Invasive Species as Rivals: Invasive Potential and Distribution Pattern of Xanthium strumarium L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Zones and Distribution Points
2.1.2. Climate, Physiography and Vegetation
2.2. Study of Invasive Characteristics
2.3. Field Studies of Invasive Species Distribution and Inter-Species Associations
2.4. Ecological Impact Assessment
2.4.1. Impact on Diversity
2.4.2. Soil Assessment
2.5. Data Analysis
3. Results
3.1. Invasive Potential of X. strumarium
3.2. Local Distribution of X. strumarium
3.3. X. strumarium Invasion Impacts on Diversity
3.4. Impacts on Soil and Associated Environmental Variables
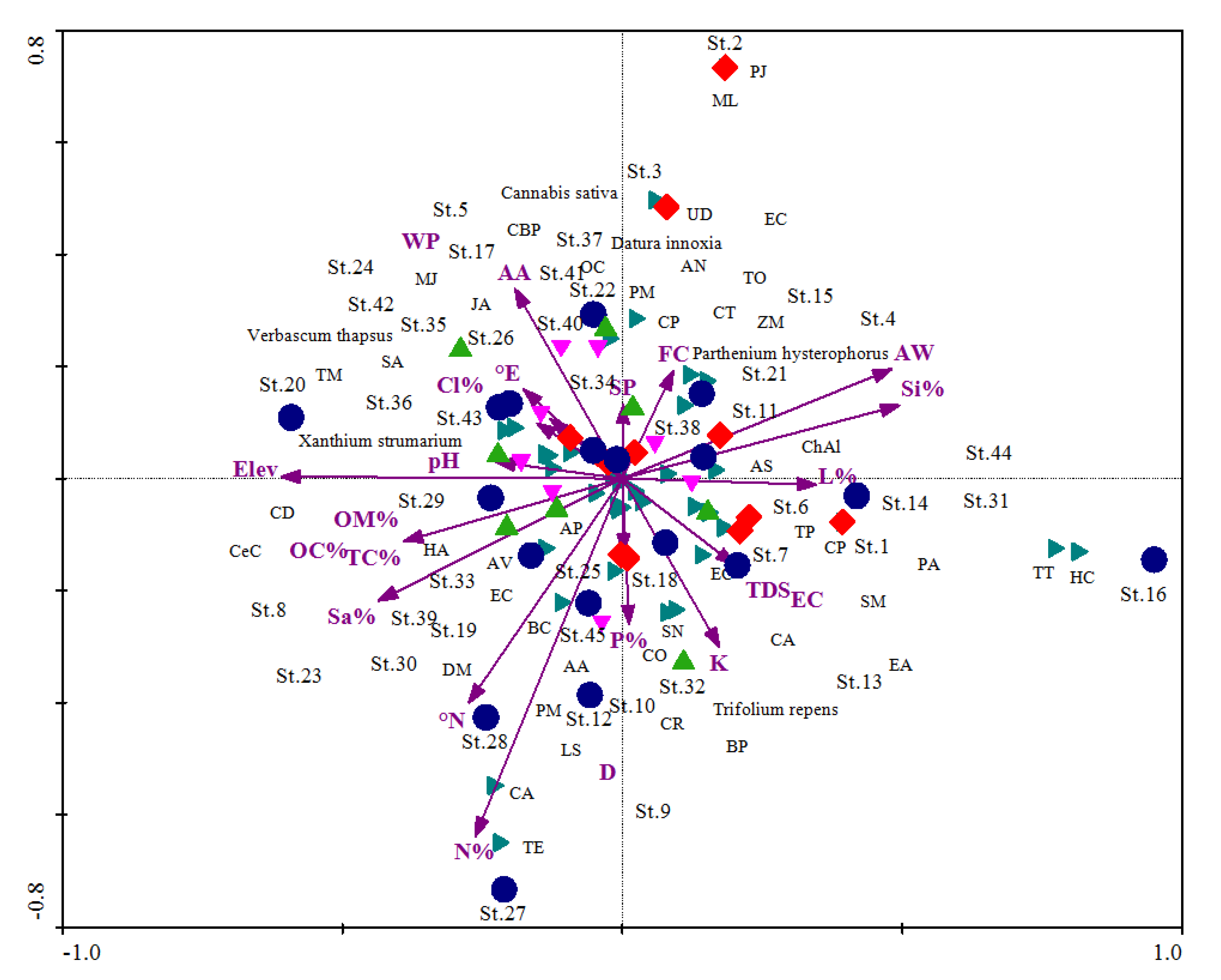
3.5. Pattern of Communities’ Composition
4. Discussion
4.1. Invasive Potential of X. strumarium
4.2. Local Distribution of X. strumarium
4.3. X. strumarium Invasion and Environmental Variables
4.4. Pattern of Communities’ Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, P.; Ouyang, Z.; Basnou, C.; Pino, J.; Park, H.; Chen, J. Nature-based solutions for urban landscapes under post-industrialization and globalization: Barcelona versus Shanghai. Environ. Res. 2017, 156, 272–283. [Google Scholar] [CrossRef] [Green Version]
- Gaertner, M.; Larson, B.M.; Irlich, U.M.; Holmes, P.M.; Stafford, L.; van Wilgen, B.W.; Richardson, D.M. Managing invasive species in cities: A framework from Cape Town, South Africa. Landsc. Urban Plan. 2016, 151, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, B.; Aldridge, D.C.; González-Moreno, P.; Pergl, J.; Pizarro, M.; Pyšek, P.; Vilà, M. Protected areas offer refuge from invasive species spreading under climate change. Glob. Change Biol. 2017, 23, 5331–5343. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.M.; Vila, M.; Antonio, C.M.D.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 775–781. [Google Scholar] [CrossRef]
- Eviner, V.T.; Garbach, K.; Baty, J.H.; Hoskinson, S.A. Measuring the effects of invasive plants on ecosystem services: Challenges and prospects. Invasive Plant Sci. Manag. 2012, 5, 125–136. [Google Scholar] [CrossRef]
- Lee, C.E. Evolutionary genetics of invasive species. Trends Ecol. Evol. 2002, 17, 386–391. [Google Scholar] [CrossRef]
- Alpert, P. The advantages and disadvantages of being introduced. Biol. Invasions 2006, 8, 1523–1534. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Pathogen accumulation and long-term dynamics of plant invasions. J. Ecol. 2013, 101, 607–613. [Google Scholar] [CrossRef]
- Rathee, S.; Ahmad, M.; Sharma, P.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R.K. Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot. 2021, 184, 104392. [Google Scholar] [CrossRef]
- Tilman, D. Community invasibility, recruitment limitation, and grassland biodiversity. Ecology 1997, 78, 81–92. [Google Scholar] [CrossRef]
- Odat, N.; Al-Khateeb, W.; Muhaidat, R.; Al-Udatt, M.; Irshiad, L. The effect of exotic Acacia saligna tree on plant biodiversity of Northern Jordan. Int. J. Agric. Biol. 2011, 13, 823–826. [Google Scholar]
- Chytrý, M.; Jarošík, V.; Pyšek, P.; Hájek, O.; Knollová, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, S.M.; Thompson, K.; Marrs, R.H.; Le Duc, M.G.; Maskell, L.C.; Firbank, L.G. Biotic homogenization and changes in species diversity across hu-man-modified ecosystems. Proceeding R. Soc. B Biol. Sci. 2006, 273, 2659–2665. [Google Scholar] [CrossRef] [Green Version]
- Morri, C.; Montefalcone, M.; Gatti, G.; Vassallo, P.; Paoli, C.; Bianchi, C.N. An Alien Invader is the cause of homogenization in the recipient eco-system: A simulation-like approach. Diversity 2019, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.R.U.; Richardson, D.M.; Rouget, M.; Procheş, Ş.; Amis, M.A.; Henderson, L.; Thuiller, W. Residence time and potential range: Crucial considerations in modelling plant invasions. Divers. Distrib. 2007, 13, 11–22. [Google Scholar] [CrossRef]
- Begum, H.A.; Hamayun, M.; Shad, N.; Khan, W.; Ahmad, J.; Khan, M.E.H.; Jones, D.A.; Ali, K. Effects of UV radiation on germination, growth, chlorophyll content, and fresh and dry weights of Brassica rapa L. and Eruca sativa L. Sarhad. J. Agric. 2021, 37, 1016–1024. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Pyšek, P.; Midgley, G.F.; Hughes, G.O.; Rouget, M. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob. Change Biol. 2005, 11, 2234–2250. [Google Scholar] [CrossRef]
- Godoy, O.; Richardson, D.M.; Valladares, F.; Castro-Díez, P. Flowering phenology of invasive alien plant species compared with native species in three Mediterranean-type ecosystems. Ann. Bot. 2009, 103, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Dudley, S.A. Plasticity and the functional ecology of plants. In Phenotypic Plasticity: Functional and Conceptual Approaches; Oxford University Press: Oxford, UK, 2004; p. 2. [Google Scholar]
- Qureshi, H.; Anwar, T.; Arshad, M.; Osunkoya, O.O.; Adkins, S.W. Impacts of Xanthium strumarium L. Invasion on vascular plant diversity in Pothwar region (Pakistan). Ann. Bot. 2019, 9, 73–82. [Google Scholar]
- Khan, S.M.; Page, S.; Ahmad, H.; Harper, D. Ethno-ecological importance of plant biodiversity in mountain ecosystems with special emphasis on indicator species of a Himalayan Valley in the northern Pakistan. Ecol. Indic. 2014, 37, 175–185. [Google Scholar] [CrossRef]
- Khan, K.; Ullah, S.; Ullah, A.; Khan, S.; Saragih, M.Y. Impact of Farmforestry on Socio-Economic Condition of Farming Communities in District Mardan, Pakistan. Bp. Int. Res. Crit. Inst. BIRCI-J. Humanit. Soc. Sci. 2020, 3, 2649–2661. [Google Scholar]
- Hussain, A.; Zarif, R.M. Invasive alien tree species—A threat to biodiversity. Pak. J. For. 2003, 53, 127–141. [Google Scholar]
- Shinwari, M.I.; Shinwari, M.I. Botanical diversity in Pakistan; past present and future. World Environ. 2010, 1, 85–104. [Google Scholar]
- Hussain, A. Pakistan. In Invasive Alien Species in South-Southeast Asia: National Reports and Directory of Resources; Pallewatta, N., Reaser, J.K., Gutierrez, A.T., Eds.; Global Invasive Species Programme: Cape Town, South Africa, 2003; pp. 70–79. [Google Scholar]
- Marwat, K.B.; Hashim, S.; Ali, H. Weed Management: A case study from North-West Pakistan. Pak. J. Bot. 2010, 42, 341–353. [Google Scholar]
- Alex, J. Development of weed problems in Canada. Biol. Ecol. Weeds 2013, 2, 309. [Google Scholar]
- Holm, L.G.; Plunknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds; East-West Center Book Univeristy Press of Hawaii: Honolulu, HI, USA, 1977; p. 609. [Google Scholar]
- Love., D.; Dansereau, P. Biosystematic studies on Xanthium: Taxonomrc appraisal and ecological status. Can. J. Bot. 1959, 37, 173–208. [Google Scholar] [CrossRef]
- Hashim, S.; Marwat, K.B.; Saeed, M.; Haroon, M.; Waqas, M.; Shah, F. Developing a sustainable and ecofriendly weed management system using organic and inorganic mulching techniques. Pak. J. Bot. 2013, 45, 483–486. [Google Scholar]
- Hussain, Z.; Marwat, K.B.; Cardina, J.; Khan, I.A. Xanthium strumarium L. impact on corn yield and yield components. Turk. J. Agric. For. 2014, 38, 39–46. [Google Scholar] [CrossRef]
- Qureshi, H.; Arshad, M.; Bibi, Y. Invasive flora of Pakistan: A critical analysis. Int. J. Biosci. 2014, 4, 407–424. [Google Scholar]
- Baloch, G.M.; Mohyuddin, A.I.; Ghani, M.A. Xanthium stmmarium L.-insects and other organisms associated with it in West Pakistan. Tech. Bull. Commonw. Inst. Biol. Control. 1968, 10, 103–111. [Google Scholar]
- Seifu, A.; Seboka, N.; Misganaw, M.; Bekele, T.; Merawi, E. Impact of Invasive Alien Plant, Xanthium Strumarium, On Species Diversity and Composition of Invaded Plant Communities in Borena Zone, Ethiopia. Biodivers. Int. J. 2017, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Iram, A.; Liu, M.C.; Feng, Y.L. Competitive approach of invasive cocklebur (Xanthium strumarium) with native weed species diversity in Northeast China. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, N.S.; Assefa, A.S.; Motbaynor, M.M.; Betsiha, E.M.; Hailu, A.A.; Beyene, G.F.; Hordofa, T.B. Invasion and impacts of Xanthium strumarium in Borena Zone of Oromia Region, Ethiopia. J. Coast. Life Med. 2017, 5, 350–355. [Google Scholar] [CrossRef]
- Ahmad, D. Khyber Pakhtunkhwa State of the Environment; Environmental Protection Agency, Government of Khyber Pakhtunkhwa: Peshawar, Pakistan, 2012.
- Ali, A.; Khan, T.A.; Ahmad, A. Analysis of Climate Data of Khyber Pakhtunkhwa, Pakistan. Int. Res. J. Eng. Technol. 2018, 5, 4266–4283. [Google Scholar] [CrossRef]
- FAO. Land Cover Atlas of Pakistan. In The Khyber Pakhtunkhwa Province and Federally Administered Tribal Areas; A joint publication by FAO, SUPARCO and Crop Reporting Service; Government of KP and FATA: Peshawar, Pakistan, 2016. [Google Scholar]
- Malkani, M.S.; Khosa, M.H.; Alyani, M.I.; Khan, K.; Somro, N.; Zafar, T.; Zahid, M.A. Mineral Deposits of Khyber Pakhtunkhwa and FATA, Pakistan. Lasbela Univ. J. Sci. Technol. 2017, 6, 23–46. [Google Scholar]
- Stewart, R.R. Annotated Catalogue of Vascular plants of West Pakistan and Kashmir; Fakhri Printing Press: Karachi, Pakistan, 1972; pp. 1–1027. [Google Scholar]
- Ahmad, H.; Khan, B. Environmental Profile of NWFP, 2nd ed.; Environmental Protection Agency: Peshawar, Pakistan, 2016.
- Rejmanek, M.; Richardson, D.M. What attributes make some plant species more invasive? Ecology 1996, 77, 1655–1661. [Google Scholar] [CrossRef]
- Morsdorf, F.; Mårell, A.; Koetz, B.; Cassagne, N.; Pimont, F.; Rigolot, E.; Allgöwer, B. Discrimination of vegetation strata in a multi-layered Mediterranean forest ecosystem using height and intensity information derived from airborne laser scanning. Remote Sens. Environ. 2010, 114, 1403–1415. [Google Scholar] [CrossRef] [Green Version]
- Curtis, J.T.; McIntosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Martínez-Falcón, A.P.; Zurita, G.A.; Ortega-Martínez, I.J.; Moreno, C.E. Populations and assemblages living on the edge: Dung beetles responses to forests-pasture ecotones. PeerJ 2018, 6, e6148. [Google Scholar] [CrossRef]
- Nasir, E.; Ali, S.I. (Eds.) Flora of West Pakistan; Fakhri Press: Karachi, Pakistan, 1972. [Google Scholar]
- Aeschimann, D.; Lauber, K.; Moser, D.M.; Theurillat, J.P. Flora Alpina; Belin: Paris, France, 2004. [Google Scholar]
- Maan, I.; Kaur, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Exotic avenue plantations turning foe: Invasive potential, distribution and impact of Broussonetia papyrifera in Chandigarh, India. Urban For. Urban Green. 2021, 59, 127010. [Google Scholar] [CrossRef]
- Castillón, E.E.; Arévalo, J.R.; Quintanilla, J.Á.V.; Rodríguez, M.M.S.; Encina-Domínguez, J.A.; Rodríguez, H.G.; Ayala, C.M.C. Classification and ordination of main plant communities along an altitudinal gradient in the arid and temperate climates of northeastern Mexico. Sci. Nat. 2015, 102, 59. [Google Scholar] [CrossRef]
- Kamrani, A.; Jalili, A.; Naqinezhad, A.; Attar, F.; Maassoumi, A.A.; Shaw, S.C. Relationships between environmental variables and vegetation across mountain wetland sites, N. Iran. Biologia 2011, 66, 76–87. [Google Scholar] [CrossRef]
- Reis, A.T.; Coelho, J.P.; Rucandio, I.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Thermo-desorption: A valid tool for mercury speciation in soils and sediments? Geoderma 2015, 237, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar]
- Yadav, R.L.; Tomar, S.S.; Sharma, U.C. Output: Input ratios and apparent balances of N, P and K inputs in a rice-wheat system in North-West India. Exp. Agric. 2002, 38, 457–468. [Google Scholar] [CrossRef]
- Arend, M.; Gessler, A.; Schaub, M. The influence of the soil on spring and autumn phenology in European beech. Tree Physiol. 2016, 36, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Gangwar, D.P.; Baskar, M. Texture Determination of Soil by Hydrometer Method for Forensic Purpose; Central Forensic Science Laboratory: Chandigarh, India, 2019.
- Hailu, H. Analysis of vegetation phytosociological characteristics and soil physicochemical conditions in Harishin Rangelands of Eastern Ethiopia. Land 2017, 6, 4. [Google Scholar] [CrossRef]
- McCune, B. Influence of noisy environmental data on canonical correspondence analysis. Ecology 1997, 78, 2617–2623. [Google Scholar] [CrossRef]
- Khan, N.; Shaukat, S.S.; Ahmad, M.; Siddiqui, M.F. Vegetation-environment relationships in the forests of Chitral District Hindukush range of Pakistan. J. For. Res. 2013, 24, 205–216. [Google Scholar] [CrossRef]
- Khan, N.; Ali, F.; Ali, K.; Shaukat, S. Composition, structure and regeneration dynamcis of Olea ferruginea Royle forests from Hindikush ranges of Pakistan. J. Mt. Sci. 2015, 12, 647–658. [Google Scholar]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Blasi, C. New insight on Mediterranean and sub-Mediterranean syntaxa included in the Vegetation Prodrome of Italy. Flora Mediterr. 2015, 25, 77–102. [Google Scholar]
- Rahman, H.U.; Nabi, G.; Ali, I.; Tahir, T.; Ahmed, M. Growth and yield of Kinnown (Citrus reticulata Blanco) and soil physical properties as affected by orchard floor management practices in Punjab, Pakistan. Soil Environ. 2012, 31, 163–170. [Google Scholar]
- Barua, N.; Aziz, M.A.I.; Tareq, A.M.; Sayeed, M.A.; Alam, N.; ul Alam, N.; Emran, T.B. In vivo and in vitro evaluation of pharmacological activities of Adenia trilobata (Roxb.). Biochem. Biophys. Rep. 2020, 23, 100772. [Google Scholar] [CrossRef]
- Irshad, M.; Khan, N.; Ali, K.; Muhammad, Z. The influence of environmental variables on Punica granatum L. assemblages in subtropical dry temperate woodland in the district of Lower Dir, Khyber Pakhtunkhwa, Pakistan. Turk. J. Bot. 2016, 40, 610–622. [Google Scholar] [CrossRef]
- Khan, N.; Bibi, K.; Ullah, R. Distribution pattern and ecological determinants of an invasive plant Parthenium hysterophorus L., in Malakand division of Pakistan. J. Mt. Sci. 2020, 17, 1670–1683. [Google Scholar] [CrossRef]
- Sherley, G. Invasive Species in the Pacific; South Pacific Regional Environment Programme: Apia, Samoa, 2000. [Google Scholar]
- Gallien, L.; Carboni, M. The community ecology of invasive species: Where are we and what’s next? Ecography 2017, 40, 335–352. [Google Scholar] [CrossRef] [Green Version]
- Simon, N. The Guiness Guide to Nature in Danger; Guiness Publishing Ltd.: London, UK, 1993. [Google Scholar]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.J.; Holmes, P.M.; Pauchard, A.; Richardson, D.M. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Ratnayake, R.M.C.S. Why plant species become invasive? In Proceedings of the National Symposium on Invasive Alien Species (IAS), Colombo, Sri Lanka, 27 November 2014; Volume 1. [Google Scholar]
- Zimmermann, H.G. Biological control of mesquite, Prosopis spp. (Fabaceae), in SouthAfrica. Agric. Ecosyst. Environ. 1991, 37, 175–186. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Feng, Y.L.; Feng, W.W.; Liu, M.C.; Lu, X.R. Ecological impacts of the invasive plant Xanthium strumarium and the impacts of three aboveground herbivores on the invader. Ecol. Indic. 2021, 131, 108140. [Google Scholar] [CrossRef]
- Awasthi, S.; Gupta, S.; Maurya, N.; Tripathi, P.; Dixit, P.; Sharma, N. Environmental risk factors for persistent asthma in Lucknow. Indian J. Pediatrics 2012, 79, 1311–1317. [Google Scholar] [CrossRef]
- Gaertner, M.; Den Breeyen, A.; Hui, C.; Richardson, D.M. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: A meta-analysis. Prog. Phys. Geogr. 2009, 33, 319–338. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Kueffer, C. Plant invasions in the Anthropocene. Sciecne 2017, 358, 724–725. [Google Scholar] [CrossRef]
- Jones, B.A. Tree shade, temperature, and human health: Evidence from invasive species-induced deforestation. Ecol. Econ. 2019, 156, 12–23. [Google Scholar] [CrossRef]
- Bartz, R.; Kowarik, I. Assessing the environmental impacts of invasive alien plants: A review of assessment approaches. NeoBiota 2019, 43, 69–99. [Google Scholar] [CrossRef] [Green Version]
- Timsina, B.; Shrestha, B.B.; Rokaya, M.B.; Münzbergová, Z. Impact of Parthenium hysterophorus L. invasion on plant species composition and soil properties of grassland communities in Nepal. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 233–240. [Google Scholar] [CrossRef]
- Zia, A.; Ahmad, S.S.; Bashir, H. Assessment of Vegetation-Edaphic Correlation of Wetland Complex of Soon Valley, Pakistan using Multivariate Techniques. Biol. Sci. PJSIR 2018, 61, 21–31. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Py_sek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jaro_s_ık, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Bekele, T. Plant Population Dynamics of Dodonaea angustifolia and Olea europaea ssp. Cuspidata in Dry Afromontane Forests of Ethiopia. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2000. [Google Scholar]
- Kosanic, A.; Anderson, K.; Harrison, S.; Turkington, T.; Bennie, J. Changes in the geographical distribution of plant species and climatic variables on the West Cornwall peninsula (South West UK). PLoS ONE 2018, 13, e0191021. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Guo, Q. Linking biotic homogenization to habitat type, invasiveness and growth form of naturalized plants in North America. Divers. Distrib. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- Tappeiner, J.; Zasada, J.; Ryan, P.; Newton, M. Salmonberry clonal and population structure: The basis for a persistent cover. Ecology 1991, 72, 609–618. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Salsa, O.E.; Lauenroth, W.K. A generalized model of the effects of grazing by large herbivores on grassland community structure. Am. Nat. 1988, 132, 87–106. [Google Scholar] [CrossRef]
- Salgado, J.; Vélez, M.I.; Caceres-Torres, L.C.; Villegas-Ibagon, J.A.; Bernal-Gonzalez, L.C.; Lopera-Congote, L.; González-Arango, C. Long-term habitat degradation drives neotropical macrophyte species loss while assisting the spread of invasive plant species. Front. Ecol. Evol. 2019, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.W.; Nafus, A.M.; Madsen, M.D. Medusahead invasion along unimproved roads, animal trails, and random transects. West. North Am. Nat. 2013, 73, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Brisson, J.; de Blois, S.; Lavoie, C. Roadside as invasion pathway for common reed (Phragmites australis). Invasive Plant Sci. Manag. 2010, 3, 506–514. [Google Scholar] [CrossRef]
- Richardson, D.M. Invasion science: The roads travelled and the roads ahead. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 397–401. [Google Scholar]
- Burleigh, B.A. Host cell signaling and Trypanosoma cruzi invasion: Do all roads lead to lysosomes? Sci. STKE 2005, 2005, pe36. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.; Edwards, P.J. Recognition that causal processes change during plant invasions helps explain conflicts in evidence. Ecology 2006, 87, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Barni, E.; Bacaro, G.; Falzoi, S.; Spanna, F.; Siniscalco, C. Establishing climatic constraints shaping the distribution of alien plant species along the elevation gradient in the Alps. Plant Ecol. 2012, 213, 757–767. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Haider, S.; Alexander, J.; Dietz, H.; Trepl, L.; Edwards, P.J.; Kueffer, C. The role of bio-climatic origin, residence time and habitat context in shaping non-native plant distributions along an altitudinal gradient. Biol. Invasions 2010, 12, 4003–4018. [Google Scholar] [CrossRef]
- Nievas, R.P.; Calderon, M.R.; Moglia, M.M. Environmental factors affecting the success of exotic plant invasion in a wildland-urban ecotone in temperate South America. Neotrop. Biol. Conserv. 2019, 14, 257. [Google Scholar] [CrossRef]
- Pauchard, A.; Alaback, P. La amenaza de plantas invasoras. Chile For. 2002, 289, 13–15. [Google Scholar]
- Koutika, L.S.; Vanderhoeven, S.; Chapuis-Lardy, L.; Dassonville, N.; Meerts, P. Assessment of changes in soil organic matter after invasion by exotic plant species. Biol. Fertil. Soils 2007, 44, 331–341. [Google Scholar] [CrossRef]
- Setälä, H.M.; Francini, G.; Allen, J.A.; Hui, N.; Jumpponen, A.; Kotze, D.J. Vegetation type and age drive changes in soil properties, nitrogen, and carbon sequestration in urban parks under cold climate. Front. Ecol. Evol. 2016, 4, 93. [Google Scholar] [CrossRef] [Green Version]
- El-Bana, M.I.; Al-Mathnani, A.S. Vegetation-soil relationships in the Wadi Al-Hayat Area of the Libyan Sahara. Australian J. Basic Appl. Sci. 2009, 3, 740–747. [Google Scholar]
- Rezaei, S.A.; Gilkes, R.J.; Andrews, S.S. A minimum data set for assessing soil quality in rangelands. Geoderma 2006, 136, 229–234. [Google Scholar] [CrossRef]
- Liu, J.; Lin, F.; Shi, S.; Ayi, Q.; Liu, S.; Zeng, B. Effects of water level regulation on the seed germination and production of annual plant Xanthium sibiricum in the water-level-fluctuating-zone of Three Gorges Reservoir. Sci. Rep. 2017, 7, 5056. [Google Scholar] [CrossRef] [Green Version]
- Champion, G.H.; Seth, S.K.; Khattak, G.M. Forest Types of Pakistan; Pakistan Forest Institute Peshawar Pakistan: Peshawar, Pakistan, 1965; p. 238. Available online: https://www.cabdirect.org/cabdirect/abstract/19950609490 (accessed on 21 October 2021).
- Khan, N.; Ahmad, M.; Shaukat, S.; Wahab, M.; Siddiqui, M.F. Structure, diversity, and regeneration potential of Monothheca Buxifolia (Falc.) A. DC. Dominated forest of Lower Dir District, Pakistan. Front. Agric. China 2011, 5, 106–121. [Google Scholar] [CrossRef]
- Haq, S.M.; Malik, A.H.; Khuroo, A.A.; Rashid, I. Floristic composition and biological spectrum of Keran-a remote valley of northwestern Himalaya. Acta Ecol. Sin. 2019, 39, 372–379. [Google Scholar] [CrossRef]
- Ullmann, I.; Heindl, B. Geographical and ecological differentiation of roadside vegetation in temperate Europe. Bot. Acta. 1989, 102, 261–269. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N.; Ali, K. Which factor explains the life-history of Xanthium strumarium L., an aggressive alien invasive plant species, along its altitudinal gradient? Plant Direct 2022, 6, e375. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N. Xanthium strumarium L. an Alien Invasive Species in Khyber Pakhtunkhwa (Pakistan): A Tool for Biomonitoring and Environmental Risk Assessment of Heavy Metal Pollutants. Arab. J. Sci. Eng. 2021, 47, 255–267. [Google Scholar] [CrossRef]
- Kapler, E.J.; Thompson, J.R.; Widrlechner, M.P. Assessing stakeholder perspectives on invasive plants to inform risk analysis. Invasive Plant Sci. Manag. 2012, 5, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Raghubanshi, A.S.; Rai, L.C.; Gaur, J.P.; Singh, J.S. Invasive Alien species and biodiversity in India. Curr. Sci. 2005, 88, 539–540. [Google Scholar]
- Dickie, I.A.; Bennett, B.M.; Burrows, L.E.; Nuñez, M.A.; Peltzer, D.A.; Porté, A.; Richardson, D.M.; Rejmánek, M.; Rundel, P.W.; van Wilgen, B.W. Conflicting values: Ecosystem services and invasive tree management. Biol. Invasions 2014, 16, 705–719. [Google Scholar] [CrossRef]

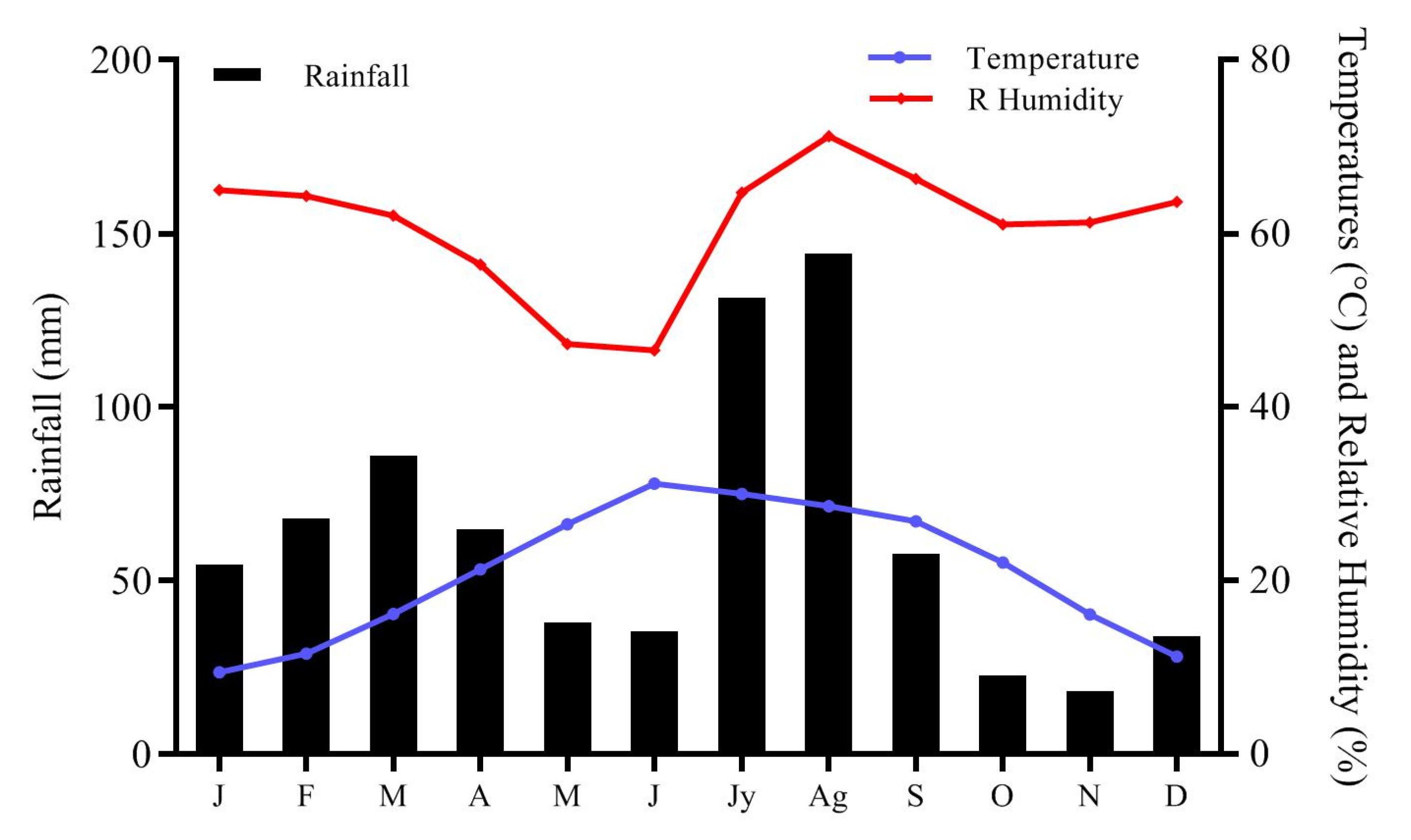
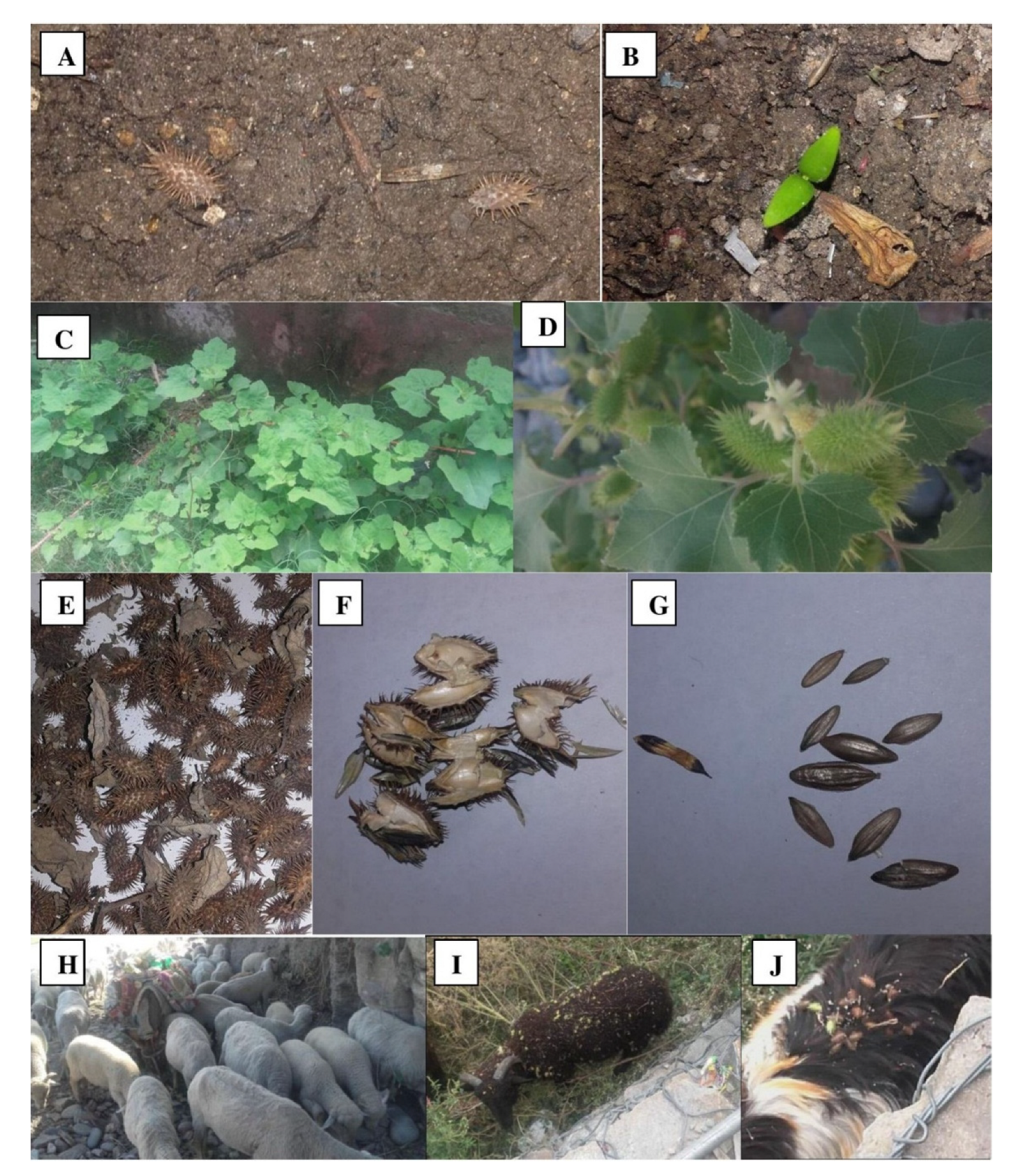

| Climatic Parameter | DLH | ADH (%) | ADR | ADMNT | ADMXT | ADMT | ADDP | DUVI |
| 12:14 ± 0.06 | 53.13 ± 13.93 | 0.43 ± 0.6 | 7.9 ± 4.02 | 34.1 ± 4.7 | 23.6 ± 3.83 | 8.23 ± 4.23 | 3 ± 2.56 | |
| Trait Parameter | DE | DI | DD | NI/P | NB/P | BB/50 | SB/100 | SBB/50 |
| 5.1 ± 0.78 | 41.65 ± 3.73 | 131 ± 12.78 | 400 ± 66 | 324 ± 56 | 25.5 ± 5 | 14.9 ± 5.76 | 39.45 ± 10.5 |
| Communities | Species | AH/Plant | AIVI (%) | H-Index | J-Index | AD/st. | AC/st. | AV/st. |
|---|---|---|---|---|---|---|---|---|
| Group-I | 29 | 26.4 ± 4.3 | 39.7 ± 1.42 a | 1.67 ± 0.11 a | 0.78 ± 0.02 | 34.8 ± 4 a | 65 ± 27 a | 24.9 ± 2 a |
| Group-II | 28 | 23.6 ± 1.2 | 45.4.94 ab | 1.44 ± 0.04 ab | 0.74 ± 0.01 | 38.3 ± 2.0 a | 38.6 ± 3 ab | 25.5 ± 1.9 a |
| Group-III | 26 | 31.9 ± 6 | 47.0 ± 2.3 b | 1.42 ± 0.09 b | 0.67 ± 0.04 | 48 ± 7.8 ab | 95.4 ± 43 a | 39 ± 7.4 b |
| Group-IV | 18 | 32.0 ± 4.1 | 50.9 ± 3 b | 1.19 ± 0.11 c | 0.71 ± 0.03 | 50 ± 7.8 ab | 84.4 ± 38 a | 31.6 ± 4.4 b |
| F-value | -- | 1.1584 | 6.47 | 5.45 | 2.30 | 3.184 | 2.512 | 2.920 |
| p-value | -- | 0.3402 | 0.001 | 0.0029 | 0.090 | 0.03 | 0.05 | 0.04 |
| Factors | Groups I | II | III | IV | H-Values | p-Values |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |||
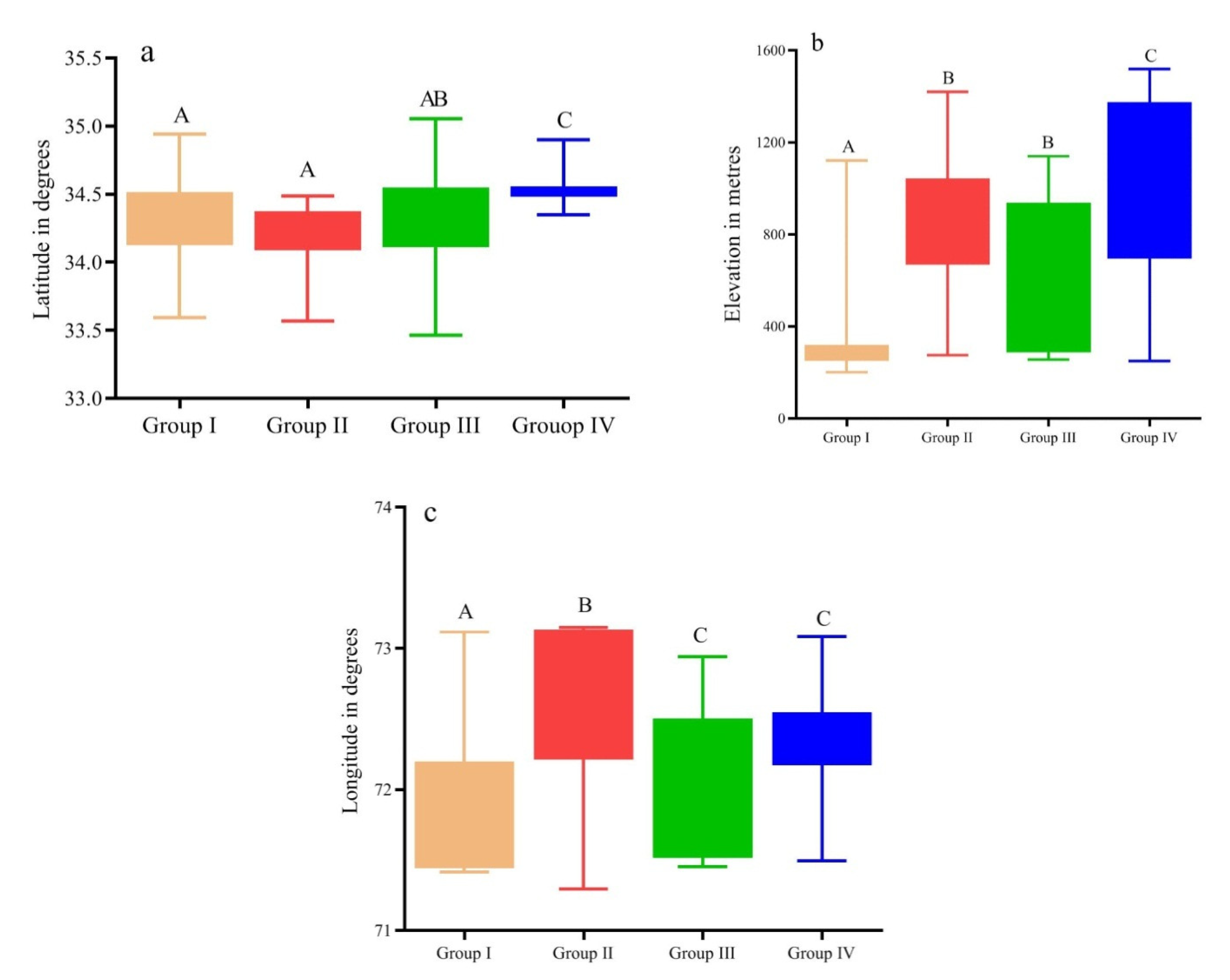
| Elev | 390.4 ± 87 | 843.1 ± 70 | 614.3 ± 123 | 981.1 ± 146 | 13.663 | 0.0034 ** |
| N° | 34.29 ± 0.11 | 34.16 ± 0.06 | 34.3 ± 0.16 | 34.55 ± 0.05 | 11.972 | 0.007 ** |
| E° | 71.84 ± 0.17 | 72.62 ± 0.12 | 71.9 ± 0.19 | 72.3 ± 0.15 | 12.538 | 0.005 ** |
| AA | 142.27 ± 32 | 193.72 ± 22 | 129.2 ± 37.9 | 126 ± 37.67 | 3.885 | 0.274 |
| Cl% | 24.76 ± 3.7 | 32.70 ± 2.5 | 21.7 ± 1.44 | 26.07 ± 3.43 | 7.17 | 0.066 * |
| Si% | 35.88 ± 5.9 | 36.33 ± 3 | 44.3 ± 4.89 | 43.2 ± 6.31 | 4.497 | 0.212 |
| Sa% | 39.35 ± 5.4 | 30.96 ± 3 | 33.8 ± 4.77 | 30.7 ± 4.36 | 2.195 | 0.532 |
| pH1:5 | 6.74 ± 0.13 | 6.91 ± 0.09 | 6.7 ± 0.092 | 6.71 ± 0.15 | 2.3041 | 0.511 |
| OM% | 1.02 ± 0.18 | 1.67 ± 0.1 | 1.48 ± 0.23 | 1.18 ± 0.16 | 5.672 | 0.128 |
| OC% | 0.57 ± 0.10 | 0.97 ± 0.1 | 0.85 ± 0.13 | 0.68 ± 0.09 | 2.458 | 0.076 |
| L% | 10.02 ± 1.0 | 8.22 ± 0.4 | 8.33 ± 0.95 | 8.82 ± 1.88 | 2.905 | 0.406 |
| TC% | 1.5 ± 0.10 | 1.98 ± 0.1 | 1.87 ± 0.13 | 1.7 ± 0.09 | 2.458 | 0.076 |
| N | 0.030 ± 0.007 | 0.064 ± 0.01 | 0.052 ± 0.019 | 0.030 ± 0.009 | 1.082 | 0.781 |
| P | 4.72 ± 0.33 | 4.65 ± 0.2 | 5.11 ± 0.59 | 5.04 ± 0.43 | 1.034 | 0.793 |
| K | 114.18 ± 9.4 | 102.2 ± 8.8 | 110.87 ± 14.81 | 100.2 ± 13.4 | 1.580 | 0.663 |
| EC | 249.72 ± 36 | 324.2 ± 20.67 | 308.62 ± 32.75 | 334.1 ± 13.3 | 7.759 | 0.051 * |
| TDS | 159.82 ± 23 | 207.50 ± 13.2 | 197.52 ± 10.96 | 213.8 ± 8.53 | 7.759 | 0.051 * |
| WP | 0.153 ± 0.017 | 0.18 ± 0.012 | 0.13 ± 0.0058 | 0.154 ± 0.01 | 7.651 | 0.053 * |
| FC | 0.28 ± 0.018 | 0.328 ± 0.01 | 0.27 ± 0.008 | 0.301 ± 0.013 | 7.232 | 0.064 * |
| BD | 1.38 ± 0.02 | 1.32 ± 0.016 | 1.38 ± 0.014 | 1.35 ± 0.017 | 6.481 | 0.090 * |
| SP | 0.47 ± 0.009 | 0.49 ± 0.006 | 0.47 ± 0.0054 | 0.487 ± 0.0066 | 6.388 | 0.094 * |
| AW | 0.13 ± 0.008 | 0.14 ± 0.005 | 0.14 ± 0.0085 | 0.14 ± 0.0068 | 1.686 | 0.639 |
| Total Variance (“Inertia”) in the Species Data: 3.3001 | Axis 1 | Axis 2 | Axis 3 |
|---|---|---|---|
| Eigenvalues | 0.28 | 0.22 | 0.19 |
| % of variance explained | 8.7 | 6.8 | 5.9 |
| Cumulative % explained | 8.7 | 15.4 | 21.3 |
| Pearson Correlation, Spp-Envt * | 0.769 | 0.924 | 0.926 |
| Kendall (Rank) Corr., Spp-Envt | 0.453 | 0.697 | 0.762 |
| Variable | Inter-Set Correlations * | Biplot Scores | ||||
|---|---|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 3 | Axis 1 | Axis 2 | Axis 3 | |
| Elev | 0.174 | −0.609 *** | −0.339 ** | 0.093 | −0.288 * | −0.149 |
| N° | 0.298 | −0.191 | 0.338 ** | 0.159 | −0.09 | 0.149 |
| E° | 0.119 | −0.241 | −0.052 | 0.064 | −0.114 | −0.023 |
| AA | −0.039 | −0.26 | −0.255 | −0.021 | −0.123 | −0.112 |
| Cl% | 0.163 | −0.21 | 0.022 | 0.087 | −0.099 | 0.01 |
| Si% | −0.284 | 0.489 ** | −0.056 | −0.152 | 0.231 | −0.024 |
| Sa% | 0.194 | −0.386 | 0.045 | 0.104 | −0.182 | 0.02 |
| P1:5 | −0.019 | −0.218 | 0.084 | −0.01 | −0.103 | 0.037 |
| OM% | 0.574 *** | −0.429 ** | −0.221 | 0.307 * | −0.203 | −0.097 |
| OC% | 0.574 *** | −0.429 ** | −0.221 | 0.307 * | −0.203 | −0.097 |
| L % | −0.273 * | 0.35 ** | 0.359 ** | −0.146 | 0.165 | 0.158 |
| TC% | 0.574 *** | −0.429 ** | −0.221 | 0.307 * | −0.203 | −0.097 |
| N | 0.367 ** | −0.274 * | 0.06 | 0.196 | −0.13 | 0.026 |
| P | 0.114 | 0.051 | −0.206 | 0.061 | 0.024 | −0.091 |
| K | 0.032 | 0.201 | 0.337 * | 0.017 | 0.095 | 0.148 |
| EC | 0.013 | 0.12 | −0.246 | 0.007 | 0.057 | −0.108 |
| TDS | 0.013 | 0.12 | −0.246 | 0.007 | 0.057 | −0.108 |
| WP | 0.155 | −0.19 | 0.006 | 0.083 | −0.09 | 0.003 |
| FC | 0.029 | 0.021 | −0.009 | 0.016 | 0.01 | −0.004 |
| D | −0.071 | 0.059 | −0.024 | −0.038 | 0.028 | −0.01 |
| SP | 0.07 | −0.056 | 0.023 | 0.037 | −0.026 | 0.01 |
| AW | −0.286 * | 0.459 ** | −0.034 | −0.153 | 0.217 | −0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, R.; Khan, N.; Hewitt, N.; Ali, K.; Jones, D.A.; Khan, M.E.H. Invasive Species as Rivals: Invasive Potential and Distribution Pattern of Xanthium strumarium L. Sustainability 2022, 14, 7141. https://doi.org/10.3390/su14127141
Ullah R, Khan N, Hewitt N, Ali K, Jones DA, Khan MEH. Invasive Species as Rivals: Invasive Potential and Distribution Pattern of Xanthium strumarium L. Sustainability. 2022; 14(12):7141. https://doi.org/10.3390/su14127141
Chicago/Turabian StyleUllah, Rafi, Nasrullah Khan, Nina Hewitt, Kishwar Ali, David Aaron Jones, and Muhammad Ezaz Hasan Khan. 2022. "Invasive Species as Rivals: Invasive Potential and Distribution Pattern of Xanthium strumarium L." Sustainability 14, no. 12: 7141. https://doi.org/10.3390/su14127141
APA StyleUllah, R., Khan, N., Hewitt, N., Ali, K., Jones, D. A., & Khan, M. E. H. (2022). Invasive Species as Rivals: Invasive Potential and Distribution Pattern of Xanthium strumarium L. Sustainability, 14(12), 7141. https://doi.org/10.3390/su14127141








