Supervision, Investigation, M.Z.; editing, Methodology, M.M.; Visualization, Software, M.M.A.; Formal analysis, S.S.; Validation, R.; Funding acquisition, resources, S.E.E.; Project administrator, M.E.M.S.; Funding acquisition, review editing M.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ameen, M.; Zafar, M.; Ahmad, M.; Shaheen, A.; Yaseen, G. Wild melon: A novel non-edible feedstock for bioenergy. Pet. Sci. 2018, 15, 405–411. [Google Scholar] [CrossRef]
- Ahmad, M.; Zafar, M. Conversion of waste seed oil of Citrus aurantium into methyl ester via green and recyclable nanoparticles of zirconium oxide in the context of circular bioeconomy approach. Waste Manag. 2021, 136, 310–320. [Google Scholar]
- Ahmad, M.; Zafar, M.; Yousaf, Z.; Ullah, S.A.; Sultana, S.; Bibi, F. Identification of novel, non-edible oil seeds via scanning electron microscopy as potential feedstock for green synthesis of biodiesel. Microsc. Res. Tech. 2021, 85, 708–720. [Google Scholar]
- Paryanto, I.; Prakoso, T.; Suyono, E.A.; Gozan, M. Determination of the upper limit of monoglyceride content in biodiesel for B30 implementation based on the measurement of the precipitate in a Biodiesel–Petrodiesel fuel blend (BXX). Fuel 2019, 258, 116104. [Google Scholar] [CrossRef]
- Guo, M. The global scenario of biofuel production and development. In Practices and Perspectives in Sustainable Bioenergy; Springer: New Delhi, India, 2020; pp. 29–56. [Google Scholar]
- Badday, A.S.; Abdullah, A.Z.; Lee, K.T.; Khayoon, M.S. Intensification of biodiesel production via ultrasonic-assisted process: A critical review on fundamentals and recent development. Renew. Sustain. Energy Rev. 2012, 16, 4574–4587. [Google Scholar] [CrossRef]
- Malek, M.N.F.A.; Hussin, N.M.; Embong, N.H.; Bhuyar, P.; Rahim, M.H.A.; Govindan, N.; Maniam, G.P. Ultrasonication: A process intensification tool for methyl ester synthesis: A mini review. Biomass Convers. Biorefinery 2020, 1–11. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- De Blasio, C. Biodiesel. In Fundamentals of Biofuels Engineering and Technology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 253–265. [Google Scholar]
- Narasimhan, M.; Chandrasekaran, M.; Govindasamy, S.; Aravamudhan, A. Heterogeneous nanocatalysts for sustainable biodiesel production: A review. J. Environ. Chem. Eng. 2021, 9, 104876. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Atabani, A.; Shobana, S.; Mohammed, M.; Uğuz, G.; Kumar, G.; Arvindnarayan, S.; Aslam, M.; Ala’a, H. Integrated valorization of waste cooking oil and spent coffee grounds for biodiesel production: Blending with higher alcohols, FT–IR, TGA, DSC and NMR characterizations. Fuel 2019, 244, 419–430. [Google Scholar] [CrossRef]
- Chai, M.; Tu, Q.; Lu, M.; Yang, Y.J. Esterification pretreatment of free fatty acid in biodiesel production, from laboratory to industry. Fuel Process. Technol. 2014, 125, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Sharma, M.; Dwivedi, G. Impact of alcohol on biodiesel production and properties. Renew. Sustain. Energy Rev. 2016, 56, 319–333. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters. Energies 2015, 2, 362–376. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Lee, J.W. In-situ transesterification of wet spent coffee grounds for sustainable biodiesel production. Bioresour. Technol. 2016, 221, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Talha, N.S.; Sulaiman, S. Overview of catalysts in biodiesel production. ARPN J. Eng. Appl. Sci. 2016, 11, 439–442. [Google Scholar]
- Krope, J.; Olabi, A.G.; Goričanec, D.; Božičnik, S. Biodiesel Production. A Brief Review. In Proceedings of the 10th International Conference on Sustainable Energy and Environmental Protection Bioenergy and Biofuels, Bled, Slovenia, 27–30 June 2017. [Google Scholar]
- Ma, X.; Liu, F.; Helian, Y.; Li, C.; Wu, Z.; Li, H.; Chu, H.; Wang, Y.; Wang, Y.; Lu, W. Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review. Energy Convers. Manag. 2021, 229, 113760. [Google Scholar] [CrossRef]
- Bohlouli, A.; Mahdavian, L. Catalysts used in biodiesel production: A review. Biofuels 2021, 12, 885–898. [Google Scholar] [CrossRef]
- Bhoi, R.; Singh, D.; Mahajani, S. Investigation of mass transfer limitations in simultaneous esterification and transesterification of triglycerides using a heterogeneous catalyst. React. Chem. Eng. 2017, 2, 740–753. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.; Ong, H.; Mahlia, T.; Mofijur, M.; Silitonga, A.; Rahman, S.; Ahmad, A. State of the art of catalysts for biodiesel production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Waste cooking oil and waste chicken eggshells derived solid base catalyst for the biodiesel production: Optimization and kinetics. Waste Manag. 2018, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-M.; Wang, Y.-F.; Chen, C.-C. Synthesis and characterization of magnetic LiFe5O8-LiFeO2 as a solid basic catalyst for biodiesel production. Catal. Commun. 2018, 106, 20–24. [Google Scholar] [CrossRef]
- Salinas, D.; Sepúlveda, C.; Escalona, N.; Gfierro, J.; Pecchi, G. Sol–gel La2O3–ZrO2 mixed oxide catalysts for biodiesel production. J. Energy Chem. 2018, 27, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Kandaramath Hari, T.; Yaakob, Z. Effect of calcination temperature on the application of sodium zirconate solid base catalyst for biodiesel production from Jatropha curcas oil. Int. J. Green Energy 2017, 14, 1163–1171. [Google Scholar] [CrossRef]
- Kaur, M.; Malhotra, R.; Ali, A. Tungsten supported Ti/SiO2 nanoflowers as reusable heterogeneous catalyst for biodiesel production. Renew. Energy 2018, 116, 109–119. [Google Scholar] [CrossRef]
- Mofijur, M.; Siddiki, S.Y.A.; Shuvho, M.B.A.; Djavanroodi, F.; Fattah, I.R.; Ong, H.C.; Mahlia, T.M.I. Effect of nanocatalysts on the transesterification reaction of first, second and third generation biodiesel sources-A mini-review. Chemosphere 2021, 270, 128642. [Google Scholar] [CrossRef]
- Yaqoob, I.; Rashid, U.; Nadeem, F. Alumina supported catalytic materials for biodiesel production-a detailed review. Int. J. Chem. Biochem. Sci. 2019, 16, 41–53. [Google Scholar]
- Benjapornkulaphong, S.; Ngamcharussrivichai, C.; Bunyakiat, K. Al2O3-supported alkali and alkali earth metal oxides for transesterification of palm kernel oil and coconut oil. Chem. Eng. J. 2009, 145, 468–474. [Google Scholar] [CrossRef]
- Navajas, A.; Campo, I.; Moral, A.; Echave, J.; Sanz, O.; Montes, M.; Odriozola, J.A.; Arzamendi, G.; Gandía, L. Outstanding performance of rehydrated Mg-Al hydrotalcites as heterogeneous methanolysis catalysts for the synthesis of biodiesel. Fuel 2018, 211, 173–181. [Google Scholar] [CrossRef]
- Marwaha, A.; Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Waste materials as potential catalysts for biodiesel production: Current state and future scope. Fuel Process. Technol. 2018, 181, 175–186. [Google Scholar] [CrossRef]
- Bano, S.; Ganie, A.S.; Sultana, S.; Sabir, S.; Khan, M.Z. Fabrication and optimization of nanocatalyst for biodiesel production: An overview. Front. Energy Res. 2020, 350. [Google Scholar] [CrossRef]
- Erturk, K.M. Synthesis and Characterization of Sulfated Heterogeneous Catalyst for Biodiesel Transesterification. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2021. [Google Scholar]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Fu, J.; Chen, L.; Lv, P.; Yang, L.; Yuan, Z. Free fatty acids esterification for biodiesel production using self-synthesized macroporous cation exchange resin as solid acid catalyst. Fuel 2015, 154, 1–8. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Kaewchada, A.; Ngamcharussrivichai, C.; Jaree, A. Biodiesel production via Interesterification of palm oil and ethyl acetate using ion-exchange resin in a packed-bed reactor. BioEnergy Res. 2020, 13, 542–551. [Google Scholar] [CrossRef]
- Kurhade, A.; Dalai, A.K. Physiochemical characterization and support interaction of alumina—Supported heteropolyacid catalyst for biodiesel production. Asia-Pac. J. Chem. Eng. 2018, 13, e2249. [Google Scholar] [CrossRef]
- Kaur, N.; Ali, A. Preparation and application of Ce/ZrO2−TiO2/SO42—As solid catalyst for the esterification of fatty acids. Renew. Energy 2015, 81, 421–431. [Google Scholar] [CrossRef]
- Andrijanto, E.; Dawson, E.; Brown, D. Hypercrosslinked polystyrene sulphonic acid catalysts for the esterification of free fatty acids in biodiesel synthesis. Appl. Catal. B Environ. 2012, 115, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Khan, Y.; Badruddin, I.A.; Ankalgi, R.; Badarudin, A.; Hungund, B.; Ankalgi, F.R. Biodiesel production by direct transesterification process via sequential use of Acid–Base catalysis. Arab. J. Sci. Eng. 2018, 43, 5929–5936. [Google Scholar] [CrossRef]
- Ullah, F.; Dong, L.; Bano, A.; Peng, Q.; Huang, J. Current advances in catalysis toward sustainable biodiesel production. J. Energy Inst. 2016, 89, 282–292. [Google Scholar] [CrossRef]
- Jitputti, J.; Kitiyanan, B.; Rangsunvigit, P.; Bunyakiat, K.; Attanatho, L.; Jenvanitpanjakul, P. Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem. Eng. J. 2006, 116, 61–66. [Google Scholar] [CrossRef]
- Macario, A.; Giordano, G.; Onida, B.; Cocina, D.; Tagarelli, A.; Giuffrè, A.M. Biodiesel production process by homogeneous/heterogeneous catalytic system using an acid—Base catalyst. Appl. Catal. A Gen. 2010, 378, 160–168. [Google Scholar] [CrossRef]
- Du, L.; Ding, S.; Li, Z.; Lv, E.; Lu, J.; Ding, J. Transesterification of castor oil to biodiesel using NaY zeolite-supported La2O3 catalysts. Energy Convers. Manag. 2018, 173, 728–734. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, P.; Ansari, F.A.; Singh, B.; Bux, F. Biodiesel synthesis from microalgal lipids using tungstated zirconia as a heterogeneous acid catalyst and its comparison with homogeneous acid and enzyme catalysts. Fuel 2017, 187, 180–188. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mahmoud, H.R.; El-Molla, S.A. Influence of support on physicochemical properties of ZrO2 based solid acid heterogeneous catalysts for biodiesel production. Catal. Commun. 2019, 122, 10–15. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R.; Soumiya, S.; Mohanapriya, N.; Nivetha, S.R. Recent Advances in Heterogeneous Catalysts for Biodiesel Production. J. Energy Environ. Sustain. 2017, 4, 1–5. [Google Scholar] [CrossRef]
- Ramos, M.; Dias, A.P.S.; Puna, J.F.; Gomes, J.; Bordado, J.C. Biodiesel production processes and sustainable raw materials. Energies 2019, 12, 4408. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, R.F.; Rashid, U.; Hazmi, B.; Ibrahim, M.L.; Tsubota, T.; Alharthi, F.A. Potential heterogeneous nano-catalyst via integrating hydrothermal carbonization for biodiesel production using waste cooking oil. Chemosphere 2022, 286, 131913. [Google Scholar] [CrossRef]
- Singh, S.B.; Tandon, P.K. Catalysis: A brief review on nano-catalyst. J. Energy Chem. Eng. 2014, 2, 106–115. [Google Scholar]
- Zhu, Z.; Liu, Y.; Cong, W.; Zhao, X.; Janaun, J.; Wei, T.; Fang, Z. Soybean biodiesel production using synergistic CaO/Ag nano catalyst: Process optimization, kinetic study, and economic evaluation. Ind. Crops Prod. 2021, 166, 113479. [Google Scholar] [CrossRef]
- Hadadian, Y.; Ramos, A.P.; Pavan, T.Z. Role of zinc substitution in magnetic hyperthermia properties of magnetite nanoparticles: Interplay between intrinsic properties and dipolar interactions. Sci. Rep. 2019, 9, 18048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abusweireh, R.S.; Rajamohan, N.; Vasseghian, Y. Enhanced production of biodiesel using nanomaterials: A detailed review on the mechanism and influencing factors. Fuel 2022, 319, 123862. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Rajput, N. Methods of preparation of nanoparticles-a review. Int. J. Adv. Eng. Technol. 2015, 7, 1806. [Google Scholar]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Co-precipitation, impregnation and so-gel preparation of Ni catalysts for pyrolysis-catalytic steam reforming of waste plastics. Appl. Catal. B Environ. 2018, 239, 565–577. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Haghighi, M.; Saghatoleslami, N.; Tabasizadeh, M. Influence of Fuel to Oxidizer Ratio on Microwave-Assisted Combustion Preparation of Nanostructured KOH/Ca12Al14O33 Catalyst Used in Efficient Biodiesel Production. Front. Energy Res. 2020, 8, 106. [Google Scholar] [CrossRef]
- Vahid, B.R.; Haghighi, M. Urea-nitrate combustion synthesis of MgO/MgAl2O4 nanocatalyst used in biodiesel production from sunflower oil: Influence of fuel ratio on catalytic properties and performance. Energy Convers. Manag. 2016, 126, 362–372. [Google Scholar] [CrossRef]
- Ashok, A.; Kennedy, L.J.; Vijaya, J.J.; Aruldoss, U. Optimization of biodiesel production from waste cooking oil by magnesium oxide nanocatalyst synthesized using coprecipitation method. Clean Technol. Environ. Policy 2018, 20, 1219–1231. [Google Scholar] [CrossRef]
- Rasouli, H.; Esmaeili, H. Characterization of MgO nanocatalyst to produce biodiesel from goat fat using transesterification process. 3 Biotech 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Yeganeh, G.; Esmaeilzadeh, F. Optimization of biodiesel production from Moringa oleifera seeds oil in the presence of nano-MgO using Taguchi method. Int. Nano Lett. 2019, 9, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, S.; Haghighi, M. Sono-dispersed MgO over cerium-doped MCM-41 nanocatalyst for biodiesel production from acidic sunflower oil: Surface evolution by altering Si/Ce molar ratios. Waste Manag. 2019, 95, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Feyzi, M.; Hosseini, N.; Yaghobi, N.; Ezzati, R. Preparation, characterization, kinetic and thermodynamic studies of MgO-La2O3 nanocatalysts for biodiesel production from sunflower oil. Chem. Phys. Lett. 2017, 677, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Rafati, A.; Tahvildari, K.; Nozari, M. Production of biodiesel by electrolysis method from waste cooking oil using heterogeneous MgO-NaOH nano catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1062–1074. [Google Scholar] [CrossRef]
- Niju, S.; Raj, F.R.; Anushya, C.; Balajii, M. Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil. Green Process. Synth. 2019, 8, 756–775. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Fan, M.; Jiang, P. Biodiesel production from soybean oil catalyzed by magnetic nanoparticle MgFe2O4@ CaO. Fuel 2016, 164, 314–321. [Google Scholar] [CrossRef]
- ANR, R.; Saleh, A.A.; Islam, M.S.; Hamdan, S.; Maleque, M.A. Biodiesel production from crude Jatropha oil using a highly active heterogeneous nanocatalyst by optimizing transesterification reaction parameters. Energy Fuels 2016, 30, 334–343. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Farhadi, K.; Mansourpanah, Y.; Nikbakht, A.M.; Molaei, R.; Forough, M. Application of CaO-based/Au nanoparticles as heterogeneous nanocatalysts in biodiesel production. Fuel 2016, 164, 119–127. [Google Scholar] [CrossRef]
- Bharti, P.; Singh, B.; Dey, R. Process optimization of biodiesel production catalyzed by CaO nanocatalyst using response surface methodology. J. Nanostructure Chem. 2019, 9, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Mihankhah, T.; Delnavaz, M.; Khaligh, N.G. Application of TiO2 nanoparticles for eco-friendly biodiesel production from waste olive oil. Int. J. Green Energy 2018, 15, 69–75. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H.; Rehan, M. Biodiesel production from used cooking oil using a novel surface functionalised TiO2 nano-catalyst. Appl. Catal. B Environ. 2017, 207, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl. Catal. A Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Baskar, G.; Gurugulladevi, A.; Nishanthini, T.; Aiswarya, R.; Tamilarasan, K. Optimization and kinetics of biodiesel production from Mahua oil using manganese doped zinc oxide nanocatalyst. Renew. Energy 2017, 103, 641–646. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Borah, R.; Deka, D. Synthesis and application of Co doped ZnO as heterogeneous nanocatalyst for biodiesel production from non-edible oil. Renew. Energy 2019, 133, 512–519. [Google Scholar] [CrossRef]
- Thangaraj, B.; Piraman, S. Heteropoly acid coated ZnO nanocatalyst for Madhuca indica biodiesel synthesis. Biofuels 2016, 7, 13–20. [Google Scholar] [CrossRef]
- Baskar, G.; Selvakumari, I.A.E.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Mapossa, A.; Cornejo, D.; Costa, A. Magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 doped with Cu and performance evaluation in transesterification reaction for biodiesel production. Fuel 2017, 191, 463–471. [Google Scholar] [CrossRef]
- Nagaraju, G.; Prashanth, S.; Shastri, M.; Yathish, K.; Anupama, C.; Rangappa, D. Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Mater. Res. Bull. 2017, 94, 54–63. [Google Scholar] [CrossRef]
- Salimi, Z.; Hosseini, S.A. Study and optimization of conditions of biodiesel production from edible oils using ZnO/BiFeO3 nano magnetic catalyst. Fuel 2019, 239, 1204–1212. [Google Scholar] [CrossRef]
- Mahdavi, M.; Abedini, E.; hosein Darabi, A. Correction: Biodiesel synthesis from oleic acid by nano-catalyst (ZrO2/Al2O3) under high voltage conditions. RSC Adv. 2015, 5, 58566. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, K.; Tyagi, B.; Shukla, R.S.; Bajaj, H.C. Solvent free synthesis of methyl palmitate over sulfated zirconia solid acid catalyst. Fuel 2016, 165, 298–305. [Google Scholar] [CrossRef]
- Takase, M.; Zhang, M.; Feng, W.; Chen, Y.; Zhao, T.; Cobbina, S.J.; Yang, L.; Wu, X. Application of zirconia modified with KOH as heterogeneous solid base catalyst to new non-edible oil for biodiesel. Energy Convers. Manag. 2014, 80, 117–125. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Nizami, A.-S.; Sultana, S.; Zafar, M.; Rehan, M.; Srinivasan, G.R.; Ali, A.M. Biodiesel production from novel non-edible caper (Capparis spinosa L.) seeds oil employing Cu–Ni doped ZrO2 catalyst. Renew. Sustain. Energy Rev. 2021, 138, 110558. [Google Scholar] [CrossRef]
- Nawaz, S.; Ahmad, M.; Asif, S.; Klemeš, J.J.; Mubashir, M.; Munir, M.; Zafar, M.; Bokhari, A.; Mukhtar, A.; Saqib, S. Phyllosilicate derived catalysts for efficient conversion of lignocellulosic derived biomass to biodiesel: A review. Bioresour. Technol. 2021, 343, 126068. [Google Scholar] [CrossRef]
- Munir, M.; Saeed, M.; Ahmad, M.; Waseem, A.; Sultana, S.; Zafar, M.; Srinivasan, G.R. Optimization of novel Lepidium perfoliatum Linn. Biodiesel using zirconium-modified montmorillonite clay catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 1–16. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Rehan, M.; Nizami, A.-S.; Zafar, M.; Arshad, M.; Sultana, S. Sustainable production of bioenergy from novel non-edible seed oil (Prunus cerasoides) using bimetallic impregnated montmorillonite clay catalyst. Renew. Sustain. Energy Rev. 2019, 109, 321–332. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Mubashir, M.; Asif, S.; Waseem, A.; Mukhtar, A.; Saqib, S.; Munawaroh, H.S.H.; Lam, M.K.; Khoo, K.S. A practical approach for synthesis of biodiesel via non-edible seeds oils using trimetallic based montmorillonite nano-catalyst. Bioresour. Technol. 2021, 328, 124859. [Google Scholar] [CrossRef]
- Jia, L.; Li, Y.; Chen, J.; Guo, X.; Lou, S.; Duan, H. Montmorillonite-supported KF/CaO: A new solid base catalyst for biodiesel production. Res. Chem. Intermed. 2016, 42, 1791–1807. [Google Scholar] [CrossRef]
- Olutoye, M.; Wong, S.; Chin, L.; Amani, H.; Asif, M.; Hameed, B. Synthesis of fatty acid methyl esters via the transesterification of waste cooking oil by methanol with a barium-modified montmorillonite K10 catalyst. Renew. Energy 2016, 86, 392–398. [Google Scholar] [CrossRef]
- Negm, N.A.; Sayed, G.H.; Yehia, F.Z.; Habib, O.I.; Mohamed, E.A. Biodiesel production from one-step heterogeneous catalyzed process of Castor oil and Jatropha oil using novel sulphonated phenyl silane montmorillonite catalyst. J. Mol. Liq. 2017, 234, 157–163. [Google Scholar] [CrossRef]
- Ayoub, M.; Bhat, A.H.; Ullah, S.; Ahmad, M.; Uemura, Y. Optimization of biodiesel production over alkaline modified clay catalyst. J. Jpn. Inst. Energy 2017, 96, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Yahya, S.; Wahab, S.K.M.; Harun, F.W. Optimization of biodiesel production from waste cooking oil using Fe-Montmorillonite K10 by response surface methodology. Renew. Energy 2020, 157, 164–172. [Google Scholar] [CrossRef]
- Aslan, S.; Aka, N.; Karaoglu, M.H. NaOH impregnated sepiolite based heterogeneous catalyst and its utilization for the production of biodiesel from canola oil. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 290–297. [Google Scholar] [CrossRef]
- Junior, E.G.S.; Barcelos, L.F.T.; Perez, V.H.; Justo, O.R.; Ramirez, L.C.; de Moraes Rêgo Filho, L.; de Castro, M.P.P. Biodiesel production from non-edible forage turnip oil by extruded catalyst. Ind. Crops Prod. 2019, 139, 111503. [Google Scholar]
- Silveira Junior, E.G.; Justo, O.R.; Perez, V.H.; Reyero, I.; Serrano-Lotina, A.; Campos Ramirez, L.; dos Santos Dias, D.F. Extruded catalysts with magnetic properties for biodiesel production. Adv. Mater. Sci. Eng. 2018, 2018, 3980967. [Google Scholar] [CrossRef] [Green Version]
- Shan, R.; Shi, J.; Yan, B.; Chen, G.; Yao, J.; Liu, C. Transesterification of palm oil to fatty acids methyl ester using K2CO3/palygorskite catalyst. Energy Convers. Manag. 2016, 116, 142–149. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y. Preparation of a palygorskite supported KF/CaO catalyst and its application for biodiesel production via transesterification. RSC Adv. 2018, 8, 16013–16018. [Google Scholar] [CrossRef] [Green Version]
- Doyle, A.M.; Albayati, T.M.; Abbas, A.S.; Alismaeel, Z.T. Biodiesel production by esterification of oleic acid over zeolite Y prepared from kaolin. Renew. Energy 2016, 97, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Đặng, T.-H.; Chen, B.-H.; Lee, D.-J. Optimization of biodiesel production from transesterification of triolein using zeolite LTA catalysts synthesized from kaolin clay. J. Taiwan Inst. Chem. Eng. 2017, 79, 14–22. [Google Scholar] [CrossRef]
- Onukwuli, O.D.; Ude, C.N. Kinetics of African pear seed oil (APO) methanolysis catalyzed by phosphoric acid-activated kaolin clay. Appl. Petrochem. Res. 2018, 8, 299–313. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Sayed, M.A. K+ trapped kaolinite (Kaol/K+) as low cost and eco-friendly basic heterogeneous catalyst in the transesterification of commercial waste cooking oil into biodiesel. Energy Convers. Manag. 2018, 177, 468–476. [Google Scholar] [CrossRef]
- Liu, J.; Yun, Z.; Gui, X. Ce/kaolin clay as an active catalyst for fatty acid methyl esters production from cottonseed oil in a new integrated apparatus. Braz. J. Chem. Eng. 2018, 35, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Fatimah, I.; Rubiyanto, D. Effect of KF Modification to Kaolinite Catalytic Activity in Microwave-Assisted Biodiesel Conversion. Egypt. J. Chem. 2018, 61, 213–223. [Google Scholar] [CrossRef]
- Setiadji, S.; Sundari, C.; Munir, M.; Fitriyah, S. Synthesis of solid catalyst from egg shell waste and clay for biodiesel production. J. Phys. Conf. Ser. 2018, 103, 012199. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Yusup, S.; Quitain, A.T.; Alnarabiji, M.S.; Kamil, R.N.M.; Kida, T. Synthesis of novel graphene oxide/bentonite bi-functional heterogeneous catalyst for one-pot esterification and transesterification reactions. Energy Convers. Manag. 2018, 171, 1801–1812. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Ibrahim, S.M.; Yakout, S.M.; El-Zaidy, M.E.; Abdeltawab, A.A. Synthesis of Na+ trapped bentonite/zeolite-P composite as a novel catalyst for effective production of biodiesel from palm oil; Effect of ultrasonic irradiation and mechanism. Energy Convers. Manag. 2019, 196, 739–750. [Google Scholar] [CrossRef]
- de Oliveira, A.d.N.; Barbosa de Lima, M.A.; de Oliveira Pires, L.H.; Rosas da Silva, M.; Souza da Luz, P.T.; Angélica, R.S.; da Rocha Filho, G.N.; F da Costa, C.E.; Luque, R.; Santos do Nascimento, L.A. Bentonites modified with phosphomolybdic heteropolyacid (HPMo) for biowaste to biofuel production. Materials 2019, 12, 1431. [Google Scholar] [CrossRef] [Green Version]
- Naik, B.D.; Meivelu, U. Experimental studies on sodium methoxide supported bentonite catalyst for biodiesel preparation from waste sunflower oil. Environ. Prog. Sustain. Energy 2020, 39, e13390. [Google Scholar] [CrossRef]
- Changmai, B.; Vanlalveni, C.; Ingle, A.P.; Bhagat, R.; Rokhum, L. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef] [PubMed]
- Gohain, M.; Devi, A.; Deka, D. Musa balbisiana Colla peel as highly effective renewable heterogeneous base catalyst for biodiesel production. Ind. Crops Prod. 2017, 109, 8–18. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Paes, O.A.; Maia, P.J.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S., Jr.; de Freitas, F.A. New heterogeneous catalyst for biodiesel production from waste tucumã peels (Astrocaryum aculeatum Meyer): Parameters optimization study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Barros, S.d.S.; Junior, W.A.P.; Sá, I.S.; Takeno, M.L.; Nobre, F.X.; Pinheiro, W.; Manzato, L.; Iglauer, S.; de Freitas, F.A. Pineapple (Ananás comosus) leaves ash as a solid base catalyst for biodiesel synthesis. Bioresour. Technol. 2020, 312, 123569. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Peng, L.; Xie, Q.; Xing, K.; Lu, M.; Ji, J. Sulfonated Sargassum horneri carbon as solid acid catalyst to produce biodiesel via esterification. Bioresour. Technol. 2021, 324, 124614. [Google Scholar] [CrossRef] [PubMed]
- Malani, R.S.; Sardar, H.; Malviya, Y.; Goyal, A.; Moholkar, V.S. Ultrasound-intensified biodiesel production from mixed nonedible oil feedstock using heterogeneous acid catalyst supported on rubber de-oiled cake. Ind. Eng. Chem. Res. 2018, 57, 14926–14938. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Naeem, A. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Nisar, J.; Razaq, R.; Farooq, M.; Iqbal, M.; Khan, R.A.; Sayed, M.; Shah, A.; ur Rahman, I. Enhanced biodiesel production from Jatropha oil using calcined waste animal bones as catalyst. Renew. Energy 2017, 101, 111–119. [Google Scholar] [CrossRef]
- Hebbar, H.H.; Math, M.; Yatish, K. Optimization and kinetic study of CaO nano-particles catalyzed biodiesel production from Bombax ceiba oil. Energy 2018, 143, 25–34. [Google Scholar] [CrossRef]
- Ali, C.H.; Asif, A.H.; Iqbal, T.; Qureshi, A.S.; Kazmi, M.A.; Yasin, S.; Danish, M.; Mu, B.-Z. Improved transesterification of waste cooking oil into biodiesel using calcined goat bone as a catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1076–1083. [Google Scholar] [CrossRef]
- Kara, K.; Ouanji, F.; El Mahi, M.; Kacimi, M.; Mahfoud, Z. Biodiesel synthesis from vegetable oil using eggshell waste as a heterogeneous catalyst. Biofuels 2019, 12, 1083–1089. [Google Scholar] [CrossRef]
- Guldhe, A.; Moura, C.V.; Singh, P.; Rawat, I.; Moura, E.M.; Sharma, Y.; Bux, F. Conversion of microalgal lipids to biodiesel using chromium-aluminum mixed oxide as a heterogeneous solid acid catalyst. Renew. Energy 2017, 105, 175–182. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, L. Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv. 2018, 8, 20131–20142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basumatary, S.; Nath, B.; Das, B.; Kalita, P.; Basumatary, B. Utilization of renewable and sustainable basic heterogeneous catalyst from Heteropanax fragrans (Kesseru) for effective synthesis of biodiesel from Jatropha curcas oil. Fuel 2021, 286, 119357. [Google Scholar] [CrossRef]
- Sahu, O. Characterisation and utilization of heterogeneous catalyst from waste rice-straw for biodiesel conversion. Fuel 2021, 287, 119543. [Google Scholar] [CrossRef]
- Aleman-Ramirez, J.; Moreira, J.; Torres-Arellano, S.; Longoria, A.; Okoye, P.U.; Sebastian, P. Preparation of a heterogeneous catalyst from moringa leaves as a sustainable precursor for biodiesel production. Fuel 2021, 284, 118983. [Google Scholar] [CrossRef]
- Ghanei, R.; Dermani, R.K.; Salehi, Y.; Mohammadi, M. Waste animal bone as support for CaO impregnation in catalytic biodiesel production from vegetable oil. Waste Biomass Valorization 2016, 7, 527–532. [Google Scholar] [CrossRef]
- Nath, B.; Kalita, P.; Das, B.; Basumatary, S. Highly efficient renewable heterogeneous base catalyst derived from waste Sesamum indicum plant for synthesis of biodiesel. Renew. Energy 2020, 151, 295–310. [Google Scholar] [CrossRef]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J. Clean. Prod. 2019, 239, 118112. [Google Scholar] [CrossRef]
- Sai, B.A.; Subramaniapillai, N.; Mohamed, M.S.B.K.; Narayanan, A. Optimization of continuous biodiesel production from rubber seed oil (RSO) using calcined eggshells as heterogeneous catalyst. J. Environ. Chem. Eng. 2020, 8, 103603. [Google Scholar]
- Vargas, M.; Niehus, X.; Casas-Godoy, L.; Sandoval, G. Lipases as biocatalyst for biodiesel production. Lipases Phospholipases 2018, 1835, 377–390. [Google Scholar]
- Santos, S.; Puna, J.; Gomes, J. A review on bio-based catalysts (immobilized enzymes) used for biodiesel production. Energies 2020, 13, 3013. [Google Scholar] [CrossRef]
- Puri, M.; Barrow, C.J.; Verma, M.L. Enzyme immobilization on nanomaterials for biofuel production. Trends Biotechnol. 2013, 31, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Bilal, M.; Li, X.; Ju, F.; Teng, Y.; Iqbal, H.M. Nanomaterial-immobilized lipases for sustainable recovery of biodiesel—A review. Fuel 2022, 316, 123429. [Google Scholar] [CrossRef]
- Marín-Suárez, M.; Méndez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Aguieiras, E.C.; Cavalcanti-Oliveira, E.D.; Cammarota, M.C.; Freire, D.M. Solid-state fermentation for the production of lipases for environmental and biodiesel applications. Curr. Dev. Biotechnol. Bioeng. 2018, 123–168. [Google Scholar] [CrossRef]
- Zheng, J.; Wei, W.; Wang, S.; Li, X.; Zhang, Y.; Wang, Z. Immobilization of Lipozyme TL 100L for methyl esterification of soybean oil deodorizer distillate. 3 Biotech 2020, 10, 51. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.-M.; Pugazhendhi, A.; Awasthi, M.K.; Kumar, D.; Kumar, G.; Yoon, J.-J.; Yang, Y.-H. An overview on advancements in biobased transesterification methods for biodiesel production: Oil resources, extraction, biocatalysts, and process intensification technologies. Fuel 2021, 285, 119117. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of biodiesel production using waste cooking oil and applying single and mixed immobilised lipases on polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Chang, J.S. Biodiesel production using immobilized lipase: Feasibility and challenges. Biofuels Bioprod. Biorefining 2016, 10, 896–916. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Torrestiana-Sánchez, B.; Dal Magro, L.; Virgen-Ortíz, J.J.; Suárez-Ruíz, F.J.; Rodrigues, R.C.; Fernandez-Lafuente, R. Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew. Energy 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Rodrigues, J.; Canet, A.; Rivera, I.; Osório, N.; Sandoval, G.; Valero, F.; Ferreira-Dias, S. Biodiesel production from crude Jatropha oil catalyzed by non-commercial immobilized heterologous Rhizopus oryzae and Carica papaya lipases. Bioresour. Technol. 2016, 213, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.; Zhang, Y.; Wei, T.; Wu, J.; Li, Q.; Liu, Y. Immobilization of candida cylindracea lipase by covalent attachment on glu-modified bentonite. Appl. Biochem. Biotechnol. 2019, 187, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Safaryan, S.M.; Yakovlev, A.V.; Pidko, E.A.; Vinogradov, A.V.; Vinogradov, V.V. Reversible sol–gel–sol medium for enzymatic optical biosensors. J. Mater. Chem. B 2017, 5, 85–91. [Google Scholar] [CrossRef]
- Kumari, V.; Shah, S.; Gupta, M.N. Preparation of biodiesel by lipase-catalyzed transesterification of high free fatty acid containing oil from Madhuca indica. Energy Fuels 2007, 21, 368–372. [Google Scholar] [CrossRef]
- Sánchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Burkholderia cepacia lipase: A versatile catalyst in synthesis reactions. Biotechnol. Bioeng. 2018, 115, 6–24. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).