Abstract
Mechanochemical treatment is an effective and ecological way to treat medium and low-grade phosphate rock (PR) for fertilizer. To explore the phosphorus (P) solubility mechanisms of mechanochemically activated phosphate rock (MAPR) and its P supply capacity, we investigated the chemical properties and infrared spectroscopy (IR) characteristics of different types of MAPR and soil-available P using the chemical extraction method (2% citric acid), IR analysis and soil incubation experiment in this study. The results showed that the P solubility of magmatic, metamorphic and sedimentary types of MAPR increased by 7.76–13.67, 0.6–1.64 and 0.91–2.68 times, respectively, compared with the initial PR. The IR analysis demonstrated that the isomorphous substitution (B-type of CO32− for PO43− and OH− for F−) occurred in the apatite and β-Ca(PO3)2 was generated with better P solubility. The dynamic changes in soil-available P (Bray and Kurtz method) treated by MAPR significantly increased by 3.81–6.57-, 2.49–5.4- and 4.98–8.39-fold, respectively, within 100 days in red soil (pH 5.94), compared with the initial PR. In conclusion, the significant increase in P solubility of MAPR and soil-available P in weakly acidic soil was due to the improved reactivity of MAPR during the process of mechanochemical activation.
1. Introduction
Phosphorus (P) is one of the three essential elements for crops and is affected by resource constraints [1,2]. The application of P chemical fertilizer has become a conventional agricultural production management measure to maintain a high crop yield [2]. Phosphate rock (PR) is the raw material used to manufacture P fertilizer. The depletion of high-grade PR and the increased harm to the environment during the production of P chemical fertilizer are the main issues faced by countries worldwide. The strategy of directly using medium- and low-grade (P < 6.11%) PR to develop P fertilizer substitutes is an inevitable choice for many countries in the world [3,4]. Due to its very low solubility and reactivity, the direct application of medium- and low-grade PR is limited [5], especially when applied to crops in the short growing period, which is usually ineffective [6]. The P dissolution and plant availability of PR to crops are the main characteristics required for the successful usage of these P sources for direct applications in agriculture usage.
Mechanochemistry technology refers to the method used to prepare innovative materials or modify materials using mechanical energy to induce chemical reactions and changes in the structure and properties of materials [7,8]. Compared to the traditional acid method used to activate PR, mechanochemical activation treatment has the advantages of a simple process, ecological cleanness, and no flotation or chemicals [9], which usually improves the reactivity of natural or synthetic apatite minerals [9,10]. Therefore, many researchers have paid attention to mechanochemical activation to directly treat medium- and low-grade PR [9,10,11,12,13]. Studies have shown that the extractable P content and P solubility rate (in 2% citric acid) significantly increased [9,13]. This is the reactivity of natural or synthetic apatite minerals, which increases due to mechanochemical activation [11,12,13]. A previous study was performed to determine the physical and chemical properties of Huangmailing phosphate rock using scanning electron microscopy (SEM) and X-ray diffraction (XRD) methods before and after mechanochemical activation (milling for 21 min) [10]. This concluded that mechanochemical activation changed the crystal structure of apatite and replaced the channel ions but did not involve the specific type and process of isomorphous substitution. Similarly, Fang et al. [13] analyzed the correlation between the P solubility and the physical, chemical properties of sedimentary (Yichang) mechanochemically activated phosphate rock (MAPR) using SEM, XRD and infrared spectroscopy (IR). There are three major types of original phosphate deposits, including magmatic, metamorphic, and sedimentary phosphate deposits [14]. The mineral composition of sedimentary phosphate deposits is mainly collophanite with relatively dense structure, often containing a small amount of quartz, dolomite, etc. The composition of magmatic phosphate deposits is mainly pyrogenic apatite with a low grade and coarse crystallization [15], associated with orthoclase and pyroxene, etc. The composition of metamorphic deposits is mainly apatite with a high weathering degree, loose structure and high mud content, and contains low amounts of amphibole, mica and so on [16]. Hence, it is very necessary to evaluate the effectiveness and study the mechanisms of mechanochemical activation among different types of PR, because of the considerable differences in mineral composition and P solubility.
Few studies have focused on the agronomic effects that MAPR has on soil and crops. Our previous results showed that a combined application treated with 10–20% MAPR and 80–90% TSP had the same effectivity as 100% triple superphosphate (TSP) in increasing maize yield, phosphorus uptake and P efficiency, although it could not significantly increase the soil-available P in Luvisol (pH 6.47) soil in Northeast China for three consecutive years [17]. Another study reported improvements in the activation properties of PR with different lignite (as an activator) ratios (1%, 2%, 3%, and 5%) during the grinding process, to prepare for the microcrystallized PR. The dry matter of rapeseed and the soil-available P increased by 56.1% and 89.6%, respectively, with the direct use of PR and micro-crystallized PR in an aqui-cinnamon soil (pH 8.2), compared with the control test without the addition of phosphate minerals [18]. We proved that this was a feasible solution, with a 10–20% substitution of MAPR for P fertilizer on spring maize in the Northeast China for nearly neutral soil (pH 6.47). For soil-available P, a 10% substitution of MAPR was equally operative, compared with 100% TSP [17]. Further studies are needed to establish the performance of MAPR and its P supply capacity in different soils.
In the present study, three different types of medium- and low-grade PR, including magmatic, metamorphic and sedimentary PR, will be milled by a high-energy planetary ball in the short timeframe of 0–60 min. To explore the P-solubility mechanisms in the process of mechanochemical activation, the chemical properties of different types of MAPR, their effects on soil-available phosphorus and their infrared spectroscopy (IR) characteristics will be determined by chemical extraction methods, soil incubation experiment and IR analysis.
2. Materials and Methods
2.1. Phosphorus Solubility in Citric Acid Determination
The elementary chemical composition of magmatic (A, from Beipiao in Liaoning Province, China), metamorphic (B, from Shimen in Hunan Province, China) and sedimentary PR (C, from Shimen in Hunan Province, China) is given in Table 1.

Table 1.
Elementary chemical composition of different types of phosphate rock.
The processing schedule of all of the types of MAPR was as follows.
Firstly, the large PR was crushed by the jaw crusher and turned into the small pieces shown below (<6 cm). Then, the small pieces were finely ground by the ball mill and screened through 100 mesh as inactivated PR. The mechanochemical activation equipment used in this study included a planetary ball mill with two 420 cm3 grinding chambers at a grinding speed of 1500 rpm, custom-built by the Transformation Center of China and Russia in Jiaxing Zhejiang Province in 2011. Each grinding chamber was filled with 5 small steel balls (totally weighed 150 g) and 5 g PR with a grinding medium of 18 mm in diameter. The grinding times were set as 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 and 60 minutes (min).
The extractable P content (Pext) in PR was determined by the dissolution of P in 2% citric acid and the colorimetric method of vanadium and molybdenum by spectrophotometer (UV1100II, Techcomp Co., Ltd., Shanghai, China) [19]. The total P (TP) of PR was established by citric acid extraction after digestion with 10–15 mL HNO3 and colorimetric method of vanadium and molybdenum by spectrophotometer (UV1100II, Techcomp Co., Ltd., Shanghai, China) [19]. The P solubility rate in citric acid (RPext) in PR was calculated according to the following formula [9]:
Pext refers to extractable P content and TP refers to total P.
2.2. Infrared Spectroscopy Analysis
The spectral characteristics of MAPR obtained using KBr pellets were examined in the spectral range of 450–4000 cm−1 using infrared spectrometer (Perkin-Elmer-FT-1730, California, USA). A resolution of 4 cm-1 was used, with 16 scans collected for each sample.
2.3. Soil Incubation Experiment
The tested soil (red soil, 0–20 cm) was collected from Hunan Academy of Agricultural Sciences, with pH 5.94, total N 0.75 g kg−1, total P 0.07 g kg−1, total K 0.26 g kg−1, available N 10.53 mg kg−1, available P 20.36 mg kg−1, available K 92.51mg kg−1 (Table 2). The tested PR was the initiated magmatic PR (A), the initial metamorphic PR (B), the initial sedimentary PR (C), the magmatic MAPR activated for 30 min (MA), the metamorphic MAPR activated for 30 min (MB) and the sedimentary MAPR activated for 30 min (MC). Treatments included: no fertilizers (CK), A, MA, B, MB, C and MC (Table 3). A total of 500g of air-dried soil (through a 2-mm sieve) was put into a plastic pot with a diameter of 15cm. According to the amount of equal pure P (491.20 kg P ha−1), MAPR and PR were added, respectively, at a constant temperature (25 °C). The soil water content was maintained at about 20% using the weight method. Soil samples were collected from each pot 10 times on the 5th, 15th, 25th, 35th, 45th, 55th, 65th, 75th, 85th and 100th day from 12 April to 21 July in 2018. Then, soil-available P was extracted by Bray and Kurtz method using a mixed solution of 0.025 mol L−1HCl and 0.03mol L−1NH4F [20]. The P in the filtrate was then calorimetrically determined using the molybdate method by spectrophotometer (UV1100II, Techcomp Co., Ltd., Shanghai, China) [21].

Table 2.
Physiochemical properties of the tested soil.

Table 3.
Dosages of phosphate rock applied in different treatments: (g pot−1).
2.4. Data Analysis and Statistics
An analysis of variance (ANOVA) was used to test the effect of treatments on soil-available P. The relationships between P solubility and milling times was tested through non-linear regression model. All of the statistical analysis was conducted by SPSS 16.0 (Chicago, IL, USA) and the accepted statistical significance level was P < 0.05. All data were processed and mapped by OriginLab 8.6 program (Northampton, MA, USA). The infrared spectrum of MAPR and PR was mapped using the wave number and transmittance data by OriginLab 8.6 program. Then, the different vibration modes of functional groups of PR (such as PO43−, CO32−, OH− ions) were compared between the determined spectrum and the standard ones [9] to identify the existence of the functional groups.
3. Results
3.1. Variations in the Extractable P Content and P Solubility Rate of Mechanochemically Activated Phosphate Rock
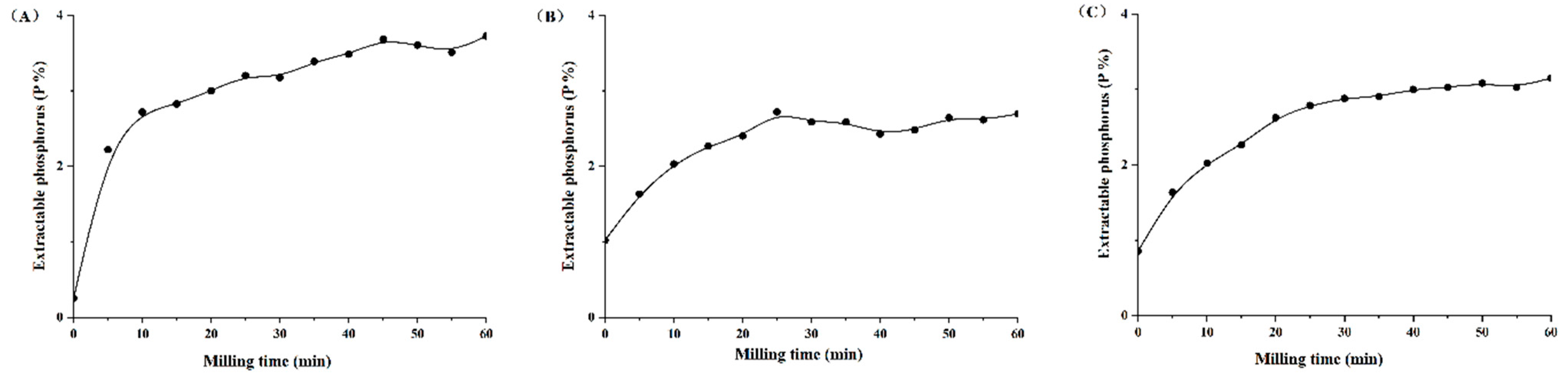
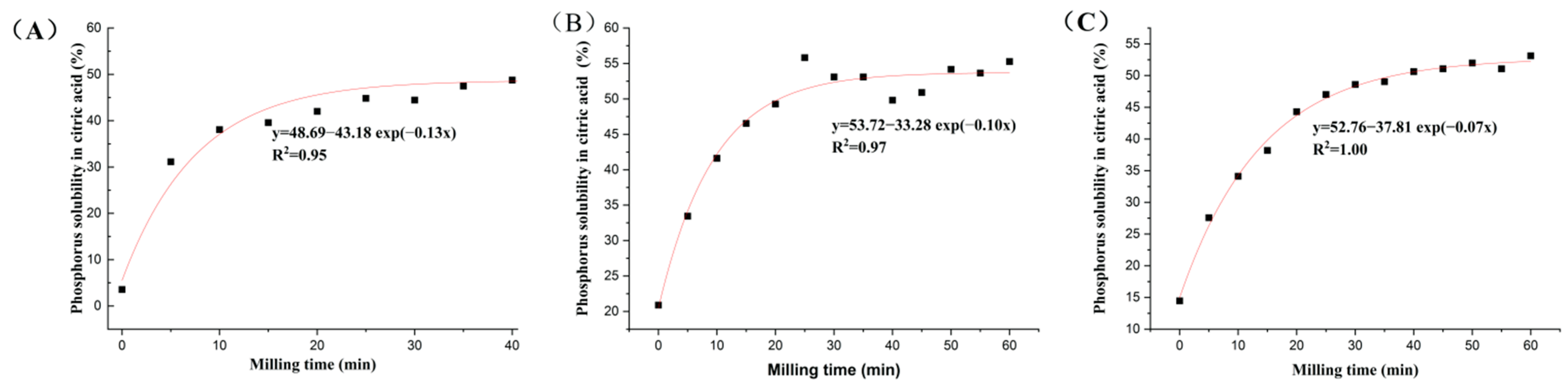
Both the extractable P content (Pext) and P solubility rate (RPext) were significantly increased after mechanochemical activation, with 0–60 min of different types of PR (Figure 1 and Figure 2). The Pext and RPext rapidly increased with the grinding time over the initial 30 min, and the growth rate slowed down from 30 to 60 min. This showed that grinding time had an important effect on Pext and RPext with the accurate processing parameters, such as the number of revolutions (1500 rpm), material and grinding medium ratio (30:1) and grinding medium diameter (18 mm). With the extension of mechanochemical activation times for magmatic, metamorphic and sedimentary PR, Pext was in the range of 0.25–3.72%, 1.02–2.72% and 0.86–3.15%, and RPext was within the scope of 4.35–63.83%, 20.91–55.80% and 14.44–53.14%, respectively. The P solubility in PR increased by 7.76–13.67-, 0.6–1.64- and 0.91–2.68-fold, respectively, compared with the initial PR. There were significant exponential relationships between P solubility rate (y) and milling times (x) (Figure 2, R2 = 0.95, 0.97, 1.00).
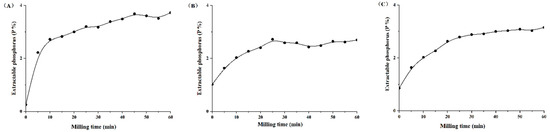
Figure 1.
Variations in extractable phosphorus content (Pext) in citric acid of mechanically activated phosphate rock with different grinding time. (A) Magmatic phosphate rock; (B) metamorphic phosphate rock; (C) sedimentary phosphate rock.
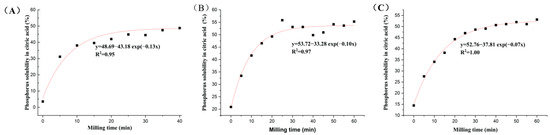
Figure 2.
The exponential relationships between P solubility rate in citric acid of mechanochemically activated phosphate rock and milling time. (A) Magmatic phosphate rock; (B) metamorphic phosphate rock; (C) sedimentary phosphate rock.
3.2. Changes in Surface Groups of Mechanochemically Activated Phosphate Rock
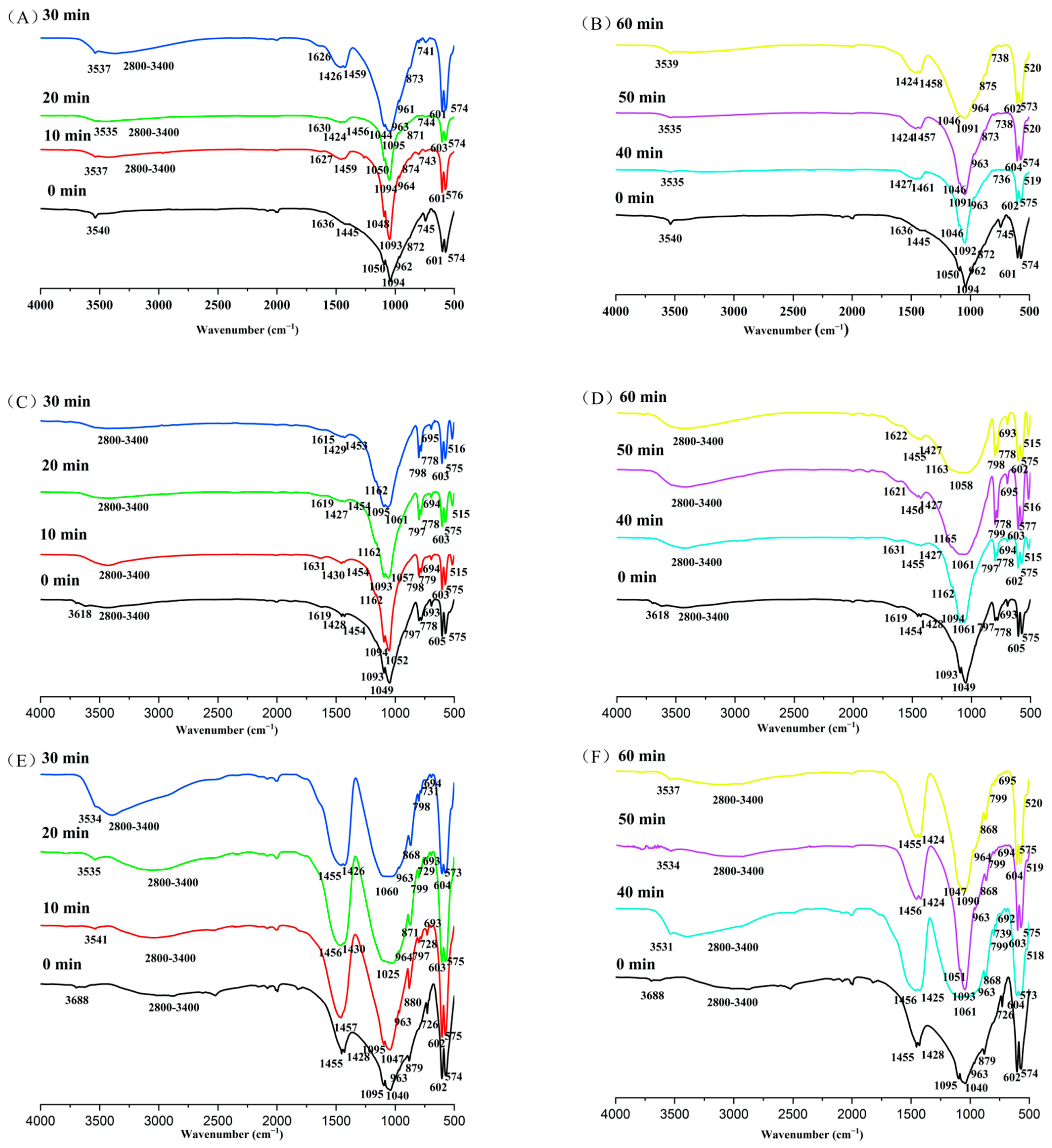
The infrared spectrum (IR) analysis (Figure 3, Table 4, Table 5 and Table 6) detected PR surface groups, such as OH−, CO32−, PO43− ions, as well as new peaks at different mechanical activation times in natural PR.
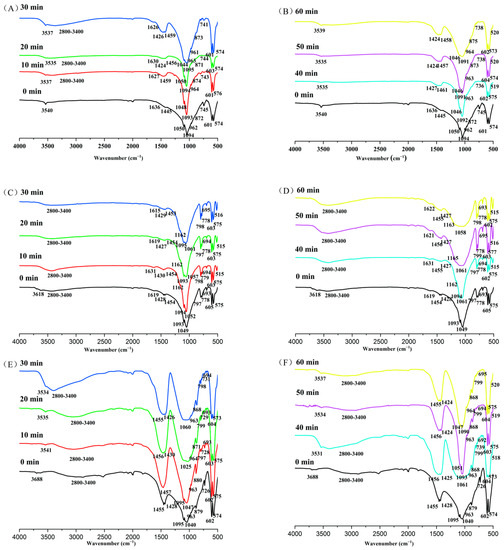
Figure 3.
The main IR spectra adsorption of phosphate rock with milling time. (A) Magmatic phosphate rock activated with 0–30 min; (B) magmatic phosphate rock activated within 40–60 min; (C) metamorphic phosphate rock activated within 0–30 min; (D) metamorphic phosphate rock activated within 40–60 min; (E) sedimentary phosphate rock activated within 0–30 min; (F) sedimentary phosphate rock activated within 40–60 min.

Table 4.
The main IR spectra adsorption of magmatic phosphate rock (A) with milling time.

Table 5.
The main IR spectra adsorption of metamorphic phosphate rock (B) with milling time.

Table 6.
The main IR spectra adsorption of sedimentary phosphate rock (C) with milling time.
The IR spectra of initial magmatic PR (Figure 3A, Table 4) showed that PO43− ions were found in the absorption bands of 960 cm−1 (ν1), and the two split absorption bands of 575–605 cm−1 (ν4) and 1050–1100 cm−1 (ν3). The 1600 cm−1 absorption bands proved the existence of adsorbed water. The OH− ion absorption bands (ν1 = 3540 cm−1) proved the existence of crystal structure water, indicating that there was similar hydroxyapatite (Ca5(PO4)3OH) in the magmatic PR. The Si–O–Si (ν1) absorption bands appeared near 770–800 cm−1, which proved that quartz was discovered in PR. With the extension of the activation time, the absorption bands of functional groups in the samples obviously changed (Figure 3A,B, Table 3). The strength of the stretching vibration absorption bands (ν4 = 572–604 cm−1 and ν1 = 960 cm−1) of PO43− ions decreased with the increase in the milling time. The asymmetric O–P–O (ν4) bending mode in β-Ca(PO3)2 appeared at 517 cm−1 after mechanochemical activation, within 40–60 min. The doubly degenerate asymmetric O–C–O (ν3 = 1400–1470 cm−1) stretching mode of B-type CO32− in CFAp (and/or CFOHAp) and/or CaCO3 appeared with mechanochemical activation from 20 to 60 min. With the increase in mechanical activation time (10, 20, 30 min), a symmetric OH− (ν1 = 2800–3390 cm−1) stretching mode was generated in crystal water. The degenerate symmetric OH− (ν2 = 1600 cm−1) bending mode in crystal water disappeared after grinding for more than 40 min (40, 50, 60 min).
The IR spectra of the initial metamorphic PR (Figure 3C, Table 5) showed that PO43− ions were also noted in the two absorption bands of 575–605 cm−1 (ν4) and 1050–1100 cm−1 (ν3). The absorption bands of carbonate ions were 1400–1470 cm−1 (ν3). The existence of 690 cm−1 absorption bands shows the vibration mode of OH− of hydroxyapatite (OHFAp) and/or fluorocarbon hydroxyapatite (CFOHAp). The OH− ion stretching absorption bands (ν1 = 3600 cm−1) demonstrated the existence of crystal structure water. 1600 cm−1 absorption bands proved the existence of adsorbed water. The IR results of the metamorphic PR after activation (Figure 3C,D, Table 4) showed that the O–P–O (ν4) stretching absorption bands appeared at 517 cm−1 with the increase in grinding time (10–60 min). The absorption bands of 960 cm−1 (ν1) emerged, and the absorption bands of OH− ion (3600 cm−1) disappeared in the apatite structure after mechanical activation within 10–60 min. The Si–O–Si (ν3) absorption bands were near 1162 cm−1 in the sample after grinding for more than 10 min.
The IR spectra of the initial sedimentary PR (Figure 3E, Table 6) proved that PO43− ions were similarly found in the absorption bands of 960 cm−1 (ν1), and the two split absorption bands of 575–605 cm−1 (ν4) and 1050–1100 cm−1 (ν3). The absorption bands of carbonate ions were 860–880 cm−1 (ν2) and 1400–1470 cm−1 (ν3). The absorption bands of 2800–3400 cm−1, 470 cm−1 and 726 cm−1 certified the presence of adsorbed water and SiO2, respectively. The results IR of the sedimentary PR after activation (Figure 3E,F, Table 5) showed that the O–P–O (ν4) stretching absorption bands came out at 517 cm−1 with the activation time (0–60 min). CO32− of the stretching vibration absorption bands (ν3 = 1400–1470 cm−1) divided into two peaks in the process of mechanochemical activation at 0, 20, 30, 40, 50, and 60 min. The 690 cm−1 absorption bands arose for mechanochemical activation within 10–60 min. New hydroxyl ion absorption bands (ν1 = 3530 cm−1) were detected in the apatite structure of MAPR in over 10 min. The absorption bands of Si–O–Si (ν1) emerged near 770–800 cm−1 after grinding, which provided evidence of quartz in PR.
3.3. Dynamic Release Characteristics of Soil-Available Phosphorus Applied with Mechanochemically Activated Phosphate Rock
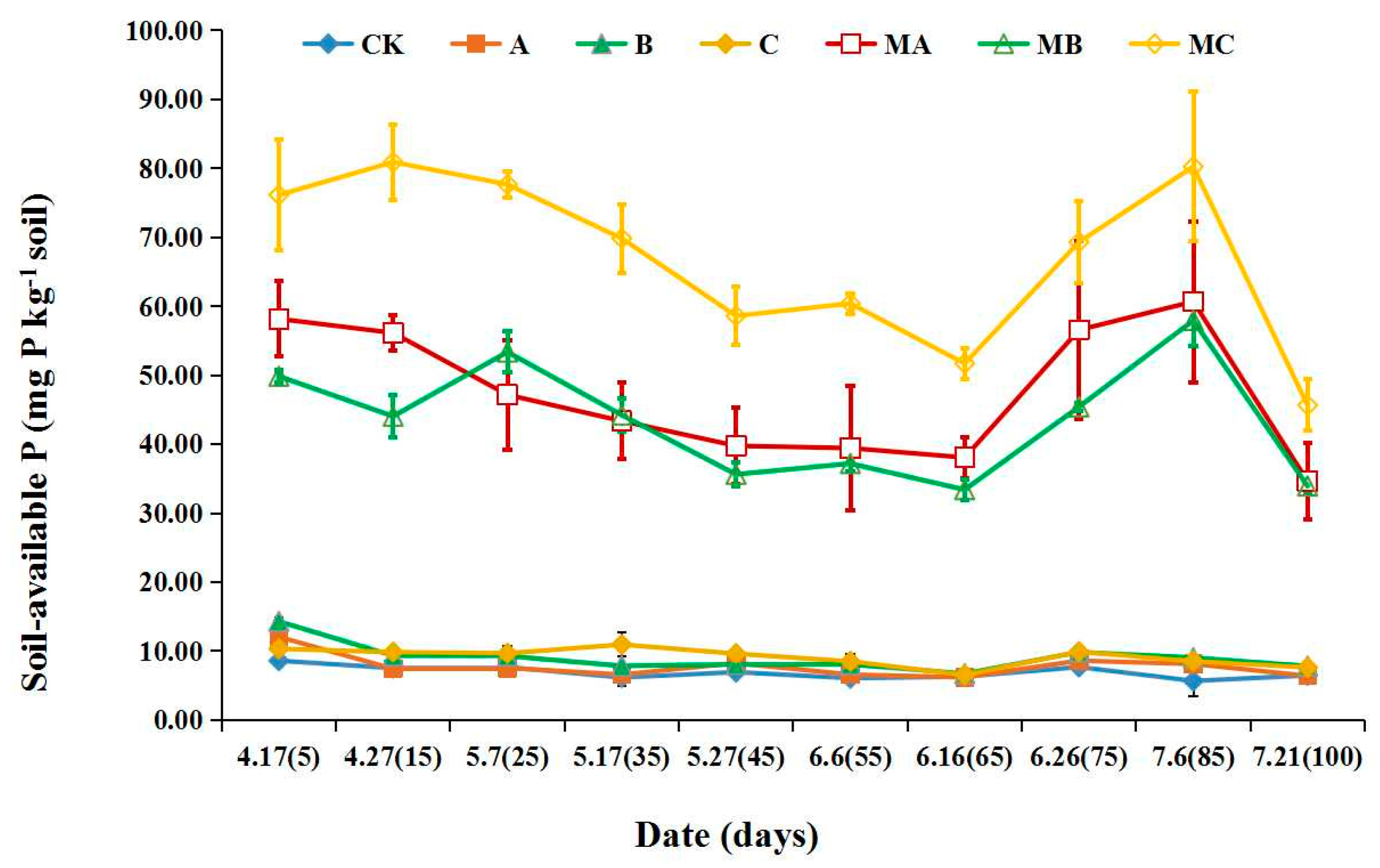
The dynamic changes in soil-available P within 100 days were significantly different with the application of initial PR and MAPR in red soil (pH 5.94) (Figure 4). The range of soil-available P was 6.41–12.06 mg P kg−1 soil, 6.73–14.03 mg P kg−1 soil, 6.54–10.95 mg P kg−1 soil after the application of initial PR (treatments of A, B, C). This was almost the same as the control treatment (CK), which was in the scope of 5.67–8.60 mg kg−1. When the three types of MAPR (MA, MB and MC) were added to red soil, soil- available P ranged from 34.64 to 60.67 mg kg−1 soil, 33.93–57.93 mg kg−1 soil and 45.65–80.90 mg kg−1 soil, which reached the highest values at the 85th, 85th and 15th days, respectively. Soil-available P decreased slowly with the incubation time, then increased at the 75th day and subsequently decreased. At the 100th day, the soil-available P were 34.64 mg kg−1 soil, 33.93 mg kg−1 soil and 45.65 mg kg−1 soil with the application of MA, MB and MC, respectively. The soil-available P with the MAPR (MA, MB and MC) treatments significantly increased by 3.81–6.57-, 2.49–5.4- and 4.98–8.39-fold, respectively, compared with the initial PR (A, B and C). This increased by 4.35–9.70-, 4.24–6.16- and 6.06–13.15-fold, respectively, compared with blank control (CK). For soil-available P, the treatment of sedimentary MAPR (MC) was the best, which was 0.23–0.65- and 0.39–0.84-fold higher than that of magmatic (MA) and metamorphic (MB) PR, respectively.
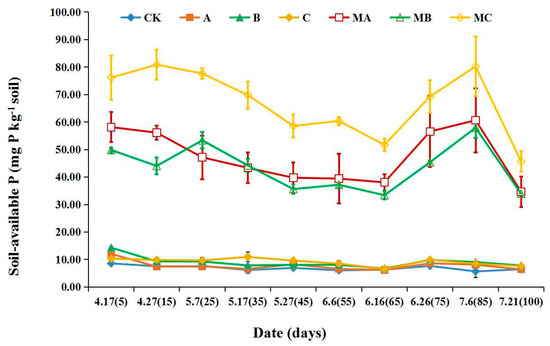
Figure 4.
Effects of mechanochemically activated phosphate rock applied on soil-available phosphorus. CK, Blank control; A, initial magmatic phosphate rock; B, initial metamorphic phosphate rock; C, initial sedimentary phosphate rock; MA, magmatic phosphate rock activated for 30 min; MB, metamorphic phosphate rock activated for 30 min; MC, sedimentary phosphate rock activated for 30 min.
4. Discussion
4.1. Effects of Mechanochemically Activated Phosphate Rock on P Solubility
MAPR can be directly used as phosphate fertilizer according to the calcium superphosphate national standard (GB20413-2006) with available P requirements of more than 2.62%. And with RPext regulation of more than 30%, it was considered to be reactive PR in New Zealand [22]. When the magmatic, metamorphic and sedimentary PR grinding with 10 min, 20 min, and 25 min, respectively, Pext reached 2.72%, 2.62%, and 2.72%, and RPext came up to 46.61%, 44.30% and 55.82%. Therefore, these three types of medium- and low-grade PR have the possibility of being applied as phosphate fertilizer after mechanochemical activation. The grinding efficiency in this study is much greater than that in previous studies (grinding time > 150 min) [9,11]. In our previous study, Yichang (sedimentary type) and huangmailing phosphate rock (metamorphic type) were milled by a planetary ball mill for 30 min and 21 min, respectively, and the maximum P solubility rates were 57.51% and 46.53%, respectively [10,13]. Furthermore, the grinding superiority was magmatic and metamorphic PR > sedimentary PR. This was consistent with the results of Jin et al. [10]. This could be related to the metallogenic conditions. The magmatic PR has a low grade and coarse crystallization, while the metamorphic PR has a high weathering degree, loose structure and high mud content. Sedimentary PR belongs to collophanite with a relatively dense structure [15], which is difficult to grind. Meanwhile, the effects of MAPR equivalent to P chemical fertilizer still need to be verified by further field experiments in different types of soil with various climates.
4.2. Effect of Mechanical Activation on Changes in the Surface Groups of Phosphate Rock
IR results showed that the surface functional groups of three types of PR changed before and after mechanochemical activation. The initial magmatic (A) and sedimentary (C) PR were mainly fluorapatite-containing CO32−. After mechanochemical activation for 20–60 min, an asymmetric O–C–O (ν3 = 1400–1470 cm−1) stretching mode of B-type CO32− in CFAp (and/or CFOHAp) and/or CaCO3 appeared, respectively, which demonstrated the B-type isomorphous substitution of CO32− [9,11,13], namely, the substitution of CO32− for PO43− tetrahedron. This was evidence of the incorporation of CO32− due to the destruction of calcite and dolomite crystals (CaCO3), not just CO2, from the air (2300 cm−1) during mechanochemical activation [9,13]. The emergence of 690 cm−1 absorption bands after mechanochemical activation for 10, 20, 30, 40, 50 and 60 min indicated the conversion of fluorapatite (FAp) to hydroxyapatite (OHAp) or/and carbonfluoro-hydroxyapatite (CFOHAp) [9,23]. This could be due to the incorporation of OH− into apatite, not only the absorption of water vapor in air, but also the isomorphous substitution of channel ion F− in fluorapatite (substitution of OH− for F−) [12]. Since hydroxyapatite was more soluble than fluorapatite, the existence of hydroxyapatite increased the P solubility in PR. This is consistent with our previous results [10,13]. For magmatic PR that was mechanochemically activated for 40–60 min, metamorphic PR activated for 10–60 min and sedimentary PR activated for 40–60 min, the asymmetric O–P–O (ν4) bending mode in β-Ca(PO3)2 at 517 cm−1 appeared. This indicated that the new phase β-Ca(PO3)2 was generated in PR after mechanochemical activation, which increased the P solubility. This is consistent with the results of Yaneva et al. (2009) [9]. In the process of mechanochemical activation, the absorption of Si–O–Si (ν1) of the three types of PR increased to near 800 cm−1, which is due to the high hardness of quartz (Mohs’scale of hardness, MSH = 7), thus maintaining its stability in the process of mechanochemical activation [24].
4.3. Effects of Mechanochemically Activated Phosphate Rock on Soil-Available P
Soil-available P content is an indicator of the level of soil phosphorus nutrient supply [25], which has direct guiding significance for fertilization. The results showed that MAPR (MA, MB and MC), when applied to red soil (pH, 5.94), could significantly increase soil-available P content by 3.81–6.57-, 2.49–5.4- and 4.98–8.39-fold, respectively, compared with the initial PR (A, B and C). This is mainly due to the fact that both the extractable P content (Pext) and P solubility rate (RPext) of MAPR significantly increased, and the isomorphous substitution (B-type of CO32− for PO43− and OH− for F−) occurred in all the apatite and β-Ca(PO3)2 generated with better P solubility during the process of mechanochemical activation [9,12,13]. Our previous results also verified that the combination of MAPR and triple superphosphate (at a ratio of 1:9) was comparable to that of 100% triple superphosphate treatment in a field experiment using spring maize in Luvisol (pH 6.47) soil in Northeast China for three consecutive years. In addition, soil-available P could reach 33.93–49.37 mg P kg−1 on the 100th day, which is considered to be the most suitable range of soil-available P content for crops [17]. As PR is more alkaline in general, and a low soil pH is generally favorable for PR dissolution [26], MAPR is more suitable for acidic soils. However, this needs further improvement.
5. Conclusions
In sum, the results of chemical extraction showed that the P solubility of magmatic, metamorphic and sedimentary type of MAPR was vastly improved, respectively, compared with the initial PR. This was due to the isomorphous substitution (B-type of CO32− for PO43− and OH− for F−) in apatite, and the β-Ca(PO3)2 was generated with better P solubility during the process of mechanochemical activation. This resulted in structural defects or a lattice distortion of apatite with decreased particle size. However, the quantitative analysis of isomorphous substitution type in apatite and the changes in the apatite crystal structure require further study. The soil-available P significantly increased with the application of MAPR in weakly acidic soil (pH 5.94), compared with the initial PR. This was for the ascending reactive of MAPR with increased P solubility. The quantitative mechanism of P dissolution of MAPR and its P supply capacity in different types of soil also require further study.
Author Contributions
Conceptualization, N.F. and G.L.; methodology, S.L.; software, X.H.; validation, N.F. and H.D.; formal analysis, S.L.; investigation, S.L.; resources, H.X.; data curation, X.H.; writing—original draft preparation, N.F.; writing—review and editing, G.L.; visualization, H.X.; supervision, X.H.; project administration, H.X.; funding acquisition, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shenyang Center of China Geological Survey, grant number IGCP665, DD20190520 and 121201007000161312 and supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28020302).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Wenlong Duan and Gongyao Tan for the technical support and phosphate rock used for experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bünemann, E.K.; Condron, L.M. Phosphorus and sulphur cycling in terrestrial ecosystems. In Nutrient Cycling in Terrestrial Ecosystems; Marschner, P., Rengel, Z., Eds.; Springer: Berlin, Germany, 2007; pp. 65–92. [Google Scholar]
- Condron, L.M.; Turner, B.L.; Cade-Menun, B.J.; Sims, J.; Sharpley, A. Chemistry and dynamics of soil organic phosphorus, In Phosphorus: Agriculture and the Environment; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America; Sims, J.T., Sharpley, A.N., Eds.; Soil Science Society of America: Madison, WI, USA, 2005; pp. 87–121. [Google Scholar]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Zhang, T.F. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, H.; Withers, P.J.A.; Baas, P.; Chan, N.L.; Doody, D.; Holiman, J.; Jacobs, B.; Li, H.; MacDonald, G.K.; McDowell, R.; et al. Integrating legacy soil phosphorus into sustainable nutrient management practices on farms. Nutr. Cycl. Agroecosys. 2016, 104, 393–412. [Google Scholar] [CrossRef]
- Koppelaar, R.H.; Weikard, H.P. Assessing phosphate rock depletion and phosphorus recycling options. Glob. Environ. Change 2013, 23, 1454–1466. [Google Scholar] [CrossRef]
- Friesen, D.K.; Sale PW, G.; Blair, G.J. Long-term greenhouse evaluation of partially acidulated phosphate rock fertilizers. II Effect of cogranulation with elemental S on availability of P from two phosphate rocks. Fertil Res. 1987, 13, 45–54. [Google Scholar] [CrossRef]
- Fernandez-Bertran, J.F. Mechanochemistry: An overview. Pure Appl. Chem. 1999, 71, 581–586. [Google Scholar] [CrossRef]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; pp. 297–405. [Google Scholar]
- Yaneva, V.; Petrov, O.; Petkova, V. Structural and spectroscopic studies of mechanochemically activated nanosized apatite from Syria. Mater. Res. Bull. 2009, 44, 693–699. [Google Scholar] [CrossRef]
- Jin, L.; Sun, L.; Wang, L.; Shi, Y. Studies on the mechanical activation of Huangmailing phosphorite. Res. J. Chem. Environ. 2013, 17, S156–S162. [Google Scholar]
- Tõnsuaadu, K.; Kaljuvee, T.; Petkova, V.; Traksmaa, R.; Bender, V.; Kirsimäe, K. Impact of mechanical activation on physical and chemical properties of phosphorite concentrates. Int. J. Miner. Process. 2011, 100, 104–109. [Google Scholar] [CrossRef]
- Petkova, V.; Koleva, V.; Kostova, B.; Sarov, S. Structural and thermal transformations on high energy milling of natural apatite. J. Therm. Anal. Calorim. 2015, 121, 217–225. [Google Scholar] [CrossRef]
- Fang, N.; Shi, Y.; Chen, Z.; Sun, X.; Zhang, L.; Yi, Y. Effect of mechanochemical activation of natural phosphorite structure as well as phosphorus solubility. PLoS ONE 2019, 14, e0224423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerald, S.; Bernhard, G.; Ingrid, W.; Michael, C.M. Efficiency developments in phosphate rock mining over the last three decades. Resour. Conserv. Recy. 2015, 105, 235–245. [Google Scholar]
- Wang, S.D.; Zhang, H.Y. Phosphate rock resources and phosphate fertilizer production and its consumption in China. Ind. Miner. Processing 2007, 9, 30–32. (In Chinese) [Google Scholar]
- Xue, K.; Zhang, R.Y. Advances of researches on the distribution and metallogenic characteristics of phosphorus deposits in China. Acta Miner. Sin. 2019, 39, 7–14. [Google Scholar]
- Fang, N.N.; Chen, Z.H.; Liu, Z.Q.; Dai, H.M.; Yang, X.M.; Wang, W. Effects of mechanochemically activated phosphate rock on maize growth and phosphorus use. Plant Soil Environ. 2022, 68, 155–161. [Google Scholar] [CrossRef]
- Zhang, X.M.; Li, Y.; Hu, C.; He, Z.Q.; Wen, M.X.; Gai, G.S.; Huang, Z.H.; Yang, Y.F.; Hao, X.Y.; Li, X.Y. Enhanced Phosphorus Release from Phosphate Rock Activated with Lignite by Mechanical Microcrystallization: Effects of Several Typical Grinding Parameters. Sustainability 2019, 11, 1068. [Google Scholar] [CrossRef] [Green Version]
- Li, Y. Soil Agricultural Chemical Routine Analytical Method, 1st ed.; China Science Publishing and Media Ltd.: Beijing, China, 1983. (In Chinese) [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 26, 31–36. [Google Scholar] [CrossRef]
- Bolan, N.S.; White, R.E.; Hedley, M.J. A review of the use of phosphate rocks as fertilizers for direct application in australia and new zealand. Aust. J. Exp. Agric. 1990, 30, 297–313. [Google Scholar] [CrossRef]
- Winand, L.; Dallemagne, M.J.; Duyckaerts, G. Hydrogen bonding in apatitic calcium phosphates. Nature 1961, 190, 164–165. [Google Scholar] [CrossRef]
- Chaikina, M.V. Mechanochemistry of Natural and Synthetic Apatites; Publishing House of SB RAS, Branch “GEO”: Novosibirsk, Russia, 2002; pp. 11–15, 105–107, 114–115, 139. [Google Scholar]
- Pierzynski, G.M.; McDowell, R.W.; Sims, J.T. Chemistry, Cycling, and Potential Movement of Inorganic Phoshphorus in Soils. In Phorphorus: Agriculture and the Environment; Sims, J.R., Sharpley, A.N., Eds.; American Society of Agronomy, Inc.; Crop Sciencce Society of America, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 2005; pp. 53–86. [Google Scholar]
- Hagin, J.; Harrison, R. Phosphate rocks and partially-acidulated phosphate rocks as controlled release P fertilizers. Fertil Res. 1993, 35, 25–32. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).