A Comparative Photographic Review on Higher Plants and Macro-Fungi: A Soil Restoration for Sustainable Production of Food and Energy
Abstract
:1. Introduction
2. Methodology of the Review
3. Plant and Human Nutrition for Sustainability
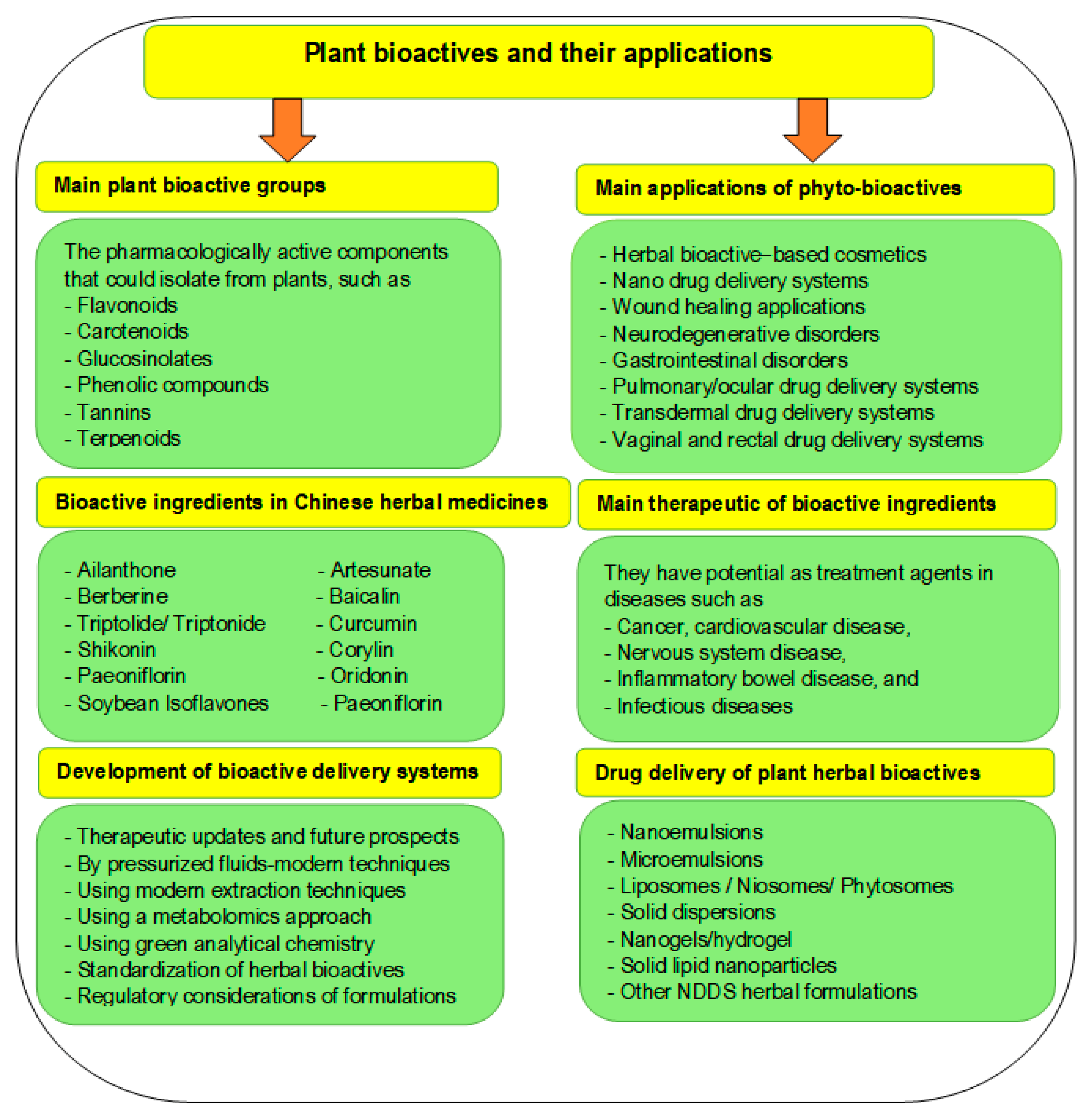
4. Phytomedicine and Human Health
5. Higher Plants and Mushrooms: A General Comparison
6. Unconventional Foods of Plants and Mushrooms
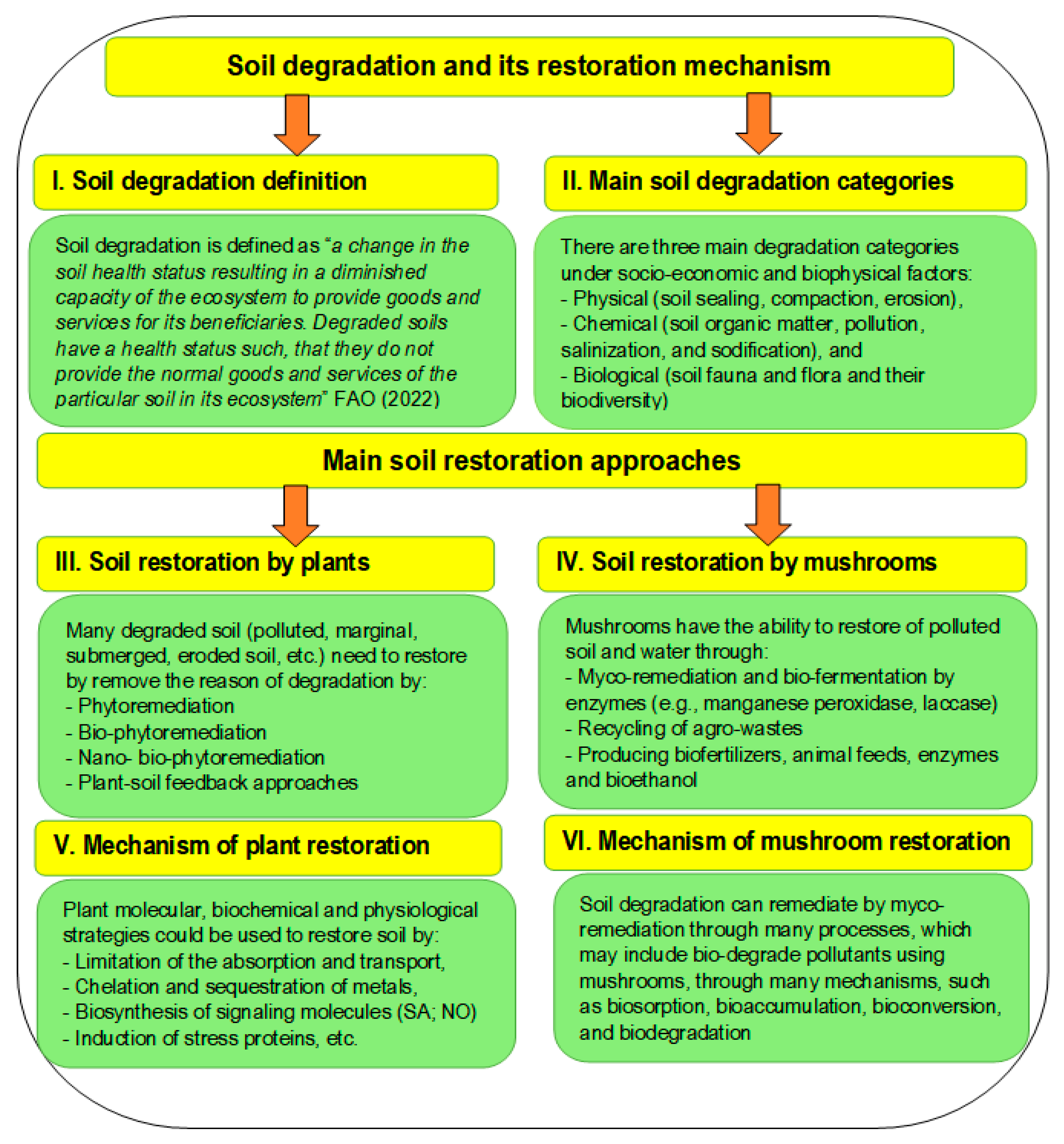
7. Soil Restoration by Plants and Mushrooms
7.1. Integrated Production of Food and Energy
7.2. Integrating Food Crops and Mushrooms
- (1)
- Improving mushroom (Pleurotus ostreatus) production cultivated on staple crop residues including banana, cassava, common bean, and maize [188],
- (2)
- Utilizing fruit waste substrates (peels of avocado, orange, and pineapple) in mushroom (Pleurotus eryngii and P. ostreatus) production [189],
- (3)
- Using hulls of faba bean as substrate for mushroom (Pleurotus ostreatus) cultivation and for animal feed production [190],
- (4)
- Producing spawns from banana leaf-midribs for cultivation of oyster (Pleurotus ostreatus) mushrooms [191],
- (5)
- Integrating mushroom cultivation and production in a circular agro-system into food chains [192], and
- (6)
- Using spent mushroom compost of mushroom (Agaricus subrufescens and A. bisporus) for cultivation of lettuce, tomato, and/or cucumber in a sustainable system in the same container under greenhouse conditions [193].
8. General Discussion
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant Nutrition under Climate Change and Soil Carbon Sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant Nutrition for Human Nutrition: Hints from Rice Research and Future Perspectives. Mol. Plant 2020, 13, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Kadirvel, V.; Subramaniyan, T. Seaweeds, an Aquatic Plant-Based Protein for Sustainable Nutrition—A Review. Future Foods 2022, 5, 100142. [Google Scholar] [CrossRef]
- Larson-Meyer, E.D.; Ruscigno, M. Plant-Based Sports Nutrition: Expert Fueling Strategies for Training, Recovery, and Performance, 1st ed.; Human Kinetics: Champaign, IL, USA, 2020. [Google Scholar]
- Agidew, M.G. Phytochemical Analysis of Some Selected Traditional Medicinal Plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar] [CrossRef]
- Alam, T.; Khan, S.A.; Dhanalekshmi, U.M. Traditional Uses, Phytochemistry, and Pharmacological Profile of Salvadora Persica Linn. In Edible Plants in Health and Diseases; Masoodi, M.H., Rehman, M.U., Eds.; Springer: Singapore, 2022; pp. 95–134. ISBN 9789811649585. [Google Scholar]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Kaur, M.; Shivay, Y.S.; Nisar, S.; Gaber, A.; Brestic, M.; Barek, V.; et al. Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security. Molecules 2022, 27, 1340. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Elbasiouny, H.; Elbehiry, F.; Elsakhawy, T.; Omara, A.E.-D.; Amer, M.; Bayoumi, Y.; Shalaby, T.A.; Eid, Y.; et al. Nano-Biofortification of Different Crops to Immune against COVID-19: A Review. Ecotoxicol. Environ. Saf. 2021, 222, 112500. [Google Scholar] [CrossRef]
- El-Ramady, H.; Brevik, E.C.; Elbasiouny, H.; Elbehiry, F.; El-Henawy, A.; Faizy, S.E.-D.; Elsakhawy, T.; Omara, A.E.-D.; Amer, M.; Eid, Y. Soils, Biofortification, and Human Health Under COVID-19: Challenges and Opportunities. Front. Soil Sci. 2021, 1, 732971. [Google Scholar] [CrossRef]
- El-Ramady, H.; Faizy, S.E.-D.; Abdalla, N.; Taha, H.; Domokos-Szabolcsy, É.; Fari, M.; Elsakhawy, T.; Omara, A.E.-D.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and Nano-Selenium Biofortification for Human Health: Opportunities and Challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]
- Kumar, S.; Dikshit, H.K.; Mishra, G.P.; Singh, A. (Eds.) Biofortification of Staple Crops; Springer: Singapore, 2022; ISBN 9789811632792. [Google Scholar]
- Kumar, S.; Dikshit, H.K.; Mishra, G.P.; Singh, A.; Aski, M.; Virk, P.S. Biofortification of Staple Crops: Present Status and Future Strategies. In Biofortification of Staple Crops; Kumar, S., Dikshit, H.K., Mishra, G.P., Singh, A., Eds.; Springer: Singapore, 2022; pp. 1–30. ISBN 9789811632792. [Google Scholar]
- González-Ball, R.; Bermúdez-Rojas, T.; Romero-Vargas, M.; Ceuterick, M. Medicinal Plants Cultivated in Urban Home Gardens in Heredia, Costa Rica. J. Ethnobiol. Ethnomed. 2022, 18, 7. [Google Scholar] [CrossRef]
- Masoodi, M.H.; Rehman, M.U. (Eds.) Edible Plants in Health and Diseases: Volume 1: Cultural, Practical and Economic Value; Springer Nature: Singapore, 2022; ISBN 9789811648793. [Google Scholar]
- Masoodi, M.H.; Rehman, M.U. (Eds.) Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties; Springer: Singapore, 2022; ISBN 9789811649585. [Google Scholar]
- Iqbal, Y.; Ponnampalam, E.N.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Extraction and Characterization of Polyphenols from Non-Conventional Edible Plants and Their Antioxidant Activities. Food Res. Int. 2022, 157, 111205. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, F.; Zhang, J.; Wang, W.; Li, L.; Yan, J. Modulatory Effects of Polysaccharides from Plants, Marine Algae and Edible Mushrooms on Gut Microbiota and Related Health Benefits: A Review. Int. J. Biol. Macromol. 2022, 204, 169–192. [Google Scholar] [CrossRef]
- Nath, H.; Samtiya, M.; Dhewa, T. Beneficial Attributes and Adverse Effects of Major Plant-Based Foods Anti-Nutrients on Health: A Review. Hum. Nutr. Metab. 2022, 28, 200147. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.S.; et al. Plant-Based Proteins and Their Multifaceted Industrial Applications. LWT 2022, 154, 112620. [Google Scholar] [CrossRef]
- Lu, Q.; Ruan, H.-N.; Sun, X.-Q.; Luo, J.-Y.; Yang, M.-H. Contamination Status and Health Risk Assessment of 31 Mycotoxins in Six Edible and Medicinal Plants Using a Novel Green Defatting and Depigmenting Pretreatment Coupled with LC-MS/MS. LWT 2022, 161, 113401. [Google Scholar] [CrossRef]
- Xia, Y.; Yan, B. Energy-Food Nexus Scarcity Risk and the Synergic Impact of Climate Policy: A Global Production Network Perspective. Environ. Sci. Policy 2022, 135, 26–35. [Google Scholar] [CrossRef]
- Terneus Páez, C.F.; Viteri Salazar, O. Analysis of Biofuel Production in Ecuador from the Perspective of the Water-Food-Energy Nexus. Energy Policy 2021, 157, 112496. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, S. Food-Energy-Emission Nexus of Rice Production in China. Crop Environ. 2022, 1, 59–67. [Google Scholar] [CrossRef]
- Luqman, M.; Al-Ansari, T. Energy, Water and Food Security through a Waste-Driven Polygeneration System for Sustainable Dairy Production. Int. J. Hydrog. Energy 2022, 47, 3185–3210. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of Soil Polluted with Petroleum Hydrocarbons and Its Reuse for Agriculture: Recent Progress, Challenges, and Perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef]
- Chen, Y.; Svenning, J.-C.; Wang, X.; Cao, R.; Yuan, Z.; Ye, Y. Drivers of Macrofungi Community Structure Differ between Soil and Rotten-Wood Substrates in a Temperate Mountain Forest in China. Front. Microbiol. 2018, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Fernández Ruiz, A.; Rodríguez de la Cruz, D.; Vicente Villardón, J.L.; Sánchez Durán, S.; García Jiménez, P.; Sánchez Sánchez, J. Considerations on Field Methodology for Macrofungi Studies in Fragmented Forests of Mediterranean Agricultural Landscapes. Agronomy 2022, 12, 528. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Berry, E.M.; Blaak, E.E.; Burlingame, B.; le Coutre, J.; van Eden, W.; El-Sohemy, A.; German, J.B.; Knorr, D.; Lacroix, C.; et al. Goals in Nutrition Science 2020–2025. Front. Nutr. 2021, 7, 606378. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.P.; Lovegrove, J.A. Nutritional Status of Micronutrients as a Possible and Modifiable Risk Factor for COVID-19: A UK Perspective. Br. J. Nutr. 2021, 125, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group, LLC: Abingdon, UK, 2011. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group, LLC: Abingdon, UK, 2015. [Google Scholar]
- Garcia, S.N.; Osburn, B.I.; Jay-Russell, M.T. One Health for Food Safety, Food Security, and Sustainable Food Production. Front. Sustain. Food Syst. 2020, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Cassani, L.; Gomez-Zavaglia, A. Sustainable Food Systems in Fruits and Vegetables Food Supply Chains. Front. Nutr. 2022, 9, 829061. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Ray, A.; Ray, R.; Sreevidya, E.A. How Many Wild Edible Plants Do We Eat—Their Diversity, Use, and Implications for Sustainable Food System: An Exploratory Analysis in India. Front. Sustain. Food Syst. 2020, 4, 56. [Google Scholar] [CrossRef]
- Bruulsema, T.; Cakmak, I.; Dobermann, A.; Gerard, B.; Majumdar, K.; McLaughlin, M.; Reidsma, P.; Vanlauwe, B.; Wollenberg, E.K.; Zhang, F.; et al. A New Paradigm for Plant Nutrition. 2020. Available online: https://www.apni.net/downloads/sprpnIss01.pdf (accessed on 25 April 2022).
- Dobermann, A.; Bruulsema, T.; Cakmak, I.; Gerard, B.; Majumdar, K.; McLaughlin, M.; Reidsma, P.; Vanlauwe, B.; Wollenberg, L.; Zhang, F.; et al. Responsible Plant Nutrition: A New Paradigm to Support Food System Transformation. Glob. Food Secur. 2022, 33, 100636. [Google Scholar] [CrossRef]
- Frazier, M.; Matthew, R. No Meat Athlete: A Plant-Based Nutrition and Training Guide for Athletes at Any Level; Fair Winds Press: Beverly, MA, USA, 2018. [Google Scholar]
- Goyal, M.; Ayeleso, A. Bioactive Compounds of Medicinal Plants: Properties and Potential for Human Health; Innovations in Plant Science for Better Health: From Soil to Fork Book Series; Apple Academic Press, Inc.: Palm Bay, FL, USA; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2019. [Google Scholar]
- Suleria, H.; Barrow, C. Bioactive Compounds from Plant Origin: Extraction, Applications, and Potential Health Benefits; Innovations in Plant Science for Better Health: From Soil to Fork Book Series; Apple Academic Press, Inc.: Palm Bay, FL, USA; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2020. [Google Scholar]
- Goyal, M.; Joy, P.; Suleria, H. Plant Secondary Metabolites for Human Health: Extraction of Bioactive Compounds; Innovations in Plant Science for Better Health: From Soil to Fork Book Series; Apple Academic Press, Inc.: Palm Bay, FL, USA; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2020. [Google Scholar]
- Goyal, M.; Chauhan, D. Assessment of Medicinal Plants for Human Health: Phytochemistry, Disease Management, and Novel Applications; Innovations in Plant Science for Better Health: From Soil to Fork Book Series; Apple Academic Press, Inc.: Palm Bay, FL, USA; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2022. [Google Scholar]
- Suleria, H.; Goyal, M.; Ul Ain, H. Bioactive Compounds from Multifarious Natural Foods for Human Health: Foods and Medicinal Plants; Innovations in Plant Science for Better Health: From Soil to Fork Book Series; Apple Academic Press, Inc.: Palm Bay, FL, USA; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2022. [Google Scholar]
- Goyal, M.; Birwal, P.; Chauhan, D. Herbs, Spices, and Medicinal Plants for Human Gastrointestinal Disorders: Health Benefits and Safety; Innovations in Plant Science for Better Health: From Soil to Fork Book Series; Apple Academic Press, Inc.: Palm Bay, FL, USA; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2022. [Google Scholar]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition Through Plant-Based Foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef]
- Deprá, M.C.; Dias, R.R.; Sartori, R.B.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. Nexus on Animal Proteins and the Climate Change: The Plant-Based Proteins Are Part of the Solution? Food Bioprod. Process. 2022, 133, 119–131. [Google Scholar] [CrossRef]
- Hadidi, M.; Jafarzadeh, S.; Forough, M.; Garavand, F.; Alizadeh, S.; Salehabadi, A.; Khaneghah, A.M.; Jafari, S.M. Plant Protein-Based Food Packaging Films; Recent Advances in Fabrication, Characterization, and Applications. Trends Food Sci. Technol. 2022, 120, 154–173. [Google Scholar] [CrossRef]
- Ramya, K.R.; Tripathi, K.; Pandey, A.; Barpete, S.; Gore, P.G.; Raina, A.P.; Khawar, K.M.; Swain, N.; Sarker, A. Rediscovering the Potential of Multifaceted Orphan Legume Grasspea- a Sustainable Resource With High Nutritional Values. Front. Nutr. 2022, 8, 826208. [Google Scholar] [CrossRef]
- Sagis, L.M.; Yang, J. Protein-Stabilized Interfaces in Multiphase Food: Comparing Structure-Function Relations of Plant-Based and Animal-Based Proteins. Curr. Opin. Food Sci. 2022, 43, 53–60. [Google Scholar] [CrossRef]
- Cao, Y.; Bolisetty, S.; Wolfisberg, G.; Adamcik, J.; Mezzenga, R. Amyloid Fibril-Directed Synthesis of Silica Core–Shell Nanofilaments, Gels, and Aerogels. Proc. Natl. Acad. Sci. USA 2019, 116, 4012–4017. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant Proteins as High-Quality Nutritional Source for Human Diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Zannini, E.; Arendt, E.K. Production of Pulse Protein Ingredients and Their Application in Plant-Based Milk Alternatives. Trends Food Sci. Technol. 2021, 110, 364–374. [Google Scholar] [CrossRef]
- Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Stelmasiak, A.; Wierzbicka, A.; Poltorak, A. Application of Atmospheric Pressure Cold Plasma Activated Plant Protein Preparations Solutions as an Alternative Curing Method for Pork Sausages. Meat Sci. 2022, 187, 108751. [Google Scholar] [CrossRef]
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A Systematic Review on Consumer Acceptance of Alternative Proteins: Pulses, Algae, Insects, Plant-Based Meat Alternatives, and Cultured Meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant Protein-Based Alternatives of Reconstructed Meat: Science, Technology, and Challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, X.; Yu, S.; Zhou, J.; Chen, J. Production of Meat Alternatives Using Live Cells, Cultures and Plant Proteins. Curr. Opin. Food Sci. 2022, 43, 43–52. [Google Scholar] [CrossRef]
- Bäuerle, L.; Kühn, S. Development of a Protein Supplemented Fruit Smoothie Using Pea Protein Isolate as a Plant-Based Protein Alternative. Future Foods 2022, 5, 100145. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.-C.; Mat Easa, A.; Gammoh, S.; Alu’datt, M.H. Recent Updates on Lentil and Quinoa Protein-Based Dairy Protein Alternatives: Nutrition, Technologies, and Challenges. Food Chem. 2022, 383, 132386. [Google Scholar] [CrossRef]
- Kurhekar, J.V. Ancient and Modern Practices in Phytomedicine. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 55–75. ISBN 978-0-12-820284-5. [Google Scholar]
- Nigam, M. Phytomedicine: Scope and Current Highlights. In Preparation of Phytopharmaceuticals for the Management of Disorders: The Development of Nutraceuticals and Traditional Medicine; Egbuna, C., Mishra, A.P., Goyal, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 39–54. [Google Scholar]
- Vater, C.; Bosch, L.; Mitter, A.; Göls, T.; Seiser, S.; Heiss, E.; Elbe-Bürger, A.; Wirth, M.; Valenta, C.; Klang, V. Lecithin-Based Nanoemulsions of Traditional Herbal Wound Healing Agents and Their Effect on Human Skin Cells. Eur. J. Pharm. Biopharm. 2022, 170, 1–9. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, A.P.; Kumar, S.; Negi, A.; Maurya, V.K. Herbal Wound Healing Agents. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 169–184. ISBN 978-0-12-820284-5. [Google Scholar]
- Rai, M.; Kosalec, I. (Eds.) Promising Antimicrobials from Natural Products; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-83503-3. [Google Scholar]
- Chavda, V.P.; Patel, A.B.; Vihol, D.; Vaghasiya, D.D.; Ahmed, K.M.S.B.; Trivedi, K.U.; Dave, D.J. Herbal Remedies, Nutraceuticals, and Dietary Supplements for COVID-19 Management: An Update. Clin. Complement. Med. Pharmacol. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Ugoeze, K.C.; Odeku, O.A. Herbal Bioactive–Based Cosmetics. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–226. ISBN 978-0-12-824385-5. [Google Scholar]
- Egbuna, C.; Mishra, A.P.; Goyal, M.R. Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-820284-5. [Google Scholar]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Maniecki, Ł.; Szczęsna, W.; Warszyński, P.; Wilk, K.A. Tailoring the Composition of Hydrogel Particles for the Controlled Delivery of Phytopharmaceuticals. Eur. Polym. J. 2021, 151, 110429. [Google Scholar] [CrossRef]
- Kumari, P.; Raina, K.; Thakur, S.; Sharma, R.; Cruz-Martins, N.; Kumar, P.; Barman, K.; Sharma, S.; Kumar, D.; Prajapati, P.K.; et al. Ethnobotany, Phytochemistry and Pharmacology of Palash (Butea Monosperma (Lam.) Taub.): A Systematic Review. Curr. Pharmacol. Rep. 2022, 8, 188–204. [Google Scholar] [CrossRef]
- Algın Yapar, E.; Durgun, M.E.; Esentürk, I.; Güngör, S.; Özsoy, Y. Herbal Bioactives for Ocular Drug Delivery Systems. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–61. ISBN 978-0-12-824385-5. [Google Scholar]
- Virmani, R.; Pathak, K. Herbal Bioactives for Pulmonary Drug Delivery Systems. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–92. ISBN 978-0-12-824385-5. [Google Scholar]
- Kaur, G.; Kaur, P.; Madaan, P.; Verma, R.; Chandel, P.; Salgotra, T.; Kaur, H.; Sindhu, R.K. Herbal Bioactives in Transdermal Drug Delivery System. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 93–110. ISBN 978-0-12-824385-5. [Google Scholar]
- Deshkar, S.S.; Mahore, J.G. Herbal Bioactive–Based Vaginal and Rectal Drug Delivery Systems. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 111–168. ISBN 978-0-12-824385-5. [Google Scholar]
- Maurya, A.P.; Chauhan, J.; Yadav, D.K.; Gangwar, R.; Maurya, V.K. Nutraceuticals and Their Impact on Human Health. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 229–254. ISBN 978-0-12-820284-5. [Google Scholar]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Bioactive Ingredients in Chinese Herbal Medicines That Target Non-Coding RNAs: Promising New Choices for Disease Treatment. Front. Pharmacol. 2019, 10, 515. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Mishra, G.; Pottoo, F.H.; Singh, B.; Zeleke, M.M. Zingiber Officinale: Its Ethanobotanical Uses, Phytochemistry, and Pharmacology. In Edible Plants in Health and Diseases; Masoodi, M.H., Rehman, M.U., Eds.; Springer: Singapore, 2022; pp. 1–42. ISBN 9789811649585. [Google Scholar]
- Taleuzzaman, M.; Ahmad, A.; Ahmad, M.; Gilani, S.J. Nigella Sativa: Its Ethnobotany, Phytochemistry, and Pharmacology. In Edible Plants in Health and Diseases; Masoodi, M.H., Rehman, M.U., Eds.; Springer: Singapore, 2022; pp. 175–203. ISBN 9789811649585. [Google Scholar]
- Sharma, S.; Kumar, D. Chemical Composition and Biological Uses of Crocus Sativus L. (Saffron). In Edible Plants in Health and Diseases; Masoodi, M.H., Rehman, M.U., Eds.; Springer: Singapore, 2022; pp. 249–277. ISBN 9789811649585. [Google Scholar]
- Qadir, I.; Bazaz, M.R.; Dar, R.M.; Ovais, S.; Mir, S.R.; Zargar, M.I.; Rehman, M.U. Cichorium Intybus: A Comprehensive Review on Its Pharmacological Activity and Phytochemistry. In Edible Plants in Health and Diseases; Masoodi, M.H., Rehman, M.U., Eds.; Springer: Singapore, 2022; pp. 373–398. ISBN 9789811649585. [Google Scholar]
- Aye, A.; Jeon, Y.-D.; Lee, J.-H.; Bang, K.-S.; Jin, J.-S. Anti-Inflammatory Activity of Ethanol Extract of Leaf and Leaf Callus of Basil (Ocimum Basilicum L.) on RAW 264.7 Macrophage Cells. Orient. Pharm. Exp. Med. 2019, 19, 217–226. [Google Scholar] [CrossRef]
- Jakovljević, D.; Stanković, M.; Warchoł, M.; Skrzypek, E. Basil (Ocimum L.) Cell and Organ Culture for the Secondary Metabolites Production: A Review. Plant Cell Tissue Organ Cult. PCTOC 2022, 149, 61–79. [Google Scholar] [CrossRef]
- Jiménez-Medina, E.; Garcia-Lora, A.; Paco, L.; Algarra, I.; Collado, A.; Garrido, F. A New Extract of the Plant Calendula Officinalis Produces a Dual in Vitroeffect: Cytotoxic Anti-Tumor Activity and Lymphocyte Activation. BMC Cancer 2006, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Ghavam, M.; Afzali, A.; Manconi, M.; Bacchetta, G.; Manca, M.L. Variability in Chemical Composition and Antimicrobial Activity of Essential Oil of Rosa × Damascena Herrm. from Mountainous Regions of Iran. Chem. Biol. Technol. Agric. 2021, 8, 22. [Google Scholar] [CrossRef]
- Kumar, N.; Pareek, S. Bioactive Compounds of Moringa (Moringa Species). In Bioactive Compounds in Underutilized Vegetables and Legumes; Reference Series in Phytochemistry; Murthy, H.N., Paek, K.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 503–524. ISBN 978-3-030-57414-7. [Google Scholar]
- Sohaib, M.; Al-Barakah, F.N.I.; Migdadi, H.M.; Husain, F.M. Comparative Study among Avicennia Marina, Phragmites Australis, and Moringa Oleifera Based Ethanolic-Extracts for Their Antimicrobial, Antioxidant, and Cytotoxic Activities. Saudi J. Biol. Sci. 2022, 29, 111–122. [Google Scholar] [CrossRef]
- Glasenapp, Y.; Korth, I.; Nguyen, X.-V.; Papenbrock, J. Sustainable Use of Mangroves as Sources of Valuable Medicinal Compounds: Species Identification, Propagation and Secondary Metabolite Composition. S. Afr. J. Bot. 2019, 121, 317–328. [Google Scholar] [CrossRef]
- Karthi, S.; Vinothkumar, M.; Karthic, U.; Manigandan, V.; Saravanan, R.; Vasantha-Srinivasan, P.; Kamaraj, C.; Shivakumar, M.S.; De Mandal, S.; Velusamy, A.; et al. Biological Effects of Avicennia Marina (Forssk.) Vierh. Extracts on Physiological, Biochemical, and Antimicrobial Activities against Three Challenging Mosquito Vectors and Microbial Pathogens. Environ. Sci. Pollut. Res. 2020, 27, 15174–15187. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M.; Tahoun, E.A.; AbuBakr, H.O. Ameliorative Effects of Jojoba Oil on Fipronil-Induced Hepatorenal- and Neuro-Toxicity: The Antioxidant Status and Apoptotic Markers Expression in Rats. Environ. Sci. Pollut. Res. 2021, 28, 25959–25971. [Google Scholar] [CrossRef]
- Qadir, A.; Khan, N.; Arif, M.; Mir Najib Ullah, S.N.; Khan, S.A.; Ali, A.; Imran, M. Identification of Phytobioconstituents Present in Simmondsia Chinensis L. Seeds Extract by GC-MS Analysis. J. Indian Chem. Soc. 2022, 99, 100354. [Google Scholar] [CrossRef]
- Olivia, N.U.; Goodness, U.C.; Obinna, O.M. Phytochemical Profiling and GC-MS Analysis of Aqueous Methanol Fraction of Hibiscus Asper Leaves. Future J. Pharm. Sci. 2021, 7, 59. [Google Scholar] [CrossRef]
- Kumar, S.; Walia, R.; Saxena, S.; Dey, P.; Madaan, R. Regulatory Considerations of Herbal Bioactive–Based Formulations. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 419–436. ISBN 978-0-12-824385-5. [Google Scholar]
- Zhang, J.; Hu, K.; Di, L.; Wang, P.; Liu, Z.; Zhang, J.; Yue, P.; Song, W.; Zhang, J.; Chen, T.; et al. Traditional Herbal Medicine and Nanomedicine: Converging Disciplines to Improve Therapeutic Efficacy and Human Health. Adv. Drug Deliv. Rev. 2021, 178, 113964. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Moradi, S.Z.; Akbari, V.; Aghaz, F.; Farzaei, M.H. Nanoformulated Herbal Bioactives for the Treatment of Neurodegenerative Disorders. In Herbal Bioactive-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–391. ISBN 978-0-12-824385-5. [Google Scholar]
- Cotter, T. Organic Mushroom Farming and Mycoremediation: Simple to Advanced and Experimental Techniques for Indoor and Outdoor Cultivation; Chelsea Green Publishing: White River Junction, VT, USA, 2015. [Google Scholar]
- Korman, R. Growing Mushrooms: The Complete Grower’s Guide to Becoming a Mushroom Expert and Starting Cultivation at Home; Amazon Digital Services LLC: Seattle, WA, USA, 2020; ISBN 978-1-65911-727-1. [Google Scholar]
- Elsakhawy, T.; Omara, A.E.-D.; Abowaly, M.; El-Ramady, H.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Green Synthesis of Nanoparticles by Mushrooms: A Crucial Dimension for Sustainable Soil Management. Sustainability 2022, 14, 4328. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus Ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- Aftab, T. (Ed.) Environmental Challenges and Medicinal Plants: Sustainable Production Solutions under Adverse Conditions; Environmental Challenges and Solutions; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-92049-4. [Google Scholar]
- León-Lobos, P.; Díaz-Forestier, J.; Díaz, R.; Celis-Diez, J.L.; Diazgranados, M.; Ulian, T. Patterns of Traditional and Modern Uses of Wild Edible Native Plants of Chile: Challenges and Future Perspectives. Plants 2022, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Milião, G.L.; de Oliveira, A.P.H.; de Soares, L.S.; Arruda, T.R.; Vieira, É.N.R.; de Leite Junior, B.R.C. Unconventional Food Plants: Nutritional Aspects and Perspectives for Industrial Applications. Future Foods 2022, 5, 100124. [Google Scholar] [CrossRef]
- Leal, M.L.; Alves, R.P.; Hanazaki, N. Knowledge, Use, and Disuse of Unconventional Food Plants. J. Ethnobiol. Ethnomed. 2018, 14, 6. [Google Scholar] [CrossRef]
- Peisino, M.C.O.; Zouain, M.S.; de Christo Scherer, M.M.; Schmitt, E.F.P.; Toledo e Silva, M.V.; Barth, T.; Endringer, D.C.; Scherer, R.; Fronza, M. Health-Promoting Properties of Brazilian Unconventional Food Plants. Waste Biomass Valorization 2020, 11, 4691–4700. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible Mushrooms as a Natural Source of Food Ingredient/Additive Replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef]
- Do Nascimento, V.T.; de Oliveira Campos, L.Z. Famine Foods: Thoughts from a Caatinga Research Experience. In Local Food Plants of Brazil; Ethnobiology; Jacob, M.C.M., Albuquerque, U.P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 161–176. ISBN 978-3-030-69138-7. [Google Scholar]
- Dou, J.; Weathers, P. Specialty Molecules from Plants and in Vitro Cultures as New Drugs: Regulatory Considerations from Flask to Patient. Plant Cell Tissue Organ Cult. PCTOC 2022, 149, 105–111. [Google Scholar] [CrossRef]
- Thomson, A.; Price, G.W.; Arnold, P.; Dixon, M.; Graham, T. Review of the Potential for Recycling CO2 from Organic Waste Composting into Plant Production under Controlled Environment Agriculture. J. Clean. Prod. 2022, 333, 130051. [Google Scholar] [CrossRef]
- Meyfroidt, P. Emerging Agricultural Expansion in Northern Regions: Insights from Land-Use Research. One Earth 2021, 4, 1661–1664. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Suhonen, A.; Ritala, A.; Oksman-Caldentey, K.-M. The Role of Single Cell Protein in Cellular Agriculture. Curr. Opin. Biotechnol. 2022, 75, 102686. [Google Scholar] [CrossRef] [PubMed]
- Bisconsin-Júnior, A.; Rodrigues, H.; Behrens, J.H.; da Silva, M.A.A.P.; Mariutti, L.R.B. “Food Made with Edible Insects”: Exploring the Social Representation of Entomophagy Where It Is Unfamiliar. Appetite 2022, 173, 106001. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, G.; Vatland, A.K.; Pampanin, D.M. The One-Health Approach in Seaweed Food Production. Environ. Int. 2022, 158, 106948. [Google Scholar] [CrossRef]
- Glaros, A.; Marquis, S.; Major, C.; Quarshie, P.; Ashton, L.; Green, A.G.; Kc, K.B.; Newman, L.; Newell, R.; Yada, R.Y.; et al. Horizon Scanning and Review of the Impact of Five Food and Food Production Models for the Global Food System in 2050. Trends Food Sci. Technol. 2022, 119, 550–564. [Google Scholar] [CrossRef]
- Van Vliet, S.; Kronberg, S.L.; Provenza, F.D. Plant-Based Meats, Human Health, and Climate Change. Front. Sustain. Food Syst. 2020, 4, 128. [Google Scholar] [CrossRef]
- Singh, M.; Trivedi, N.; Enamala, M.K.; Kuppam, C.; Parikh, P.; Nikolova, M.P.; Chavali, M. Plant-Based Meat Analogue (PBMA) as a Sustainable Food: A Concise Review. Eur. Food Res. Technol. 2021, 247, 2499–2526. [Google Scholar] [CrossRef]
- Klöckner, C.A.; Engel, L.; Moritz, J.; Burton, R.J.; Young, J.F.; Kidmose, U.; Ryynänen, T. Milk, Meat, and Fish From the Petri Dish—Which Attributes Would Make Cultured Proteins (Un)Attractive and for Whom? Results From a Nordic Survey. Front. Sustain. Food Syst. 2022, 6, 847931. [Google Scholar] [CrossRef]
- Stephan, A.; Ahlborn, J.; Zajul, M.; Zorn, H. Edible Mushroom Mycelia of Pleurotus Sapidus as Novel Protein Sources in a Vegan Boiled Sausage Analog System: Functionality and Sensory Tests in Comparison to Commercial Proteins and Meat Sausages. Eur. Food Res. Technol. 2018, 244, 913–924. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent Trends in Submerged Cultivation of Mushrooms and Their Application as a Source of Nutraceuticals and Food Additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Cutroneo, S.; Angelino, D.; Tedeschi, T.; Pellegrinin, N.; Martini, D.; Group, S.Y.W. Nutritional Quality of Meat Analogues: Results From the Food Labelling of Italian Products (FLIP) Project. Front. Nutr. 2022, 9, 852831. [Google Scholar] [CrossRef]
- Ramos Diaz, J.M.; Kantanen, K.; Edelmann, J.M.; Suhonen, H.; Sontag-Strohm, T.; Jouppila, K.; Piironen, V. Fibrous Meat Analogues Containing Oat Fiber Concentrate and Pea Protein Isolate: Mechanical and Physicochemical Characterization. Innov. Food Sci. Emerg. Technol. 2022, 77, 102954. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Pérez-Alvarez, J.A. Quinoa and Chia Products as Ingredients for Healthier Processed Meat Products: Technological Strategies for Their Application and Effects on the Final Product. Curr. Opin. Food Sci. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. The Science of Plant-Based Foods: Approaches to Create Nutritious and Sustainable Plant-Based Cheese Analogs. Trends Food Sci. Technol. 2021, 118, 207–229. [Google Scholar] [CrossRef]
- Flores, M.; Piornos, J.A. Fermented Meat Sausages and the Challenge of Their Plant-Based Alternatives: A Comparative Review on Aroma-Related Aspects. Meat Sci. 2021, 182, 108636. [Google Scholar] [CrossRef]
- Muchekeza, J.T.; Jombo, T.Z.; Magogo, C.; Mugari, A.; Manjeru, P.; Manhokwe, S. Proximate, Physico-Chemical, Functional and Sensory Properties OF Quinoa and Amaranth Flour AS Potential Binders in Beef Sausages. Food Chem. 2021, 365, 130619. [Google Scholar] [CrossRef]
- Bahmanyar, F.; Hosseini, S.M.; Mirmoghtadaie, L.; Shojaee-Aliabadi, S. Effects of Replacing Soy Protein and Bread Crumb with Quinoa and Buckwheat Flour in Functional Beef Burger Formulation. Meat Sci. 2021, 172, 108305. [Google Scholar] [CrossRef]
- Anzani, C.; Boukid, F.; Drummond, L.; Mullen, A.M.; Álvarez, C. Optimising the Use of Proteins from Rich Meat Co-Products and Non-Meat Alternatives: Nutritional, Technological and Allergenicity Challenges. Food Res. Int. 2020, 137, 109575. [Google Scholar] [CrossRef]
- Felix, M.; Camacho-Ocaña, Z.; López-Castejón, M.L.; Ruiz-Domínguez, M. Rheological Properties of Quinoa-Based Gels. An Alternative for Vegan Diets. Food Hydrocoll. 2021, 120, 106827. [Google Scholar] [CrossRef]
- Huang, K.; Shi, J.; Li, M.; Sun, R.; Guan, W.; Cao, H.; Guan, X.; Zhang, Y. Intervention of Microwave Irradiation on Structure and Quality Characteristics of Quinoa Protein Aggregates. Food Hydrocoll. 2022, 130, 107677. [Google Scholar] [CrossRef]
- Luo, L.; Cheng, L.; Zhang, R.; Yang, Z. Impact of High-Pressure Homogenization on Physico-Chemical, Structural, and Rheological Properties of Quinoa Protein Isolates. Food Struct. 2022, 32, 100265. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhang, X.; Li, T.; Zhou, J.; Yang, Y.; Bian, X.; Wang, L. High Internal Phase Emulsions Stabilized Solely by Sonicated Quinoa Protein Isolate at Various PH Values and Concentrations. Food Chem. 2022, 378, 132011. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, S.; Reddy Surasani, V.K. Quinoa Protein Isolate Supplemented Pasta: Nutritional, Physical, Textural and Morphological Characterization. LWT 2021, 135, 110045. [Google Scholar] [CrossRef]
- Ingrassia, R.; Busti, P.A.; Boeris, V. Physicochemical and Mechanical Properties of a New Cold-Set Emulsion Gel System and the Effect of Quinoa Protein Fortification. LWT 2022, 156, 113048. [Google Scholar] [CrossRef]
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil Degradation in the European Mediterranean Region: Processes, Status and Consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef]
- Lal, R. (Ed.) The Soil-Human Health-Nexus; Advances in Soil Science Book Series; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group, LLC: Abingdon, UK, 2021. [Google Scholar]
- FAO Soil Degredation. Available online: https://www.fao.org/soils-portal/soil-degradation-restoration/en/ (accessed on 8 May 2022).
- Wu, Y.; Li, X.; Yu, L.; Wang, T.; Wang, J.; Liu, T. Review of Soil Heavy Metal Pollution in China: Spatial Distribution, Primary Sources, and Remediation Alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Prajapati, G.S.; Rai, P.K.; Mishra, V.N.; Singh, P.; Shahi, A.P. Remote Sensing-Based Assessment of Waterlogging and Soil Salinity: A Case Study from Kerala, India. Results Geophys. Sci. 2021, 7, 100024. [Google Scholar] [CrossRef]
- Rasool, S.; Rasool, T.; Gani, K.M. A Review of Interactions of Pesticides within Various Interfaces of Intrinsic and Organic Residue Amended Soil Environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Bello-Bello, E.; López-Arredondo, D.; Rico-Chambrón, T.Y.; Herrera-Estrella, L. Conquering Compacted Soils: Uncovering the Molecular Components of Root Soil Penetration. Trends Plant Sci. 2022, S1360138522001054. [Google Scholar] [CrossRef]
- Rai, P.K. Environmental Degradation by Invasive Alien Plants in the Anthropocene: Challenges and Prospects for Sustainable Restoration. Anthr. Sci. 2022, 1, 5–28. [Google Scholar] [CrossRef]
- Fan, R.; Tian, H.; Wu, Q.; Yi, Y.; Yan, X.; Liu, B. Mechanism of Bio-Electrokinetic Remediation of Pyrene Contaminated Soil: Effects of an Electric Field on the Degradation Pathway and Microbial Metabolic Processes. J. Hazard. Mater. 2022, 422, 126959. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Jiang, W.; Xiukang, W.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U.; et al. Cadmium Phytotoxicity, Tolerance, and Advanced Remediation Approaches in Agricultural Soils; A Comprehensive Review. Front. Plant Sci. 2022, 13, 773815. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Edrisi, S.A. Developing Sustainable Measures to Restore Fly-ash Contaminated Lands: Current Challenges and Future Prospects. Land Degrad. Dev. 2021, 32, 4817–4831. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Sushkova, S.; Singh, R.K.; Verma, K.K. Nanotechnology in the Restoration of Polluted Soil. Nanomaterials 2022, 12, 769. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S.; et al. Jute: A Potential Candidate for Phytoremediation of Metals—A Review. Plants 2020, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, D.; Chen, R.; Ma, F.; Wang, G. How a Functional Soil Animal-Earthworm Affect Arbuscular Mycorrhizae-Assisted Phytoremediation in Metals Contaminated Soil? J. Hazard. Mater. 2022, 435, 128991. [Google Scholar] [CrossRef]
- Ait Elallem, K.; Sobeh, M.; Boularbah, A.; Yasri, A. Chemically Degraded Soil Rehabilitation Process Using Medicinal and Aromatic Plants: Review. Environ. Sci. Pollut. Res. 2021, 28, 73–93. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of Heavy Metals in Soil and Water: An Eco-Friendly, Sustainable and Multidisciplinary Approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation Technology and Food Security Impacts of Heavy Metal Contaminated Soils: A Review of Literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing Phytoremediation of Soils Polluted with Heavy Metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S. Plant-Assisted Metal Remediation in Mine-Degraded Land: A Scientometric Review. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Zhu, S.-C.; Zheng, H.-X.; Liu, W.-S.; Liu, C.; Guo, M.-N.; Huot, H.; Morel, J.L.; Qiu, R.-L.; Chao, Y.; Tang, Y.-T. Plant-Soil Feedbacks for the Restoration of Degraded Mine Lands: A Review. Front. Microbiol. 2022, 12, 751794. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Sharma, A.; Purkayastha, S.; Pandey, K.; Dhingra, S. Bio-Plastics and Biofuel. In Plastics to Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 365–376. ISBN 978-0-12-813140-4. [Google Scholar]
- Goria, K.; Kothari, R.; Singh, H.M.; Singh, A.; Tyagi, V.V. Biohydrogen: Potential Applications, Approaches, and Hurdles to Overcome. In Handbook of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 399–418. ISBN 978-0-12-822810-4. [Google Scholar]
- Paschalidou, A.; Tsatiris, M.; Kitikidou, K.; Papadopoulou, C. Using Energy Crops for Biofuels or Food: The Choice; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-63942-0. [Google Scholar]
- Samberger, C. The Role of Water Circularity in the Food-Water-Energy Nexus and Climate Change Mitigation. Energy Nexus 2022, 6, 100061. [Google Scholar] [CrossRef]
- Fouladi, J.; AlNouss, A.; Bicer, Y.; Al-Ansari, T. Thermodynamic Analysis of a Renewable Energy-Water-Food Nexus: A Trade-off Analysis of Integrated Desalination, Gasification and Food Systems. Case Stud. Therm. Eng. 2022, 34, 102024. [Google Scholar] [CrossRef]
- Coudard, A.; Corbin, E.; de Koning, J.; Tukker, A.; Mogollón, J.M. Global Water and Energy Losses from Consumer Avoidable Food Waste. J. Clean. Prod. 2021, 326, 129342. [Google Scholar] [CrossRef]
- Mahdavian, S.M.; Ahmadpour Borazjani, M.; Mohammadi, H.; Asgharipour, M.R.; Najafi Alamdarlo, H. Assessment of Food-Energy-Environmental Pollution Nexus in Iran: The Nonlinear Approach. Environ. Sci. Pollut. Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Deng, C.; Wang, H.; Hong, S.; Zhao, W.; Wang, C. Identifying the Drivers of Changes in Embodied Food–Energy–Water in the Bohai Mega-Urban Region, China: A Perspective of Final Demands. Environ. Sci. Pollut. Res. 2022, 1–17. [Google Scholar] [CrossRef]
- Gazal, A.A.; Jakrawatana, N.; Silalertruksa, T.; Gheewala, S.H. Water-Energy-Land-Food Nexus for Bioethanol Development in Nigeria. Biomass Convers. Biorefinery 2022, 1–14. [Google Scholar] [CrossRef]
- Haitsma Mulier, M.C.G.; Van de Ven, F.H.M.; Kirshen, P. Quantification of the Local Water Energy Nutrient Food Nexus for Three Urban Farms in Amsterdam & Boston. Energy Nexus 2022, 6, 100078. [Google Scholar] [CrossRef]
- Haitsma Mulier, M.C.G.; Van de Ven, F.H.M.; Kirshen, P. Circularity in the Urban Water-Energy-Nutrients-Food Nexus. Energy Nexus 2022, 100081. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Xu, C.; Lyu, J.; Su, Z. A Quantitative Analysis Framework for Water-Food-Energy Nexus in an Agricultural Watershed Using WEAP-MODFLOW. Sustain. Prod. Consum. 2022, 31, 693–706. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Zhang, C.; Liu, Y.; Fu, Q. Optimization of Agricultural Resources in Water-Energy-Food Nexus in Complex Environment: A Perspective on Multienergy Coordination. Energy Convers. Manag. 2022, 258, 115537. [Google Scholar] [CrossRef]
- Yuan, M.-H.; Lo, S.-L. Principles of Food-Energy-Water Nexus Governance. Renew. Sustain. Energy Rev. 2022, 155, 111937. [Google Scholar] [CrossRef]
- Núñez-López, J.M.; Rubio-Castro, E.; Ponce-Ortega, J.M. Optimizing Resilience at Water-Energy-Food Nexus. Comput. Chem. Eng. 2022, 160, 107710. [Google Scholar] [CrossRef]
- Siaw, M.N.K.; Oduro-Koranteng, E.A.; Dartey, Y.O.O. Food-Energy-Water Nexus: Food Waste Recycling System for Energy. Energy Nexus 2022, 5, 100053. [Google Scholar] [CrossRef]
- Xu, Z.; Yao, L. Opening the Black Box of Water-Energy-Food Nexus System in China: Prospects for Sustainable Consumption and Security. Environ. Sci. Policy 2022, 127, 66–76. [Google Scholar] [CrossRef]
- Andrade, M.C.; de Gorgulho Silva, C.O.; de Souza Moreira, L.R.; Ferreira Filho, E.X. Crop Residues: Applications of Lignocellulosic Biomass in the Context of a Biorefinery. Front. Energy 2021, 1–22. [Google Scholar] [CrossRef]
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of Lignocellulosic Biomass. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. ISBN 978-0-12-815936-1. [Google Scholar]
- Cao, B.; Bai, C.; Zhang, M.; Lu, Y.; Gao, P.; Yang, J.; Xue, Y.; Li, G. Future Landscape of Renewable Fuel Resources: Current and Future Conservation and Utilization of Main Biofuel Crops in China. Sci. Total Environ. 2022, 806, 150946. [Google Scholar] [CrossRef]
- Holmatov, B.; Schyns, J.F.; Krol, M.S.; Gerbens-Leenes, P.W.; Hoekstra, A.Y. Can Crop Residues Provide Fuel for Future Transport? Limited Global Residue Bioethanol Potentials and Large Associated Land, Water and Carbon Footprints. Renew. Sustain. Energy Rev. 2021, 149, 111417. [Google Scholar] [CrossRef]
- Chen, F.; Xiong, S.; Latha Gandla, M.; Stagge, S.; Martín, C. Spent Mushroom Substrates for Ethanol Production—Effect of Chemical and Structural Factors on Enzymatic Saccharification and Ethanolic Fermentation of Lentinula edodes-Pretreated Hardwood. Bioresour. Technol. 2022, 347, 126381. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Kapoor, S.; Thakur, R.; Sharma, E.; Tiwari, R.K.; Joshi, S.J. Lignocellulolytic Enzymes and Bioethanol Production from Spent Biomass of Edible Mushrooms Using Saccharomyces Cerevisiae and Pachysolen Tannophilus. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Xiong, S.; Martín, C.; Eilertsen, L.; Wei, M.; Myronycheva, O.; Larsson, S.H.; Lestander, T.A.; Atterhem, L.; Jönsson, L.J. Energy-Efficient Substrate Pasteurisation for Combined Production of Shiitake Mushroom (Lentinula edodes) and Bioethanol. Bioresour. Technol. 2019, 274, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.P.; Ravel, M.; Perumal, K. Pretreatment and Process Optimization of Bioethanol Production from Spent Biomass of Ganoderma Lucidum Using Saccharomyces cerevisiae. Fuel 2021, 306, 121680. [Google Scholar] [CrossRef]
- Ranjithkumar, M.; Uthandi, S.; Senthil Kumar, P.; Muniraj, I.; Thanabal, V.; Rajarathinam, R. Highly Crystalline Cotton Spinning Wastes Utilization: Pretreatment, Optimized Hydrolysis and Fermentation Using Pleurotus florida for Bioethanol Production. Fuel 2022, 308, 122052. [Google Scholar] [CrossRef]
- Periyasamy, S.; Karthik, V.; Senthil Kumar, P.; Isabel, J.B.; Temesgen, T.; Hunegnaw, B.M.; Melese, B.B.; Mohamed, B.A.; Vo, D.-V.N. Chemical, Physical and Biological Methods to Convert Lignocellulosic Waste into Value-Added Products. A Review. Environ. Chem. Lett. 2022, 20, 1129–1152. [Google Scholar] [CrossRef]
- Rueda, A.M.; López de los Santos, Y.; Vincent, A.T.; Létourneau, M.; Hernández, I.; Sánchez, C.I.; Molina, V.D.; Ospina, S.A.; Veyrier, F.J. Genome Sequencing and Functional Characterization of a Dictyopanus pusillus Fungal Enzymatic Extract Offers a Promising Alternative for Lignocellulose Pretreatment of Oil Palm Residues. PLoS ONE 2020, 15, e0227529. [Google Scholar] [CrossRef]
- Grimm, D.; Wösten, H.A.B. Mushroom Cultivation in the Circular Economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [Green Version]
- Kumar Sarangi, P.; Subudhi, S.; Bhatia, L.; Saha, K.; Mudgil, D.; Prasad Shadangi, K.; Srivastava, R.K.; Pattnaik, B.; Arya, R.K. Utilization of Agricultural Waste Biomass and Recycling toward Circular Bioeconomy. Environ. Sci. Pollut. Res. 2022, 1–14. [Google Scholar] [CrossRef]
- Jasinska, A.; Wojciechowska, E.; Stoknes, K.; Roszak, M. Bioconversion of Agricultural Wastes into a Value-Added Product: Straw of Norwegian Grains Composted with Dairy Manure Food Waste Digestate in Mushroom Cultivation. Horticulturae 2022, 8, 331. [Google Scholar] [CrossRef]
- Oliveira Vieira, V.; Almeida Conceição, A.; Raisa Barbosa Cunha, J.; Enis Virginio Machado, A.; Gonzaga de Almeida, E.; Souza Dias, E.; Magalhães Alcantara, L.; Neil Gerard Miller, R.; Gonçalves de Siqueira, F. A New Circular Economy Approach for Integrated Production of Tomatoes and Mushrooms. Saudi J. Biol. Sci. 2022, 29, 2756–2765. [Google Scholar] [CrossRef]
- Jasińska, A. Spent Mushroom Compost (SMC)—Retrieved Added Value Product Closing Loop in Agricultural Production. Acta Agrar. Debr. 2018, 150, 185–202. [Google Scholar] [CrossRef]
- Kazige, O.K.; Chuma, G.B.; Lusambya, A.S.; Mondo, J.M.; Balezi, A.Z.; Mapatano, S.; Mushagalusa, G.N. Valorizing Staple Crop Residues through Mushroom Production to Improve Food Security in Eastern Democratic Republic of Congo. J. Agric. Food Res. 2022, 8, 100285. [Google Scholar] [CrossRef]
- Otieno, O.D.; Mulaa, F.J.; Obiero, G.; Midiwo, J. Utilization of Fruit Waste Substrates in Mushroom Production and Manipulation of Chemical Composition. Biocatal. Agric. Biotechnol. 2022, 39, 102250. [Google Scholar] [CrossRef]
- Ivarsson, E.; Grudén, M.; Södergren, J.; Hultberg, M. Use of Faba Bean (Vicia faba L.) Hulls as Substrate for Pleurotus ostreatus—Potential for Combined Mushroom and Feed Production. J. Clean. Prod. 2021, 313, 127969. [Google Scholar] [CrossRef]
- Chouhan, P.; Koreti, D.; Kosre, A.; Chauhan, R.; Jadhav, S.K.; Chandrawanshi, N.K. Production and Assessment of Stick-Shaped Spawns of Oyster Mushroom from Banana Leaf-Midribs. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 92, 405–414. [Google Scholar] [CrossRef]
- Grimm, D.; Kuenz, A.; Rahmann, G. Integration of Mushroom Production into Circular Food Chains. Org. Agric. 2021, 11, 309–317. [Google Scholar] [CrossRef]
- Stoknes, K.; Wojciechowska, E.; Jasinska, A.; Noble, R. Amelioration of Composts for Greenhouse Vegetable Plants Using Pasteurised Agaricus Mushroom Substrate. Sustainability 2019, 11, 6779. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Mortimer, P.E.; Hyde, K.D.; Kakumyan, P.; Thongklang, N. Mushroom Cultivation for Soil Amendment and Bioremediation. Circ. Agric. Syst. 2021, 1, 11. [Google Scholar] [CrossRef]
- Jung, D.-H.; Son, J.-E. CO2 Utilization Strategy for Sustainable Cultivation of Mushrooms and Lettuces. Sustainability 2021, 13, 5434. [Google Scholar] [CrossRef]

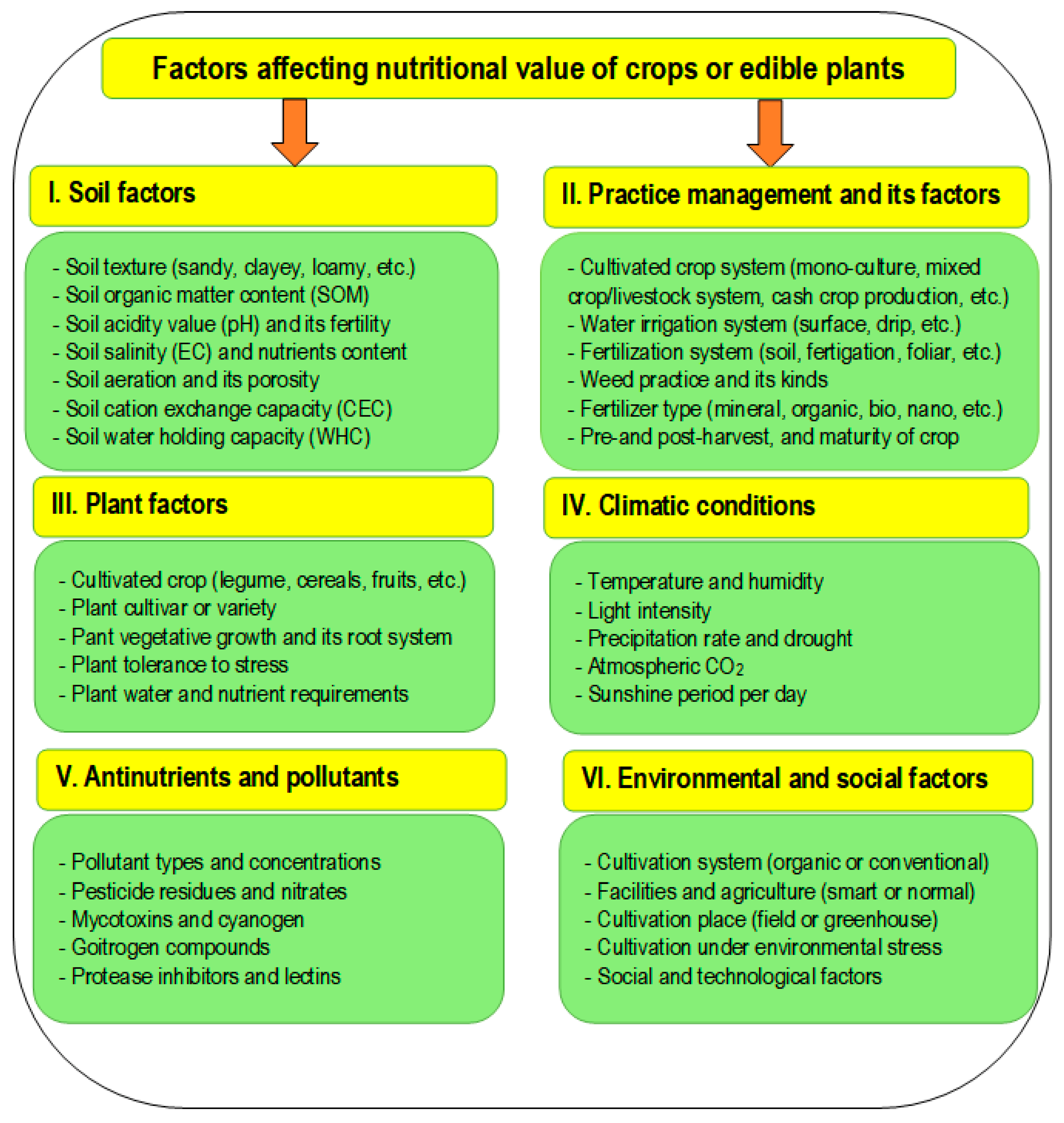
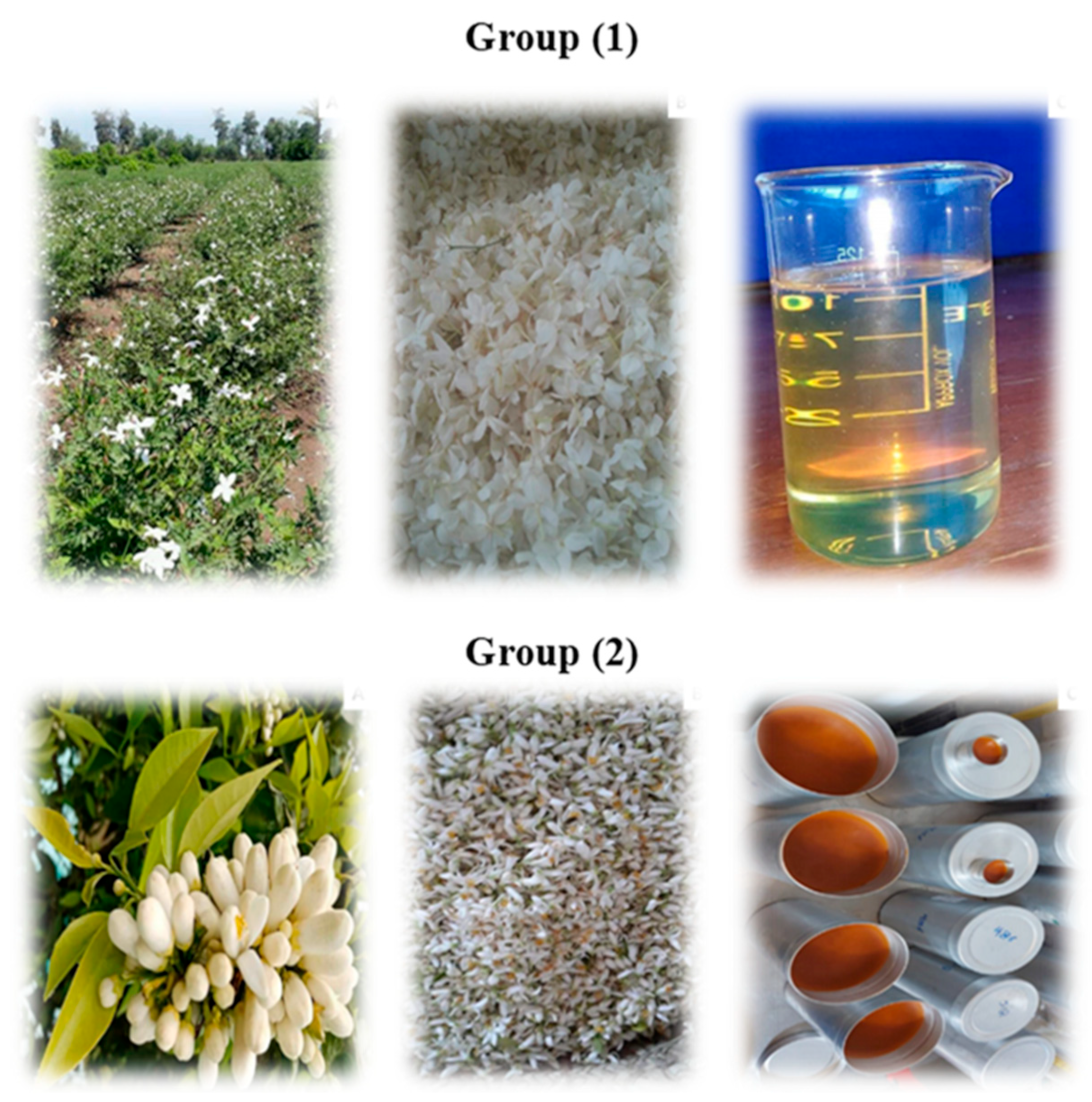









| Comparison Item | Plant Nutrition | Human Nutrition |
|---|---|---|
| Forming own food | They can because of chlorophyll | They cannot |
| Main requirements for nutrition | Plants need sunlight, CO2 and water as autotrophic | They metabolize large food molecules as heterotrophic |
| Final product from nutrition | Mainly glucose, energy, and oxygen | Amino acids, monosaccharides, fatty acids, and glycerol |
| Main organs involved in the nutrition | Leaves, including their components (chloroplasts, xylem, and phloem) | Mouth, esophagus, stomach, small intestine and the large intestine |
| Amino acids forming | Uptake of N, converted to NH3, form amino acids and then proteins | Amino acids can be obtained from the breakdown of proteins |
| Getting energy | Both photosynthesis and respiration can be used for forming energy (ATP) | Only the respiration process can produce energy (ATP) |
| Storage of carbohydrates | Plants can store glucose in the form of starch | Humans can store glucose in the form of glycogen |
| Enzymes involved | During nutritional processes, many enzymes: amylase, cellulase, lipase, phosphatase, phytase and urease | During metabolism, the main nutrients are carbohydrates, forming glucose, lipids (fatty acid) & proteins (amino acids) |
| Essential elements for both plants/humans | C, H, O, N, P, K, Ca, Mg, S, Fe, Mn, Cu, Cl, Mo, Ni, Zn | C, H, O, N, P, K, Ca, Mg, S, Fe, Mn, Cu, Cl, Mo, Ni, Zn |
| Suggested as essential | Al, Co, Se, Si, Na | As, F, Si, V, Cr, Sn, Ni |
| Essential element (only) | B | Na, Co, I, Se |
| Plant Species (Family) | Bioactive Phytochemicals | Pharmacological Activities | Refs. |
|---|---|---|---|
| Ginger: Zingiber officinale (Zingiberaceae) | Phenolic compounds (gingerols, paradols, and shogaols), flavonoids, carbohydrates, proteins, and terpenes | Antiemetic, anti-inflammatory, antidiabetic, anticancer, cardio-protective, and neuroprotective | [78] |
| Chewing stick or miswak: Salvadora persica Linn. (Salvadoraceae) | Underground parts or roots are used as toothbrushes, due to tannins (tannic acid), alkaloids (salvadorine), essential volatile oils, Vitamin C | Antimicrobial, antidiuretic, tick-repellent, anticancer, anti-inflammatory, hypolipidemic, and analgesic activities | [6] |
| Black seed: Nigella sativa (Ranunculaceae) | Seeds contain fixed oil (arachidonic, linoleic), protein, alkaloids, volatile oil (anethole, cymene), and saponin | Hepatoprotective, anti-cancer, anti-nephrotoxic, anti-diabetic, anti-parasitic, anti-malarial, anti-inflammatory and analgesic, etc. | [79] |
| Saffron: Crocus sativus L. (Iridaceae) | Flavonoids (flavone, flavonone), di-, mono-, tri-, tetra-terpenes (lycopene, crocetin) phenolics, carboxylic acids, phytosterols, vitamins (riboflavin) | Antiparasitic, antibacterial, hypotensive, antidepressant, anxiolytic, anticonvulsant, anti-Alzheimer, antitumor, anti-nociceptive, cytotoxic activity | [80] |
| Chicory: Cichorium intybus, (Asteraceae) | Vitamins (ascorbic acid, thiamine, riboflavin, retinol), carotenoids, inulin, niacin, sesquiterpenes, esculin, esculetin, cichorin A, lactucin, and lactucopicrin | Anti-inflammatory, antidiabetic, antimicrobial, gastroprotective, antioxidant, antimalarial, anthelmintic, analgesic and hepatoprotective activity | [81] |
| Basil plant: Ocimum basilicum L. (Lamiaceae) | Phenolic acids, isoprenoids, and flavonoids | Antioxidant, antibacterial, antifungal and anti-inflammatory activity | [82,83] |
| Scotch marigold: Calendula officinalis L. (Asteraceae) | Triterpenoid, carotenoids, lutein auroxanthin, zeaxanthin, saponins, beta-carotene, flavonol glycosides | Anti- genotoxic, anti-viral, and anti-inflammatory properties | [84] |
| Damask rose: Rosa damascena Mill. (Rosaceae) | Essential oil has β-citronellol, citronellol, docosane, geraniol, heneicosane, and nonadecane, | Rose oil is antiviral, anti-cancer, antioxidant, laxative, antiseptic and anti-inflammatory | [85] |
| Moringa: Moringa oleifera Lam. (Moringaceae) | Flavonoids, alkaloids, phenolics, tannins, saponins, glucosinolates vitamin A, vitamin C, Ca, and K | Anti-cancerous, cardiovascular, anti-asthmatic, antidiabetic, anti-microbial and anti-inflammatory | [86,87] |
| Mangrove: Avicennia marina (Forssk.) Vierh. (Acanthaceae) | Polyphenols, tannins, eicosanoic acid, cis9-hexadecenal, oleic acid, and di-Ndecylsulfone | Anti- antiviral, antibacterial, antifungal and antioxidant activities | [88,89] |
| Jojoba: Simmondsia chinensis (Link) C. K. (Simmondsiaceae) | Phenolic compounds like gallic acid, flavonoid, stigmast-5-en-3-ol, cis-9-octadecen-1-ol, 9-octadecen-1-ol, (Z), ergost-5-en-3-ol, (3-β)-ol, (Z)-14-tricosenyl formate, | Antidiabetic, anti-inflammatory, anthelminthic, antirheumatic, antiepileptic, antipsoriatic, antigonorrheal, analgesic, and pesticidal activities | [90,91] |
| Hibiscus: Hibiscus asper (Malvaceae) | Alkaloids, flavonoids, glycosides, phenols, saponins, steroid, tannin, terpenoids, 9, 12, 15- Octadecatrien-1-ol (Z, Z, Z) | Antiapoptotic Neuroprotective Antibacterial, anti-inflammatory, anti-ulcer, and anti-oxidative properties | [87,92] |
| Source of Proteins (the Country, if Any) | Sources of Plant Proteins | Refs. |
|---|---|---|
| Dairy-based protein alternatives (general study) | Quinoa and lentil are considered high-digestibility proteins | [61] |
| Meat analogues including steak, burgers, meatballs, and cutlets (Italy) | Plant steaks, burgers, meatballs, and cutlets | [120] |
| Fibrous meat analogues (Poland) | Pea protein isolate and oat fiber concentrate | [121] |
| Innovative approaches for meat production | Strategy of adding quinoa or chia to meat products | [122] |
| Dairy cheese analogs (the USA) | Plant-based cheese analogs | [123] |
| Fermented meat sausages (Span and Italy) | Plant-based alternatives includes flavor of plant protein isolates | [124] |
| Applied binders in meat product (sausages) processing | Quinoa flour could be applied as binder in beef sausage production | [125] |
| Producing beef burgers formed from flour of quinoa and buckwheat | Flour of both quinoa and buckwheat along with soy protein in beef burgers | [126] |
| Meat co-products as a meat replacer (general study) | Crops or seaweeds can be replaced by 20% in meat protein | [127] |
| Boiled meat sausages (Germany) | Pleurotus sapidus as protein in a vegan boiled sausage analog | [118] |
| Food-Energy Nexus | The Dimension of the Study | Refs. |
|---|---|---|
| Energy–food–water nexus | The performance of the energy–food–water nexus using solar energy under integrated production of fresh water from seawater desalination, biomass gasification and food systems in Qatar | [159] |
| Food–energy–water nexus | Reducing the losses in energy and water from consumer avoidable food wastes to increase sustainability in the food system in China | [160] |
| Food–energy nexus related to eco-pollution | Problems resulted from the production of energy and chemical fertilizers, as sources of environmental pollution due to the depletion of groundwater resources in Iran | [161] |
| Food–energy–water nexus | Identification of the change drivers in urban regions in China by a study of consumption of urban households, fixed capital formation and exports under food–energy–water system | [162] |
| Food–energy–land–water nexus | There is a need to produce a sustainable source of food, clean energy (biofuels), and water in Nigeria | [163] |
| Energy–food nexus | Collaborative management and conservation for scarcity of food and energy resources under climate policy were higher for low-income compared to high-income economies | [21] |
| Food–energy–water nexus | Mitigation of climate change and water circularity role in food–energy–water nexus for transition from a linear economy to a circular economy | [158] |
| Water–energy–nutrient–food nexus | Under urban agriculture system, water and nutrient needs at greenhouse farm and a container farm could be supplied by resources present in urban waters of wastewater and rainwater | [164] |
| Nutrient–food–energy–water nexus | Reusing urban wastewaters in urban farming can reduce energy needs for nutrient, water, irrigation, food transport, and wastewater pumping | [165] |
| Food–water–energy nexus | Integrated management in agricultural watershed and under drought can increase food production by 6% and reduce energy consumption by 3% compared to water-saving irrigation | [166] |
| Food–water–energy nexus | This nexus can contribute to sustainable and efficient management of different agricultural resources (i.e., energy, land, and water) | [167] |
| Food–energy–water nexus | This nexus governance depends on 9 principles: innovation, sharing, connectivity, participation, equitability, coordination, legitimacy, empowerment, and strategy | [168] |
| Energy–water–food nexus | Optimizing resilience can calculate to minimize emissions of CO2 based on total profits, while considering natural disaster events as interruptions | [169] |
| Food–energy–water nexus | Recycling of food wastes as a source of energy and water can perform using a mechanical presser/anaerobic digester to produce biogas | [170] |
| Food–water–energy nexus | This nexus could examine the sustainability implications in China, which needs some strategies through developing socio-economic balance and saving resources from the consumption perspectives | [171] |
| Energy Crop | The Common Meaning or Species in the Category |
|---|---|
| I. Energy crops | |
| Lignocellulosic biomass (LCB) categories | |
| 1. Annual and perennial energy grasses | Canary grass, switchgrass, Miscanthus, giant reed, alfalfa, and Napier grass |
| 2. Woody biomass | Natural forest residues, forestry wastes (wood chips, and branches from dead trees), tree bark, wood shavings, and sawdust |
| 3. Non-woody biomass | Agricultural wastes in the field (crop stubble, grasses, paddy husks, straw) and agricultural processing wastes (animal paunch waste, sugarcane bagasse, palm oil waste, cotton gin trash, etc.). |
| Lignocellulosic biomass from crop residues | |
| Biorefinery of crop residues applications | Converting biomass into bio-based products (biofuels, bioenergy, pharmaceuticals, biopolymers, surfactants) under circular bioeconomy |
| Lignocellulose-degrading enzymes from microorganisms and their biotechnological applications | |
| Biofuels | Bioethanol, biodiesel, biohydrogen, and biobutanol |
| Bioenergy | Biochar, biogas, syngas, methane, etc. |
| Recent fractionation process of lignocellulosic biomass | |
| Pyrolysis, microwave assisted deep eutectic solvents, aldehydes, organo-Cat, hydrothermal and delignification | |
| Mechanism of biofuel production by plants | |
| Novel biofuels have been produced from LCB, such as bio-hydrogen, biobutanol, dimethylfuran by enzymes of cellulases, hemicellulases, lytic polysaccharide monooxygenase, ligninase, and cellobiose dehydrogenases | |
| Main food crops and their generated residues | |
| Apple (apple pomace), cotton (cotton sheets, cotton stalks), rice (rice straw, rice hulls), coffee (coffee husks, coffee pulp, wastewater), sugarcane (sugarcane bagasse, cane straw), barley (barley straw), beans (peel beans), sorghum (sorghum straw), orange (orange peel, orange bagasse), maize (corncobs, corn straw), soybean (soybean hull), wheat (wheat straw), grapes (grape pomace) | |
| Main biorefinery applications of some major crop residues | |
| Coffee residues | Production of levulinic acid, gibberellic acid, biogas, bioethanol, biodiesel, α-amylase, pectinase, endoglucanase, and cultivation of mushrooms |
| Maize residues | Production of methane, prebiotic xylo-oligosaccharides, biosorbent, biogas |
| Soybean residues | Production of protease, β-amylase, α-amylase, biodiesel, biogas, bioethanol |
| Sugarcane residues | Production of glycosyl hydrolases, xylanases, endoglucanase, biobutanol, bioethanol, lignin, and levulinic acid |
| Rice residues | Production of cellulase, lignin degrading enzymes, biochar, nano-silica, nanocrystals, biobutanol, lignin, and cellulose |
| Wheat residues | Production of bioethanol, biogas, levulinic acid, bacterial cellulose, and mushroom cultivation |
| II. Mushrooms | |
| Most important mushroom species produce bioethanol | |
| Pleurotus florida, P. ostreatus, Ganoderma lucidum, Lentinula edodes | |
| Mechanism of biofuel production by mushroom | |
| Production of biofuels and energy from LCB is based on biochemical processes, which LCB needs C:N ratio ˂ 30 and humidity ˃ 30% through degrading enzymes (laccase, mannanase, cellulase, xylanase, etc.) | |
| Spent mushroom substrates (SMS) and its use for bioethanol production | |
| I. SMS of both mushrooms (Agaricus bisporus and Pleurotus forida) used for lignocellulolytic enzymes (hydrolytic and oxidative enzymes) | |
| II. SMS of Lentinula edodes was used for enzymatic saccharification, which resulted in high glucan digestibility (80–90%) in the SMS beside phenolics | |
| III. Using Hot-air (75–100 °C) pasteurization instead of autoclaving for SMS of Lentinula edodes by enzymatic digestibility of glucan in SMS | |
| IV. SMS of Ganoderma lucidum used by 0.2% (v/v) for fermentation using baker’s yeast (Saccharomyces cerevisiae) and incubated for 5 days at 30 °C | |
| Fermentation using mushroom for bioethanol production | |
| I. Fermentation using Pleurotus florida on cotton spinning wastes and the optimum ethanol yield (1.18 g L−1) was obtained by 64% at 60 h | |
| II. Mushroom of Dictyopanus genera can its enzyme (laccase activity 267 U L−1) from oil palm delignification process for bioethanol derived-cellulose | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ramady, H.; Törős, G.; Badgar, K.; Llanaj, X.; Hajdú, P.; El-Mahrouk, M.E.; Abdalla, N.; Prokisch, J. A Comparative Photographic Review on Higher Plants and Macro-Fungi: A Soil Restoration for Sustainable Production of Food and Energy. Sustainability 2022, 14, 7104. https://doi.org/10.3390/su14127104
El-Ramady H, Törős G, Badgar K, Llanaj X, Hajdú P, El-Mahrouk ME, Abdalla N, Prokisch J. A Comparative Photographic Review on Higher Plants and Macro-Fungi: A Soil Restoration for Sustainable Production of Food and Energy. Sustainability. 2022; 14(12):7104. https://doi.org/10.3390/su14127104
Chicago/Turabian StyleEl-Ramady, Hassan, Gréta Törős, Khandsuren Badgar, Xhensila Llanaj, Peter Hajdú, Mohammed E. El-Mahrouk, Neama Abdalla, and József Prokisch. 2022. "A Comparative Photographic Review on Higher Plants and Macro-Fungi: A Soil Restoration for Sustainable Production of Food and Energy" Sustainability 14, no. 12: 7104. https://doi.org/10.3390/su14127104
APA StyleEl-Ramady, H., Törős, G., Badgar, K., Llanaj, X., Hajdú, P., El-Mahrouk, M. E., Abdalla, N., & Prokisch, J. (2022). A Comparative Photographic Review on Higher Plants and Macro-Fungi: A Soil Restoration for Sustainable Production of Food and Energy. Sustainability, 14(12), 7104. https://doi.org/10.3390/su14127104











