The Influence of Yarrowia lipolytica Glycosylation on the Biochemical Properties and Oligomerization of Heterologous Invertase
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Culture Conditions
2.3. Purification of Extracellular Invertase
2.3.1. Ultrafiltration—Concentrate Preparation
2.3.2. Ion Exchange Chromatography
2.4. Polyacrylamide Gel Electrophoresis (PAGE, Non-Denaturing Conditions)
2.5. Enzyme Activity, Protein, and Carbon Sources Determination
2.6. Optimum of pH and Temperature, Substrate Specificity and Affinity, Thermostability and Ions Inactivation
2.7. Deglycosylation Analysis
2.8. N-Glycan Purification, Hydrolysis, and Analysis with MALDI-TOF and HPLC
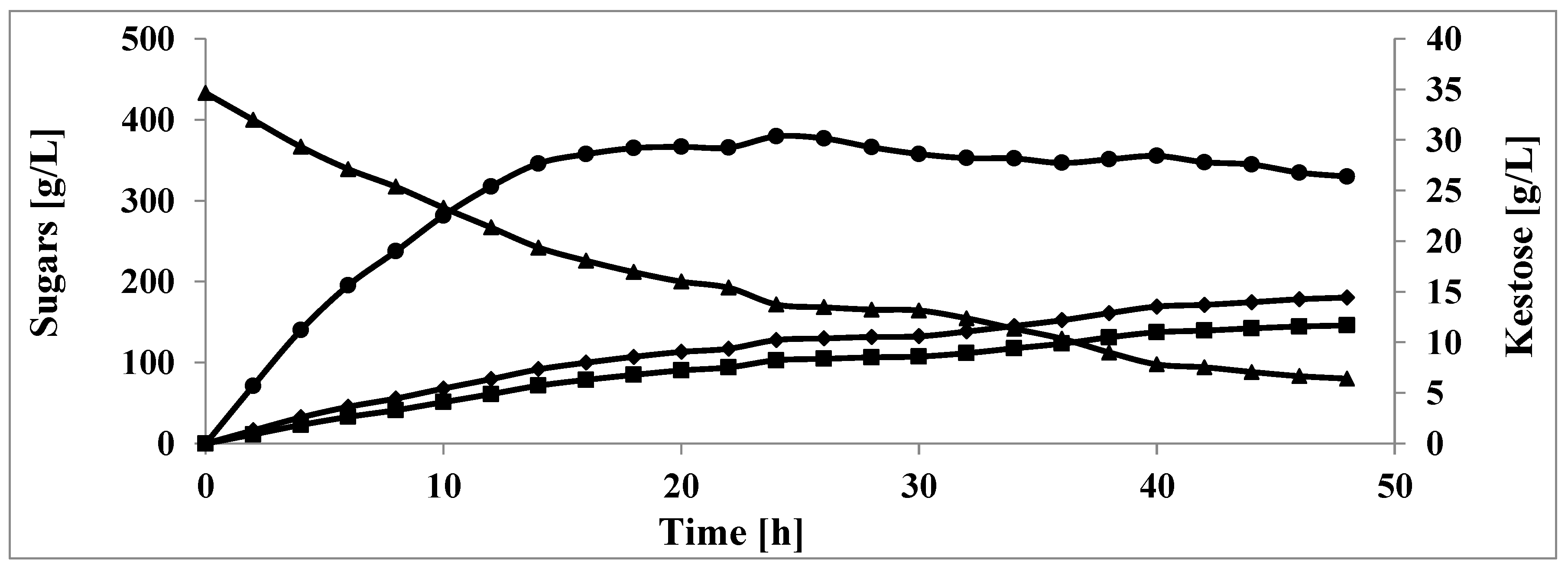
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Gutiérrez-Alonso, P.; Fernández-Arrojo, L.; Plou, F.J.; Fernández-Lobato, M. Biochemical characterization of a β-fructofuranosidase from Rhodotorula dairenensis with transfructosylating activity. FEMS Yeast Res. 2009, 9, 768–773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaheen, I.; Bhatti, H.N.; Ashraf, T. Production, purification and thermal characterization of invertase from newly isolated Fusarium sp. under solid-state fermentation. Int. J. Food Sci. Technol. 2008, 43, 1152–1158. [Google Scholar] [CrossRef]
- Zhou, J.; He, L.; Gao, Y.; Han, N.; Zhang, R.; Wu, Q.; Li, J.; Tang, X.; Xu, B.; Ding, J.; et al. Characterization of a novel low-temperature-active, alkaline and sucrose-tolerant invertase. Sci. Rep. 2016, 6, 32081. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Benito, M.; Polo, A.; González, B.; Fernández-Lobato, M.; Sanz-Aparicio, J. Structural and kinetic analysis of Schwanniomyces occidentalis invertase reveals a new oligomerization pattern and the role of its supplementary domain in substrate binding. J. Biol. Chem. 2010, 285, 13930–13941. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Polo, M.A.; Ramirez-Escudero, M.; Lafraya, A.; Gonzalez, B.; Marin-Navarro, J.; Polaina, J.; Sainz-Aparicio, J. Three-dimensional Structure of Saccharomyces Invertase. J. Biol. Chem. 2013, 288, 9755–9766. [Google Scholar] [CrossRef] [PubMed]
- Andjelković, U.; Pićurić, S.; Vujčić, Z. Purification and characterization of Saccharomyces cerevisiae external invertase isoforms. Food Chem. 2010, 120, 799–804. [Google Scholar] [CrossRef]
- Gascón, S.; Neumann, N.P.; Lampen, J.O. Comparative study of the properties of the purified internal and external invertase from yeast. J. Biol. Chem. 1968, 243, 1573–1577. [Google Scholar] [CrossRef]
- Neuman, N.P.; Lampen, J.O. Purification and properties of yeast invertase. Biochemistry 1967, 6, 468–475. [Google Scholar] [CrossRef]
- Moreno, S.; Sanchez, Y.; Rodriguez, L. Purification and characterization of the invertase from Schizosaccharomyces pombe. Biochem. J. 1990, 267, 697–702. [Google Scholar] [CrossRef]
- Aburigal, A.A.A.; Elkhalifa, E.A.; Sulieman EA, M.; Elamin, H.B. Extraction and partial kinetic properties of invertase from Schizosaccharomyces pombe. Int. J. Food Sci. Nutr. Eng. 2014, 4, 80–85. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Cell wall polysaccharides released during the alcoholic fermentation by Schizosaccharomyces pombe and S. japonicus: Quantification and characterization. Food Microb. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.C.; Runco, R.; Navarro, A.R. Invertase from a strain of Rhodotorula glutinis. Phytochemistry 2002, 61, 605–609. [Google Scholar] [CrossRef]
- Canli, O.; Erdal, S.; Taskin, M.; Kurbanoglu, E.B. Effects of extremely low magnetic field on the production of invertase by Rhodotorula glutinis. TIH 2011, 27, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Belcarz, A.; Ginalska, G.; Lobarzewski, J.; Pencel, C. The novel non-glycosylated invertase from Candida utilis (the properties and the conditions of production and purification). Acta Biochim. Biophys. 2002, 1594, 40–53. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Sabirzianova, T.A.; Celiakh, I.O.; Kalebina, T.S.; Kulaev, I.S. Export of an invertase by yeast cells (Candida utilis). Prikl. Biokhim. Mikrobiol. 2014, 50, 156–162. [Google Scholar] [PubMed]
- Rodrigo-Frutos, D.; Jiménez-Ortega, E.; Piedrabuena, D.; Ramirez-Escudero, M.; Miguez, N.; Plou, F.J.; Sanz-Aparicio, J.; Fernandez-Lobato, M. New insights into the molecular mechanism behind mannitol and erythritol fructosylation by β-fructofuranosidase from Schwanniomyces occidentalis. Sci. Rep. 2021, 11, 7158. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Nawaz, R.; Sheikh, M.A.; Asgher, M.; Abbas, A. Studies on kinetics and thermostability of a novel acid invertase from Fusarium solani. J. Agric. Food Chem. 2006, 54, 4617–4623. [Google Scholar] [CrossRef]
- Santana de Almeida, A.C.; Costa de Araújo, L.; Costa, A.M.; Moraes de Abreu, C.A.; Gomes de Andrade Lima, M.A.; de Los Angeles Perez Fernandez Palha, M. Sucrose hydrolysis catalyzed by auto-immobilized invertase into intact cells of Cladosporium cladosporioides. Electr. J. Biotechnol. 2005, 8, 54–62. [Google Scholar] [CrossRef]
- Manoochehri, H.; Hosseini, N.F.; Saidijam, M.; Taheri, M.; Rezaee, H.; Nouri, F. A review on invertase: Its potentials and applications. Biocatal. Agric. Biotechnol. 2020, 25, 101599. [Google Scholar] [CrossRef]
- Linde, D.; Macias, I.; Fernández-Arrojo, L.; Plou, F.J.; Jiménez, A.; Fernández-Lobato, M. Molecular and biochemical characterization of a β-fructofuranosidase from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2009, 75, 1065–1073. [Google Scholar] [CrossRef]
- Turkiewicz, M.; Pazgier, M.; Donachie, S.P.; Kalinowska, H. Invertase and α-glucosidase production by the endemic Antarctic Marine yeast Leucosporidium antarcticum. Pol. Polar Res. 2005, 26, 125–136. [Google Scholar]
- Uma, C.; Gomathi, D.; Muthulakshmi, C.; Gopalakrishnan, V.K. Production, purification and characterization of invertase by Aspergillus flavus using fruit peel waste as substrate. Adv. Biol. Res. 2010, 4, 31–36. [Google Scholar]
- Nadeem, H.; Rashid, M.H.; Siddique, M.H.; Azeem, F.; Muzammil, S.; Javed MRAli, M.A.; Rasul, I.; Riaz, M. Microbial invertases: A review on kinetics, thermodynamics, physiochemical properties. Process Biochem. 2015, 50, 1202–1210. [Google Scholar] [CrossRef]
- Carlson, M.; Botstein, D. Two differentially regulated mRNAs with different 5‘ ends encode secreted with intracellular forms of yeast invertase. Cell 1982, 28, 145–154. [Google Scholar] [CrossRef]
- Naumov, G.I.; Naumova, E.S. Polygenic control for fermentation of β-fructosides in the yeast Saccharomyces cerevisiae: New genes SUC9 and SUC10. Microbiology 2010, 79, 160–166. [Google Scholar] [CrossRef]
- Förster, A.; Aurich, A.; Mauersberger, S.; Barth, G. Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 75, 1409–1417. [Google Scholar] [CrossRef]
- Walczak, E.; Robak, M. Growth on sucrose of Yarrowia lipolytica yeats clones with invertase gene from Saccharomyces cerevisiae. Acta Sci. Pol. Biotechnol. 2009, 8, 25–36. [Google Scholar]
- Lazar, Z.; Rossignol, T.; Verbeke, J.; Crutz-Le Coq, A.M.; Nicaud, J.M.; Robak, M. Optimized invertase expression and secretion cassette for improving Yarrowia lipolytica growth on sucrose for industrial applications. J. Ind. Microbiol. Biotechnol. 2013, 40, 1273–1283. [Google Scholar] [CrossRef]
- Madzak, C.; Beckerich, J.M. Heterologous protein expression and secretion in Yarrowia lipolytica. In Yarrowia lipolytica; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–76. [Google Scholar]
- Vandermies, M.; Fickers, P. Bioreactor-scale strategies for the production of recombinant protein in the yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef]
- Ballou, C.E. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol. 1990, 185, 440–470. [Google Scholar] [CrossRef]
- Lazar, Z.; Walczak, E.; Robak, M. Simultaneous production of citric acid and invertase by Yarrowia lipolytica SUC+ transformants. Bioresour. Technol. 2011, 102, 6982–6989. [Google Scholar] [CrossRef] [PubMed]
- Buser, R.; Lazar, Z.; Käser, S.; Künzler, M.; Aebi, M. Identification, Characterization, and Biosynthesis of a Novel N-Glycan Modification in the Fruiting Body of the Basidiomycete Coprinopsis cinerea. J. Biol. Chem. 2010, 285, 10715–10723. [Google Scholar] [CrossRef] [PubMed]
- Lazar, Z.; Żubrowski, D.; Korzun-Chłopicka, U.; Robak, M. Recombinant Strain of Yarrowia lipolytica in Simultaneous Biosynthesis of Citrate and Invertase from Sucrose. Acta Sci. Pol. Biotechnol. 2016, 15, 25–35. [Google Scholar]
- Rashad, M.M.; Nooman, M.U. Production, purification and characterization of extracellular invertase from Saccharomyces cerevisiae NRRL Y-12632 by solid-state fermentation of red carrot residue. Aust. J. Bas. Sci. 2009, 3, 1910–1919. [Google Scholar]
- Rodriguez, J.; Perez, J.A.; Ruiz, T.; Rodriguez, L. Characterization of the invertase from Pichia anomala. Biochem. J. 1995, 306, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.M.G.; de Morais, T.P.; de Andrade Silva, C.A.; da Silva Santos, F.R.; Garcia, N.F.L.; Fonseca, G.G.; Leite, R.S.R.; da Paz, M.F. Biochemical characterization and evaluation of invertases produced from Saccharomyces cerevisiae CAT-1 and Rhodotorula mucilaginosa for the production of fructooligosaccharides. Prep. Biochem. Biotechnol. 2018, 48, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Boer, E.; Wartmann, T.; Luther, B.; Mantueffel, R.; Bode, R.; Gellissen, G.; Kunze, G. Characterization of the AINV gene and the encoded invertase from the dimorphic yeast Arxula adeninivorans. Antoine Van Leeuwenhoek 2004, 86, 121–134. [Google Scholar] [CrossRef]
- De Godoy, V.R.; Muller, G.; Stambuk, B. Efficient maltotriose fermentation through hydrolysis mediated by the intracellular invertase of Saccharomyces cerevisiae. BMC Proc. 2014, 8, 181. [Google Scholar] [CrossRef]
- Belcastro, M.; Marino, T.; Russo, N.; Toscano, M. Interaction of cysteine with Cu2+ and group IIb (Zn2+, Cd2+, Hg2+) metal cations: A theoretical study. J. Mass Spectrom. 2005, 40, 300–306. [Google Scholar] [CrossRef]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Farine, S.; Versluis, C.; Bonnici, P.J.; Heck, A.; L’homme, C.; Puigserver, A.; Biagini, A. Application of high performance anion exchange chromatography to study invertase-catalysed hydrolysis of sucrose and formation of intermediate fructan products. Appl. Microbiol. Biotechnol. 2001, 55, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, D.C.; Palai, T.; Agarwal, A.; Bhattacharya, P.K. Kinetics of sucrose conversion to fructo-oligosaccharides using enzyme (invertase) under free condition. Bioprocess Biosyst. Eng. 2014, 37, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Mano, M.C.R.; Neri-Numa, I.A.; da Silva, J.B.; Paulino, B.N.; Pessoa, M.G.; Pastore, G.M. Oligosaccharide biotechnology: An approach of prebiotic revolution on the industry. Appl. Microbiol. Biotechnol. 2018, 102, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Esmon, P.C.; Esmon, B.E.; Schauer, I.E.; Taylor, A.; Scheckman, R. Structure, assembly, and secretion of octameric invertase. J. Biol. Chem. 1987, 262, 4387–4394. [Google Scholar] [CrossRef]
- Tammi, M.; Ballou, L.; Taylor, A.; Ballou, C.E. Effect of glycosylation on yeast invertase oligomer stability. J. Biol. Chem. 1987, 262, 4395–4401. [Google Scholar] [CrossRef]
- Reddy, V.A.; Johnson, R.S.; Biemann, K.; Williams, R.S.; Ziegler, F.D.; Trimble, R.B.; Maley, F. Characterization of the glycosylation sites in yeast external invertase. I. N-linked oligosaccharide content of the individual sequins. J. Biol. Chem. 1988, 263, 6978–6985. [Google Scholar] [CrossRef]
- Trimble, R.B.; Atkinson, P.H.; Tschopp, J.F.; Townsend, R.R.; Maley, F. Structure of oligosaccharides on Saccharomyces SUC2 invertase secreted by methylotrophic yeast Pichia pastoris. J. Biol. Chem. 1991, 266, 22807–22817. [Google Scholar] [CrossRef]
- Zárate, V.; Belda, F. Characterization of the heterologous invertase produced Schizosaccharomyces pombe from the SUC2 gene of Saccharomyces cerevisiae. J. Appl. Bacteriol. 1996, 80, 45–52. [Google Scholar] [CrossRef]
- Schweigkofler, W.; Lopandic, K.; Molnár, O.; Prillinger, H. Analysis of phylogenetic relationships among Ascomycota with yeast phases using ribosomal DNA sequences and cell wall sugars. Org. Divers. Evol. 2002, 2, 1–17. [Google Scholar] [CrossRef][Green Version]
- Teparić, R.; Lozančić, M.; Mrša, V. Evolutionary Overview of Molecular Interactions and Enzymatic Activities in the Yeast Cell Walls. Int. J. Mol. Sci. 2020, 21, 8996. [Google Scholar] [CrossRef]






| Purification Step | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg of Protein) | Recovery Yield [%] | Purification (Fold) |
|---|---|---|---|---|---|
| Crude enzyme | 208 | 7120 | 34.23 | 100.0 | 1.00 |
| Ultrafiltration I | 141 | 4864 | 34.50 | 68.3 | 1.01 |
| Dialysis | 86.1 | 4998 | 58.05 | 70.2 | 1.70 |
| Ultrafiltration II | 42.0 | 3768 | 89.71 | 52.9 | 2.62 |
| Ion exchange chromatography | |||||
| I | 1.55 | 351 | 226.45 | 4.9 | 6.62 |
| II | 2.48 | 1073 | 432.66 | 15.1 | 12.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymański, K.; Hapeta, P.; Moroz, P.; Wąsik, B.; Robak, M.; Lazar, Z. The Influence of Yarrowia lipolytica Glycosylation on the Biochemical Properties and Oligomerization of Heterologous Invertase. Sustainability 2022, 14, 7926. https://doi.org/10.3390/su14137926
Szymański K, Hapeta P, Moroz P, Wąsik B, Robak M, Lazar Z. The Influence of Yarrowia lipolytica Glycosylation on the Biochemical Properties and Oligomerization of Heterologous Invertase. Sustainability. 2022; 14(13):7926. https://doi.org/10.3390/su14137926
Chicago/Turabian StyleSzymański, Kacper, Piotr Hapeta, Paweł Moroz, Bartosz Wąsik, Małgorzata Robak, and Zbigniew Lazar. 2022. "The Influence of Yarrowia lipolytica Glycosylation on the Biochemical Properties and Oligomerization of Heterologous Invertase" Sustainability 14, no. 13: 7926. https://doi.org/10.3390/su14137926
APA StyleSzymański, K., Hapeta, P., Moroz, P., Wąsik, B., Robak, M., & Lazar, Z. (2022). The Influence of Yarrowia lipolytica Glycosylation on the Biochemical Properties and Oligomerization of Heterologous Invertase. Sustainability, 14(13), 7926. https://doi.org/10.3390/su14137926






