Gait Speed as a Biomarker of Cognitive Vulnerability: A Population-Based Study with Cognitively Normal Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.2.1. Cognitive Assessment
2.2.2. Gait Speed
2.2.3. Covariates
2.2.4. Statistical Analysis
3. Results
3.1. Main Characteristics of the Participants
3.2. Performance of Usual GS and Fast GS
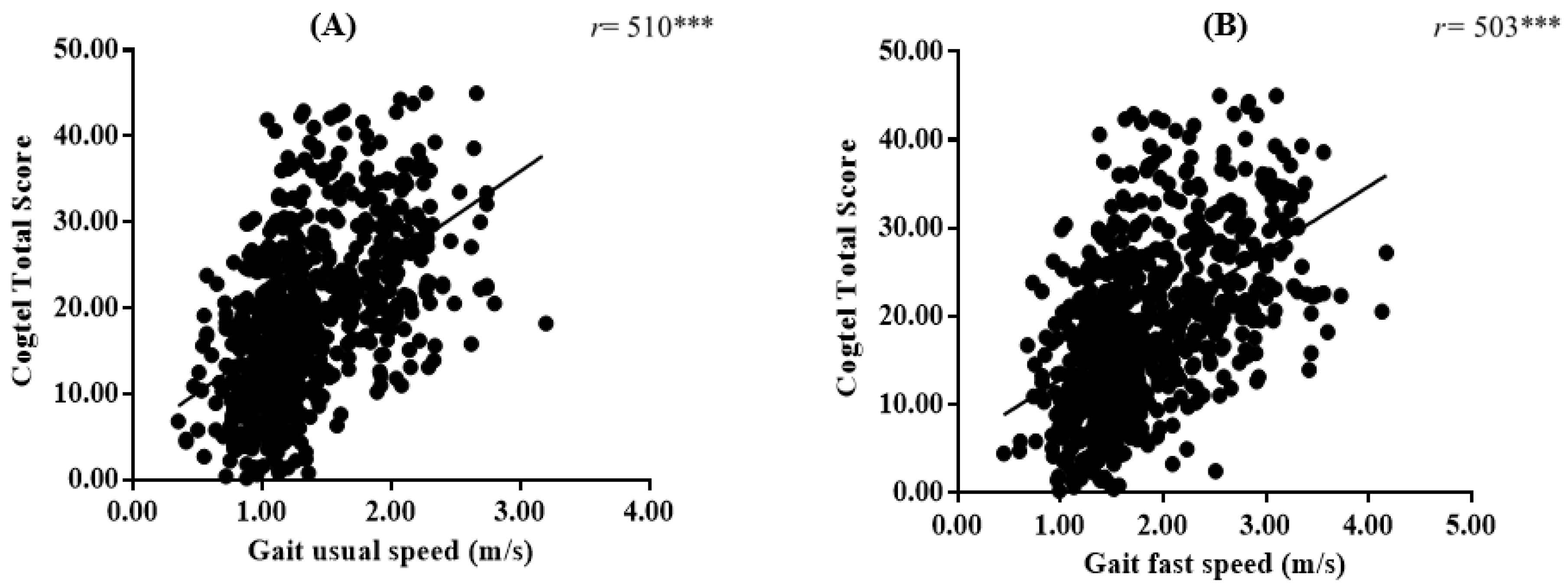
3.3. Correlations between Usual and Fast GS and Cognitive Performance
3.4. Associations between Usual GS and Fast GS (Continuous Variable) and Cognitive Vulnerability
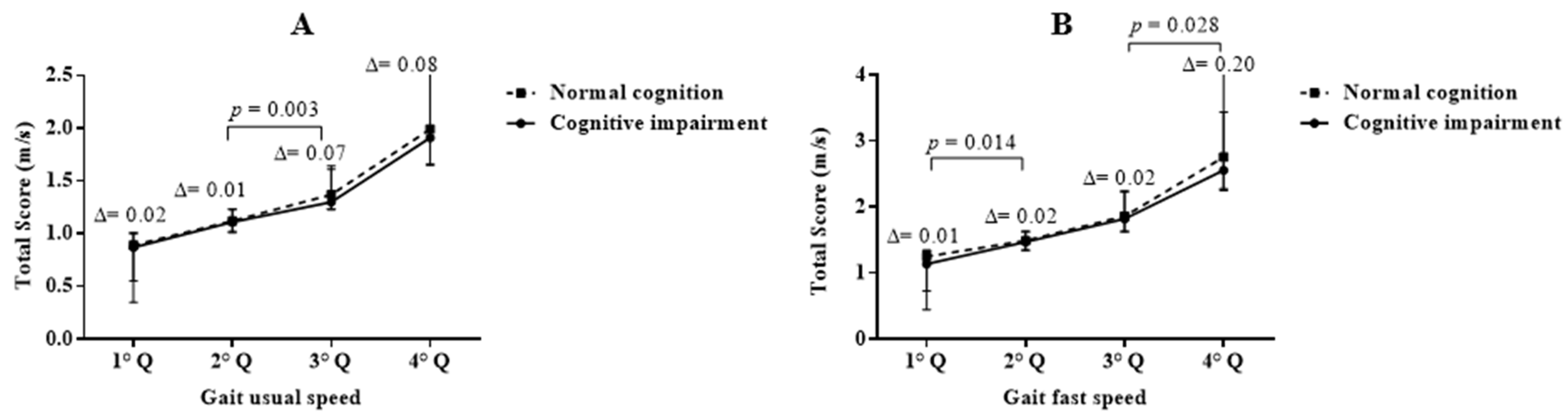
3.5. Associations between Usual GS (Quartile) and Cognitive Impairment
3.6. Associations between Fast GS (Quartile) and Cognitive Impairment
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, M.E.; Lasschuit, D.A.; Lord, S.R.; Delbaere, K.; Kurrle, S.E.; Mikolaizak, A.S.; Kvelde, T.; Close, J.C.T. Slow gait speed is associated with executive function decline in older people with mild to moderate dementia: A one year longitudinal study. Arch. Gerontol. Geriatr. 2017, 73, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Toots, A.T.M.; Taylor, M.E.; Lord, S.R.; Close, J.C.T. Associations between gait speed and cognitive domains in older people with cognitive impairment. J. Alzheimer’s Dis. 2019, 71, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Allali, G.; Montembeault, M.; Brambati, S.M.; Bherer, L.; Blumen, H.M.; Launay, C.P.; Liu-Ambrose, T.; Helbostad, J.L.; Verghese, J.; Beauchet, O. Brain structure covariance associated with gait control in aging. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2019, 74, 705–713. [Google Scholar] [CrossRef]
- Grande, G.; Triolo, F.; Nuara, A.; Welmer, A.-K.; Fratiglioni, L.; Vetrano, D.L. Measuring gait speed to better identify prodromal dementia. Exp. Gerontol. 2019, 124, 110625. [Google Scholar] [CrossRef]
- Cohen, J.A.; Verghese, J.; Zwerling, J.L. Cognition and gait in older people. Maturitas 2016, 93, 73–77. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Launay, C.; Herrmann, F.R.; Annweiler, C. Gait variability at fast-pace walking speed: A biomarker of mild cognitive impairment? J. Nutr. Health Aging 2013, 17, 235–239. [Google Scholar] [CrossRef]
- Montero-Odasso, M. Gait as a biomarker of cognitive impairment and dementia syndromes. Quo vadis? Eur. J. Neurol. 2016, 23, 437–438. [Google Scholar] [CrossRef]
- Garcia-Cifuentes, E.; Márquez, I.; Vasquez, D.; Aguillon, D.; Borda, M.G.; Lopera, F.; Cano-Gutierrez, C. The role of gait speed in dementia: A secondary analysis from the sabe colombia study. Dement. Geriatr. Cogn. Disord. 2020, 49, 565–572. [Google Scholar] [CrossRef]
- Knapstad, M.K.; Steihaug, O.M.; Aaslund, M.K.; Nakling, A.; Naterstad, I.F.; Fladby, T.; Aarsland, D.; Giil, L.M. Reduced walking speed in subjective and mild cognitive impairment: A cross-sectional study. J. Geriatr. Phys. Ther. 2019, 42, E122–E128. [Google Scholar] [CrossRef]
- Windham, B.G.; Parker, S.B.; Zhu, X.; Gabriel, K.P.; Palta, P.; Sullivan, K.J.; Parker, K.G.; Knopman, D.S.; Gottesman, R.F.; Griswold, M.E.; et al. Endurance and gait speed relationships with mild cognitive impairment and dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12281. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Verghese, J.; Beauchet, O.; Hausdorff, J.M. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012, 60, 2127–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosso, A.L.; Studenski, S.A.; Chen, W.G.; Aizenstein, H.J.; Alexander, N.B.; Bennett, D.A.; Black, S.E.; Camicioli, R.; Carlson, M.C.; Ferrucci, L.; et al. Aging, the central nervous system, and mobility. J. Gerontol. Ser. A 2013, 68, 1379–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demnitz, N.; Hogan, D.B.; Dawes, H.; Johansen-Berg, H.; Ebmeier, K.P.; Poulin, M.J.; Sexton, C.E. Cognition and mobility show a global association in middle- and late-adulthood: Analyses from the Canadian longitudinal study on aging. Gait Posture 2018, 64, 238–243. [Google Scholar] [CrossRef]
- Lau, B.; Welter, M.-L.; Belaid, H.; Fernandez Vidal, S.; Bardinet, E.; Grabli, D.; Karachi, C. The integrative role of the pedunculopontine nucleus in human gait. Brain 2015, 138, 1284–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, D.; Schoellmann, A.; Fox, M.D.; Bohnen, N.I.; Factor, S.A.; Nieuwboer, A.; Hallett, M.; Lewis, S.J.G. Freezing of gait: Understanding the complexity of an enigmatic phenomenon. Brain 2020, 143, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Launay, C.P.; Annweiler, C.; Allali, G. Hippocampal volume, early cognitive decline and gait variability: Which association? Exp. Gerontol. 2015, 61, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumurgier, J.; Crivello, F.; Mazoyer, B.; Ahmed, I.; Tavernier, B.; Grabli, D.; François, C.; Tzourio-Mazoyer, N.; Tzourio, C.; Elbaz, A. MRI atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage 2012, 60, 871–878. [Google Scholar] [CrossRef]
- Annweiler, C.; Beauchet, O.; Bartha, R.; Wells, J.L.; Borrie, M.J.; Hachinski, V.; Montero-Odasso, M. Motor cortex and gait in mild cognitive impairment: A magnetic resonance spectroscopy and volumetric imaging study. Brain 2013, 136, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Kueper, J.K.; Lizotte, D.J.; Montero-Odasso, M.; Speechley, M. Cognition and motor function: The gait and cognition pooled index. PLoS ONE 2020, 15, e0238690. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Speechley, M.; Muir-Hunter, S.W.; Sarquis-Adamson, Y.; Sposato, L.A.; Hachinski, V.; Borrie, M.; Wells, J.; Black, A.; Sejdić, E.; et al. Motor and cognitive trajectories before dementia: Results from gait and brain study. J. Am. Geriatr. Soc. 2018, 66, 1676–1683. [Google Scholar] [CrossRef]
- Abellan Van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette-Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Stuck, A.E.; Iliffe, S. Comprehensive geriatric assessment for older adults. BMJ 2011, 343, d6799. [Google Scholar] [CrossRef] [PubMed]
- Machulda, M.M.; Pankratz, V.S.; Christianson, T.J.; Ivnik, R.J.; Mielke, M.M.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Petersen, R.C. Practice effects and longitudinal cognitive change in normal aging vs. Incident mild cognitive impairment and dementia in the mayo clinic study of aging. Clin. Neuropsychol. 2013, 27, 1247–1264. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.S.; Segawa, E.; Boyle, P.A.; Anagnos, S.E.; Hizel, L.P.; Bennett, D.A. The natural history of cognitive decline in Alzheimer’s disease. Psychol. Aging 2012, 27, 1008–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielke, M.M.; Roberts, R.O.; Savica, R.; Cha, R.; Drubach, D.I.; Christianson, T.; Pankratz, V.S.; Geda, Y.E.; Machulda, M.M.; Ivnik, R.J.; et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the mayo clinic study of aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 929–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savica, R.; Wennberg, A.M.V.; Hagen, C.; Edwards, K.; Roberts, R.O.; Hollman, J.H.; Knopman, D.S.; Boeve, B.F.; Machulda, M.M.; Petersen, R.C.; et al. Comparison of gait parameters for predicting cognitive decline: The mayo clinic study of aging. J. Alzheimer’s Dis. 2016, 55, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Holtzer, R.; Verghese, J.; Xue, X.; Lipton, R.B. Cognitive processes related to gait velocity: Results from the Einstein aging study. Neuropsychology 2006, 20, 215–223. [Google Scholar] [CrossRef]
- Tasvuran Horata, E.; Cetin, S.Y.; Erel, S. Effects of individual progressive single- and dual-task training on gait and cognition among older healthy adults: A randomized-controlled comparison study. Eur. Geriatr. Med. 2021, 12, 363–370. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Buchanan, C.K.; Nahin, R.L.; Dekosky, S.T.; Atkinson, H.H.; Carlson, M.C.; Williamson, J.D. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J. Gerontol. A. Biol. Sci. Med. Sci. 2007, 62, 1244–1251. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, Y.; Yoshida, H.; Fujiwara, Y.; Motohashi, Y.; Shinkai, S. A prospective study of gait performance and subsequent cognitive decline in a general population of older japanese. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Brazilian Society of Geriatrics and Gerontology (SBGG) Aging in the North and Northeast of Brazil: What Needs to Be Done to Overcome the Challenges of Care. Available online: https://sbgg.org.br/envelhecimento-no-norte-e-nordeste-do-brasil-o-que-e-preciso-driblar-para-vencer-os-desafios-do-cuidar/ (accessed on 2 January 2021).
- Creavin, S.T.; Noel-Storr, A.H.; Smailagic, N.; Giannakou, A.; Ewins, E.; Wisniewski, S.; Cullum, S. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s dementia and other dementias in asymptomatic and previously clinically unevaluated people aged over 65 years in community and primary care populations. In Cochrane Database of Systematic Reviews; Creavin, S.T., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2016. [Google Scholar]
- Kliegel, M.; Martin, M.; Jäger, T. Development and Validation of the Cognitive Telephone Screening Instrument (COGTEL) for the assessment of cognitive function across adulthood. J. Psychol. 2007, 141, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Ihle, A.; Gouveia, É.R.; Gouveia, B.R.; Kliegel, M. The Cognitive Telephone Screening Instrument (COGTEL): A brief, reliable, and valid tool for capturing interindividual differences in cognitive functioning in epidemiological and aging studies. Dement. Geriatr. Cogn. Dis. Extra 2017, 7, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J. Fallproof!: A Comprehensive Balance and Mobility Training Program, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2010; ISBN 978-0-7360-6747-8. [Google Scholar]
- Marfell-Jones, M.; Olds, T.; Stew, A.; Carter, L. International Standards for Anthropometric Assessment; International Society for Advancement of Kinanthropometry: Potchefstroom, South Africa, 2018. [Google Scholar]
- Bischkopf, J.; Busse, A.; Angermeyer, M.C. Mild cognitive impairment–areview of prevalence, incidence and outcome according to current approaches. Acta Psychiatr. Scand. 2012, 196, 403–414. [Google Scholar]
- Cohen, J. Set correlation and contingency tables. Appl. Psychol. Meas. 1988, 12, 425–434. [Google Scholar] [CrossRef]
- Fradelos, E.; Papathanasiou, I.; Mitsi, D.; Tsaras, K.; Kleisiaris, C.; Kourkouta, L. Health based Geographic Information Systems (GIS) and their applications. Acta Inform. Med. 2014, 22, 402. [Google Scholar] [CrossRef] [Green Version]
- Miranda Goncalves, R.; Moreira Domingos, I. Riverside population in Amazonas and inequality in access to health. Rev. Estud. Const. Hermenêutica E Teor. Do Direito 2019, 11, 99–108. [Google Scholar]
- Aracaty, M.L.; de Souza Rojas, S.R. Índice de vulnerabilidade Social vulnerability index (IVS) of the metropolitan regions of Belém do Pará-PA (RMB) and Manaus-AM (RMM). Econ. E Desenv. 2021, 33, 1–22. [Google Scholar]
- Laukka, E.J.; MacDonald, S.W.S.; Fratiglioni, L.; Bäckman, L. Preclinical cognitive trajectories differ for Alzheimer’s disease and vascular dementia. J. Int. Neuropsychol. Soc. 2012, 18, 191–199. [Google Scholar] [CrossRef]
- Buracchio, T.; Dodge, H.H.; Howieson, D.; Wasserman, D.; Kaye, J. The trajectory of gait speed preceding mild cognitive impairment. Arch. Neurol. 2010, 67, 980–986. [Google Scholar] [CrossRef]
- Soumare, A.; Tavernier, B.; Alperovitch, A.; Tzourio, C.; Elbaz, A. A Cross-sectional and longitudinal study of the relationship between walking speed and cognitive function in community-dwelling elderly people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64A, 1058–1065. [Google Scholar] [CrossRef] [Green Version]
- Watson, N.L.; Rosano, C.; Boudreau, R.M.; Simonsick, E.M.; Ferrucci, L.; Sutton-Tyrrell, K.; Hardy, S.E.; Atkinson, H.H.; Yaffe, K.; Satterfield, S.; et al. Executive function, memory, and gait speed decline in well-functioning older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65A, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, L.J.H.; Caspi, A.; Ambler, A.; Broadbent, J.M.; Cohen, H.J.; D’Arbeloff, T.; Elliott, M.; Hancox, R.J.; Harrington, H.; Hogan, S.; et al. Association of neurocognitive and physical function with gait speed in midlife. JAMA Netw. Open 2019, 2, e1913123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosano, C.; Aizenstein, H.J.; Studenski, S.; Newman, A.B. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2007, 62, 1048–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allali, G.; Annweiler, C.; Predovan, D.; Bherer, L.; Beauchet, O. Brain volume changes in gait control in patients with mild cognitive impairment compared to cognitively healthy individuals; GAIT study results. Exp. Gerontol. 2016, 76, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.A.; Wise, S.P. Interactions between orbital prefrontal cortex and amygdala: Advanced cognition, learned responses and instinctive behaviors. Curr. Opin. Neurobiol. 2010, 20, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Makizako, H.; Shimada, H.; Doi, T.; Yoshida, D.; Ito, K.; Kato, T.; Shimokata, H.; Washimi, Y.; Endo, H.; Suzuki, T. The association between decline in physical functioning and atrophy of medial temporal areas in community-dwelling older adults with amnestic and nonamnestic mild cognitive impairment. Arch. Phys. Med. Rehabil. 2011, 92, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Beare, R.; Phan, T.G.; Blizzard, L.; Thrift, A.G.; Chen, J.; Srikanth, V.K. Brain structural change and gait decline: A longitudinal population-based study. J. Am. Geriatr. Soc. 2013, 61, 1074–1079. [Google Scholar] [CrossRef]
- Montero-Odasso, M.M.; Barnes, B.; Speechley, M.; Muir Hunter, S.W.; Doherty, T.J.; Duque, G.; Gopaul, K.; Sposato, L.A.; Casas-Herrero, A.; Borrie, M.J.; et al. Disentangling cognitive-frailty: Results from the gait and brain study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1476–1482. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Verghese, J.; Holtzer, R. A comparison of two walking while talking paradigms in aging. Gait Posture 2014, 40, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Breitling, L.P.; Müller, H.; Stegmaier, C.; Kliegel, M.; Brenner, H. Association of prion protein with cognitive functioning in humans. Exp. Gerontol. 2012, 47, 919–924. [Google Scholar] [CrossRef]
- Noce Kirkwood, R.; de Souza Moreira, B.; Mingoti, S.A.; Faria, B.F.; Sampaio, R.F.; Alves Resende, R. The slowing down phenomenon: What is the age of major gait velocity decline? Maturitas 2018, 115, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.; Lusardi, M. White Paper: “Walking Speed: The Sixth Vital Sign”. J. Geriatr. Phys. Ther. 2009, 32, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; An, Y.; Resnick, S.M.; Studenski, S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: Results from the Baltimore Longitudinal Study of Aging. Age Ageing 2016, 46, 445–451. [Google Scholar] [CrossRef] [Green Version]
| Variable | Full Sample (n = 697) | Cognitive Impairment (n = 331) | Normal Cognition (n = 366) | p-Value |
|---|---|---|---|---|
| Age in years (median, IQR) | 69.2 (60.00–91.84) | 79.8 (60.24–91.84) | 67.9 (60.00–89.46) | <0.001 |
| 60–69 n (%) | 339 (48.6) | 139 (42.0) | 200 (54.6) | |
| 70–79 n (%) | 274 (39.3) | 136 (41.1) | 138 (37.7) | |
| ≥80 n (%) | 84 (12.1) | 56 (16.9) | 28 (7.7) | |
| Gender n (%) women | 430 (61.7) | 187 (55.7) | 243 (67.3) | 0.002 |
| BMI (kg/m2) (median, IQR) | 27.6 (16.35–47.48) | 26.9 (16.36–47.00) | 28.5 (16.35–47.48) | <0.001 |
| Years of education (median, IQR) | 4.0 (0.00–23.00) | 0.0 (0.00–16.00) | 8.0 (0.00–23.00) | <0.001 |
| Number of meds (median, IQR) | 2.0 (0.00–13.00) | 2.0 (0.00–10.00) | 1.5 (0.00–13.00) | 0.190 |
| Falls n (%) | 227 (32.38) | 111 (33.5) | 116 (31.7) | 0.605 |
| MMSE (median, IQR) | 25.0 (11.00–30.00) | 22.0 (11.00–30.00) | 27.0 (15.00–30.00) | <0.001 |
| Comorbidities n (%) | ||||
| Heart disease | 395 (56.7) | 191 (57.7) | 204 (44.3) | 0.328 |
| Hearing impairment | 180 (25.8) | 81 (24.5) | 99 (27.0) | 0.245 |
| Visual impairment | 582 (83.5) | 268 (81.0) | 314 (85.8) | 0.054 |
| Musculoskeletal disease | 295 (42.3) | 112 (33.8) | 183 (50.0) | <0.001 |
| Cerebrovascular disease | 32 (4.6) | 16 (4.8) | 16 (4.4) | 0.455 |
| Depression | 32 (4.6) | 17 (5.1) | 15 (4.1) | 0.318 |
| Variable | Full Sample (n = 697) | Cognitive Impairment (n = 331) | Normal Cognition (n = 366) | p-Value |
|---|---|---|---|---|
| Gait usual speed (m/s) (median, IQR) | 1.23 (0.35–3.20) | 1.08 (0.35–2.62) | 1.47 (0.55–3.20) | <0.001 |
| Gait fast speed (median, IQR) | 1.64 (0.45–4.17) | 1.45 (0.45–3.44) | 1.99 (0.73–4.17) | <0.001 |
| Gait usual speed n (%) | ||||
| 1 (lowest) | 157 (22.5) | 121 (36.0) | 36 (0.9) | 0.195 |
| 2 | 191 (27.4) | 116 (32.1) | 75 (20.7) | 0.270 |
| 3 | 173 (24.8) | 67 (19.9) | 106 (29.3) | 0.003 |
| 4 (highest) | 176 (25.2) | 27 (8.0) | 149 (41.2) | 0.299 |
| Gait fast speed n (%) | ||||
| 1 (lowest) | 162 (23.2) | 115 (34.7) | 47 (12.8) | 0.014 |
| 2 | 180 (25.8) | 112 (33.8) | 68 (18.6) | 0.117 |
| 3 | 176 (25.3) | 76 (23.0) | 100 (27.3) | 0.470 |
| 4 (highest) | 179 (25.7) | 28 (8.5) | 151 (41.3) | 0.028 |
| Variable | Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Usual gait c (m/s) * | −0.451 (−0.553–0.410) | <0.001 | −0.180 (−0.264–0.120) | <0.001 |
| Fast speed c (m/s) * | −0.433 (−0.384–0.280) | <0.001 | −0.150 (−0.166–0.063) | <0.001 |
| Usual speed (m/s) † | ||||
| Quartile 4 (highest) | 1 | 1 | <0.001 | |
| Quartile 3 | 3.488 (2.091–5.817) | <0.001 | 1.773 (0.953–3.297) | 0.071 |
| Quartile 2 | 8.535 (5.165–14.106) | <0.001 | 2.977 (1.611–5.499) | <0.001 |
| Quartile 1 (lowest) | 18.548 (10.663–32.266) | <0.001 | 4.099 (2.088–8.048) | <0.001 |
| Fast speed (m/s) † | ||||
| Quartile 4 (highest) | ||||
| Quartile 3 | 4.10 (2.482–6.768) | <0.001 | 2.33 (1.266–4.312) | <0.001 |
| Quartile 2 | 8.884 (5.369–14.695) | <0.001 | 3.05 (1.636–5.705) | <0.001 |
| Quartile 1 (lowest) | 13.200 (7.790–22.352) | <0.001 | 3.15 (1.640–6.060) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, M.d.M.; Gouveia, É.R.; Marques, A.; Gouveia, B.R.; Marconcin, P.; Ihle, A. Gait Speed as a Biomarker of Cognitive Vulnerability: A Population-Based Study with Cognitively Normal Older Adults. Sustainability 2022, 14, 7348. https://doi.org/10.3390/su14127348
Nascimento MdM, Gouveia ÉR, Marques A, Gouveia BR, Marconcin P, Ihle A. Gait Speed as a Biomarker of Cognitive Vulnerability: A Population-Based Study with Cognitively Normal Older Adults. Sustainability. 2022; 14(12):7348. https://doi.org/10.3390/su14127348
Chicago/Turabian StyleNascimento, Marcelo de Maio, Élvio Rúbio Gouveia, Adilson Marques, Bruna R. Gouveia, Priscila Marconcin, and Andreas Ihle. 2022. "Gait Speed as a Biomarker of Cognitive Vulnerability: A Population-Based Study with Cognitively Normal Older Adults" Sustainability 14, no. 12: 7348. https://doi.org/10.3390/su14127348
APA StyleNascimento, M. d. M., Gouveia, É. R., Marques, A., Gouveia, B. R., Marconcin, P., & Ihle, A. (2022). Gait Speed as a Biomarker of Cognitive Vulnerability: A Population-Based Study with Cognitively Normal Older Adults. Sustainability, 14(12), 7348. https://doi.org/10.3390/su14127348










