1. Introduction
Starting from the early 20th century and the industrial revolution, an increasing trend of urbanization took place that continues to this date. According to the World Bank [
1], currently, over 55% of the world’s population (~4.2 billion inhabitants) live in urban areas while this value is projected to be increased by 1.5 times until 2045 and reach a total of 6 billion people. Although such scale of urban sprawl require major resource challenges, it is estimated to be also responsible for over 70% of the total greenhouse gas production and consume some 75% of global energy supply [
2]. The U.S. Energy Information Administration (EIA) [
3], estimates that about 30% of the total energy consumption of urban areas is directly used in buildings, mostly for cooling and heating purposes. To address this and follow sustainable means, recent advances in construction and building materials have attempted increasing the energy efficiency of buildings by utilizing highly porous composite materials that have a lower thermal conductivity [
4,
5,
6]. This includes a series of lightweight composite materials, such as lightweight and foam concretes produced through open-graded aggregates, porous aggregates, or a foaming agent [
7,
8].
Although porous composites are known to be energy efficient, their rate of energy retention is known to be considerably low [
9], since they only reduce the conductivity of materials and are not a mean to store thermal energy. This leads to inefficient use of energy for temperature control in buildings. As a result, recent studies have reported that the use of novel phase change materials (PCMs) can increase the energy retention rate significantly [
10] through their considerable capacity to retain thermal energy while changing their phases.
In general, PCMs refer to specific class of organic or inorganic compounds that have a low melting temperature and high latent heat of fusion [
11] that gives them the ability to absorb and release a large amount of thermal energy, as they change phase [
12]. This can be melting (or liquifying) and resolidifying, in case of solid-liquid PCMs, but it can also be merely softening or hardening in case of solid-solid PCMs [
13]. Nonetheless, due to certain volume change and other elemental impracticality, not all forms of PCMs can be used for construction purposes [
13]. In this sense, according to Ref. [
11], the selection of proper PCMs for successful use in buildings depends on various thermodynamic, chemical and economic criteria.
In general, recent studies (e.g., [
14]) have reported that fatty acid-based PCMs can be very suitable for thermal energy (or heat) storage applications due to their outstanding properties, such as cost effectiveness, high capacity for latent heat storage, thermal reliability, non-toxicity, low vapor pressure in the liquid phase, as well as negligible supercoiling and high stability [
15]. Nonetheless, since the melting and resolidifying temperature point of the mentioned pure PCMs are constant, using a combination of PCMs can provide further tailoring-ability to adjust the mentioned temperature points as needed, by using a mixed ratio of multiple PCMs [
15]. According to Refs. [
16,
17,
18], eutectic mixture of Capric (CA)-Palmitic acid (PA) can be used for this purpose due to their relatively high compatibility and proper performance for indoor thermoregulation in buildings. As reported by Ref. [
19], unlike certain organic PCMs, a combination of CA-PA does not produce separate freezing (or solidifying points), making the two an ideal group of PCMs to be used as composite.
Although organic PCMs have numerous benefits, they are known to be prone to leakage, and thus, requiring a specific packaging method, such as impregnation [
20], micro-encapsulation [
21], and adsorption [
22]. In either case, however, impregnating PCMs into a highly porous media, such as foam concrete, can be an effective way to avoid leakage, especially as the porous medium can adsorb the PCMs through capillary force and surface tension [
19]. To provide such conditions, this study adopted the use of recycled concrete powder which is known to be a solid waste material and have relatively high internal porosity [
23]. It is reported that concrete powder originating from construction and demolition (C&D) has an annual production of about 600 million tons, only in the United States [
24], and is considered as a solid waste material. Nevertheless, waste concrete powder is commonly considered as an inferior material for reuse, as a recycled construction material due to its high porosity and weak interfacial transition zone (ITZ) with other aggregates, resulting in reduced physico-mechanical properties [
25,
26].
Nonetheless, its use in foam concrete have been recently practiced (e.g., [
26,
27]) and often found beneficial since it generally reduces pore connectivity and settlement tendency of foams while it does not significantly increase the conductivity of the produced foams since it is a porous byproduct [
23,
28,
29]. In general, foam concrete refers to a class of lightweight concrete with significantly reduced density and very high degree of porosity [
6,
8,
30,
31] that has major uses as insulating composite materials. Nonetheless, it is known that foams are incapable of retaining a considerable amount of thermal energy and its use is merely for insulation purposes.
To date, however, no study has used recycled concrete powder to be impregnated by PCMs in foam concrete. Ref. [
32], for instance, utilized expanded clay, as lightweight aggregates to micro-encapsulate paraffin but had to polymer coat the aggregates to avoid leakage. Similarly, Ref. [
33] impregnated paraffin into expanded clay and pumice and reported that such combination can increase the life span of bridge and the major infrastructure by at least a few years due to its high effectiveness in reducing the adverse effect of freeze thawing. However, the mentioned study again used porous aggregates as a means to host PCMs and did not provide further information on the leakage of the produced materials, neither studied the actual thermal properties of the produced cementitious composites. In addition, Ref. [
34] impregnated paraffin into diatomite and reported a maximum latent heat absorption of 70.51 J/g. Similar set of materials have also been used by Ref. [
35] and reported that up to 67% of the PCM has been leaked when impregnated into diatomite, suggesting alternative means for the impregnation of PCMs. Similarly, Hunger et al. [
36] used waste marble powder as encapsulating agent of paraffin and reported the optimum rate of paraffin inclusion is 3% from which the physico-mechanical properties are significantly impacted. Other studies (e.g., [
37,
38]), impregnated PCMs similarly in porous media and reported enhanced thermal retention of the produced concretes, as well as documenting certain leakage shortcoming.
As can be seen in the above literature review, although various techniques have been used to suitably incorporate PCMs in concrete, to date, no attempt has been done to use recycled concrete powder as a means for storing a combination of CA-PA. This combination can lead to enhanced thermo-physical properties, as compared to ordinary foams and other studies. Similarly, since the inclusion of recycled concrete powder is reported to lower the pore connectivity [
39,
40,
41], this can also avoid PCMs leakage problems. As a result, this study, for the first time, studied the impregnation of a mixed combination of PCMs into recycled concrete powder, since recycled concrete powder has the ability to adsorb and host PCMs in liquid form. To suitably evaluate the properties of the produced samples, a series of physico-mechanical, thermal and microstructural tests have been conducted and reported. Additionally, specifically designed tests have been carried out to evaluate a detailed thermal performance of the produced samples. Further information, regarding the methodology of this research can be found in the following sections.
4. Conclusions
To increase energy efficiency of buildings, porous composites have been used for many decades with successful results in reducing the thermal conductivity of buildings. Nonetheless, various porous composites are known to have little capacity to store thermal energy which reduces their effectiveness in lowering the need for energy used to heat or cool the buildings. To address this, recent studies have documented favorable results when used various forms of PCMs, which are found beneficial in reducing the temperature intensity at peak hours due to their high capacity for latent heat storage. Despite this, PCMs have the potential to be leaked out of the produced porous composites when in liquid phase, which requires a specific impregnation method for their actual application. To address this and provide a more flexible melting and solidifying point, this study used a combination of Capric (CA)-Palmitic acid (PA) PCMs and impregnated into recycled concrete powder, which is known for its high content of fine-sized porosity. To evaluate the physico-mechanical, thermal and microstructural properties of the produced foamed composites, a series of tests have been conducted from which, the following conclusions can be drawn:
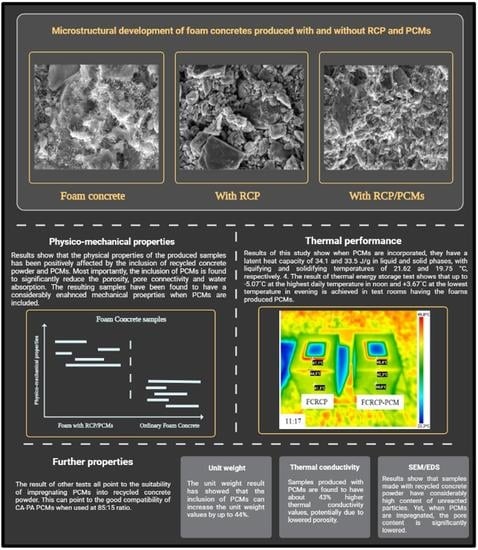
Based on the trials, the highest ratio of recycled concrete powder to PCM without leaking is found to be at about 20% (by weight). The results of DSC analysis show that the RCP/PCM composite produced this way has a latent heat capacity of 34.1 and 33.5 J/g in liquid and solid phases, with melting and solidifying temperatures of 21.62 and 19.75 °C, respectively. In addition, after about 300 cycles of liquifying and solidifying, the latent heat capacity is found to remain almost the same which shows the high cycling stability of the produced RCP/PCM composite.
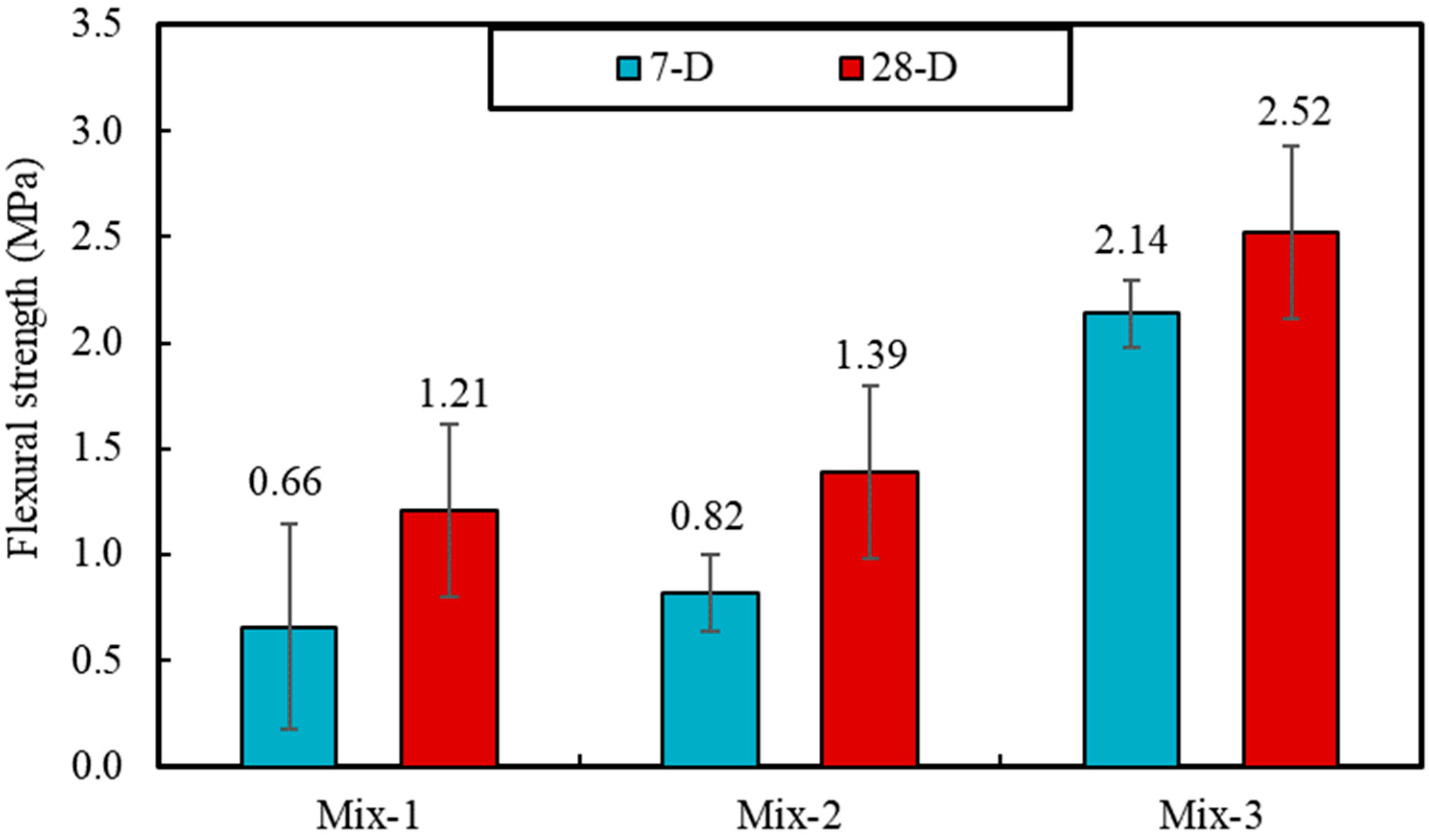
The results of materials tests including unit weight, porosity, water absorption, all point to the lowered porosity and potentially pore network connectivity for samples produced with PCMs, compared to foams with silica sand. For instance, the impregnation of PCMs is found to reduce porosity and the capillary water absorption by 32% and 360%, respectively, when it is compared to foam samples produced with silica sand. This also causes some 44% increase in unit weight values, as well as 204% and 108% increase for compressive and flexural strength values (28-day), respectively. Despite such significant changes, the result of thermal conductivity shows only ~43% increase in the thermal conductivity values of mixes produced with PCMs, which can be associated with significantly lowered porosity values.
The result of SEM shows that when foams are produced with silica sand, the pores and cracks are relatively deep and often interconnected. Nonetheless, when recycled concrete powder is used, the overall content of pores is found to become slightly lowered but a high content of unconnected particles can be seen which represents a weak ITZ. After PCMs are impregnated into the recycled concrete powder, however, the pores are found to be highly disconnected and often better dispersed. With regards to the EDS test, the foams produced with silica sand are found to have the highest content of calcium. In turn, upon the inclusion of recycled concrete powder, very high content of carbon is observed in its microstructure. The reason for this can be high carbonation tendency of the produced samples. When PCMs are impregnated, however, the Ca, C and Si content is found to shift toward the mix with silica sand, pointing to the fact that the inclusion of PCMs has reduced carbonation tendency of the produced samples.
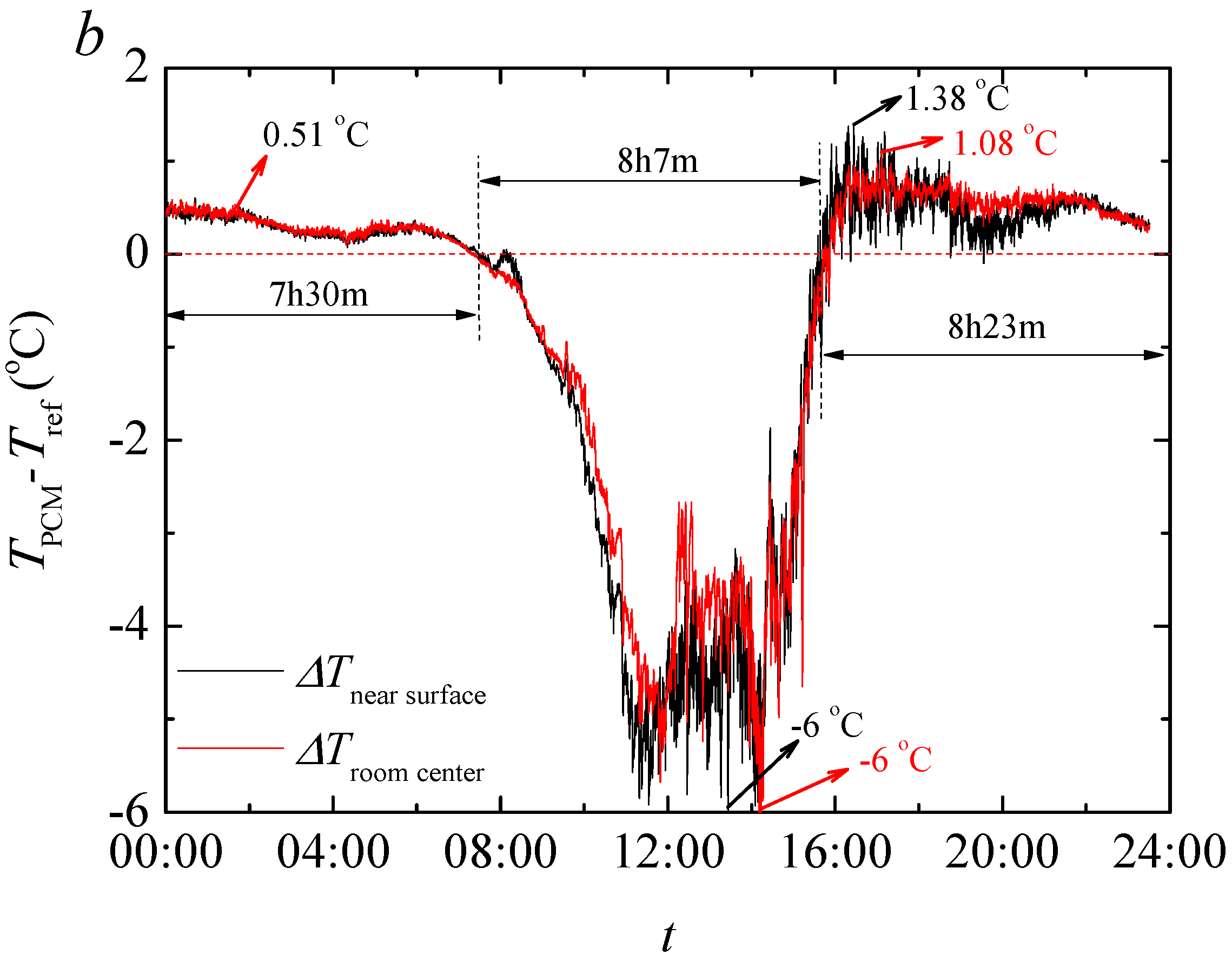
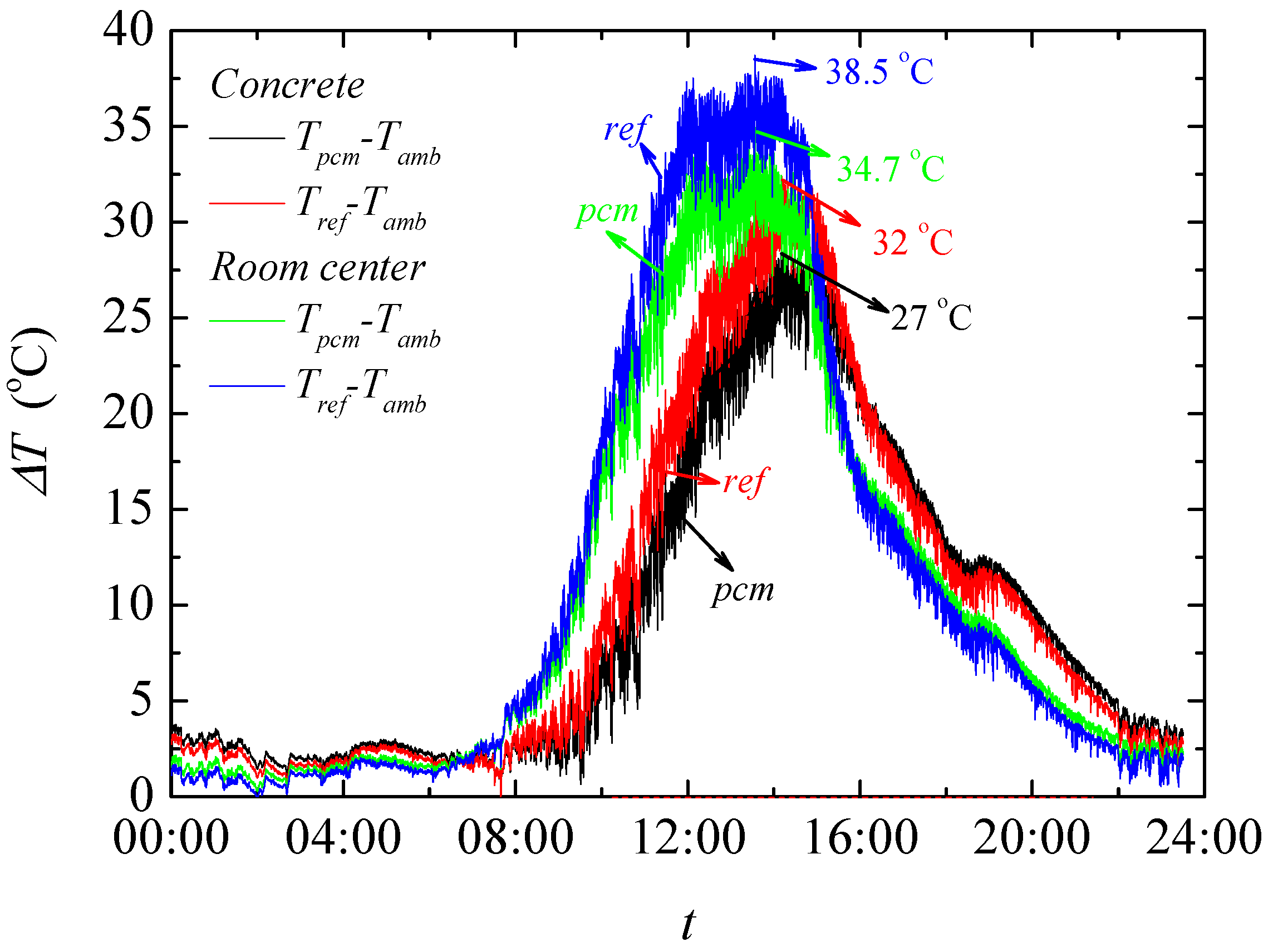
The result of temperature regulation performance test shows that up to −5.07 °C at the highest daily temperature in noon and +3.67 °C at the lowest temperature in evening is achieved in test rooms having the foams produced PCMs. It is also found that PCMs reduces the actual peak temperature time which further helps increasing the building energy efficiency in any climatic situation. This is further confirmed through the thermal camera results that showed up to 2.2 °C lower temperature on the surface of the produced rooms when PCMs are used.
The result of this study is found to be significant and point to the successful use of recycled concrete powder to host PCMs. Nonetheless, the future studies, can use alternative forms of PCMs with other hosting agents, or microencapsulation techniques to provide a comparative evaluation of impregnated PCMs and their respective impact on the actual thermo-regulative performance of buildings.