Corrosion Inhibition Evaluation of Chitosan–CuO Nanocomposite for Carbon Steel in 5% HCl Solution and Effect of KI Addition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of CHT-CuO Nanocomposites
2.3. Corrosion Inhibition Studies
2.3.1. Electrochemical Measurements
2.3.2. Weight Loss Measurements
2.3.3. Surface Analysis
3. Results and Discussion
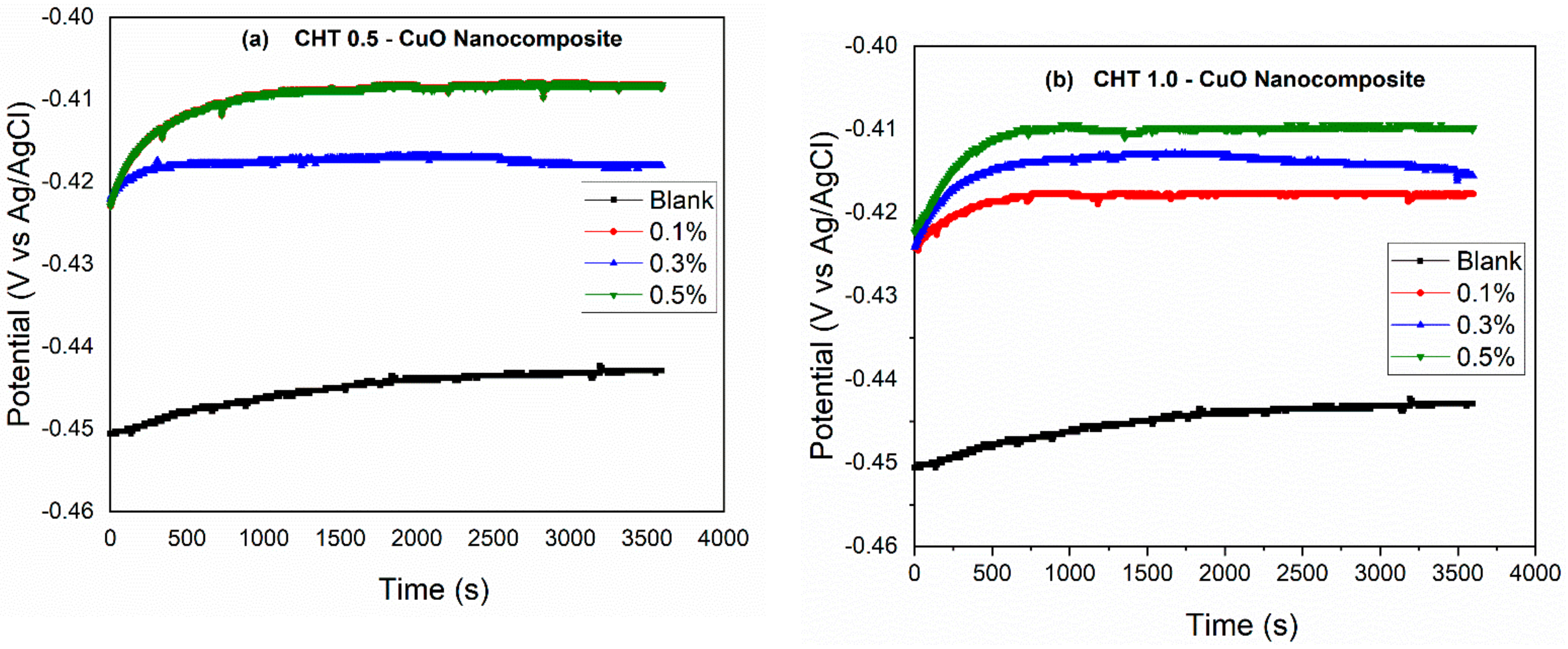
3.1. Open Circuit Potential
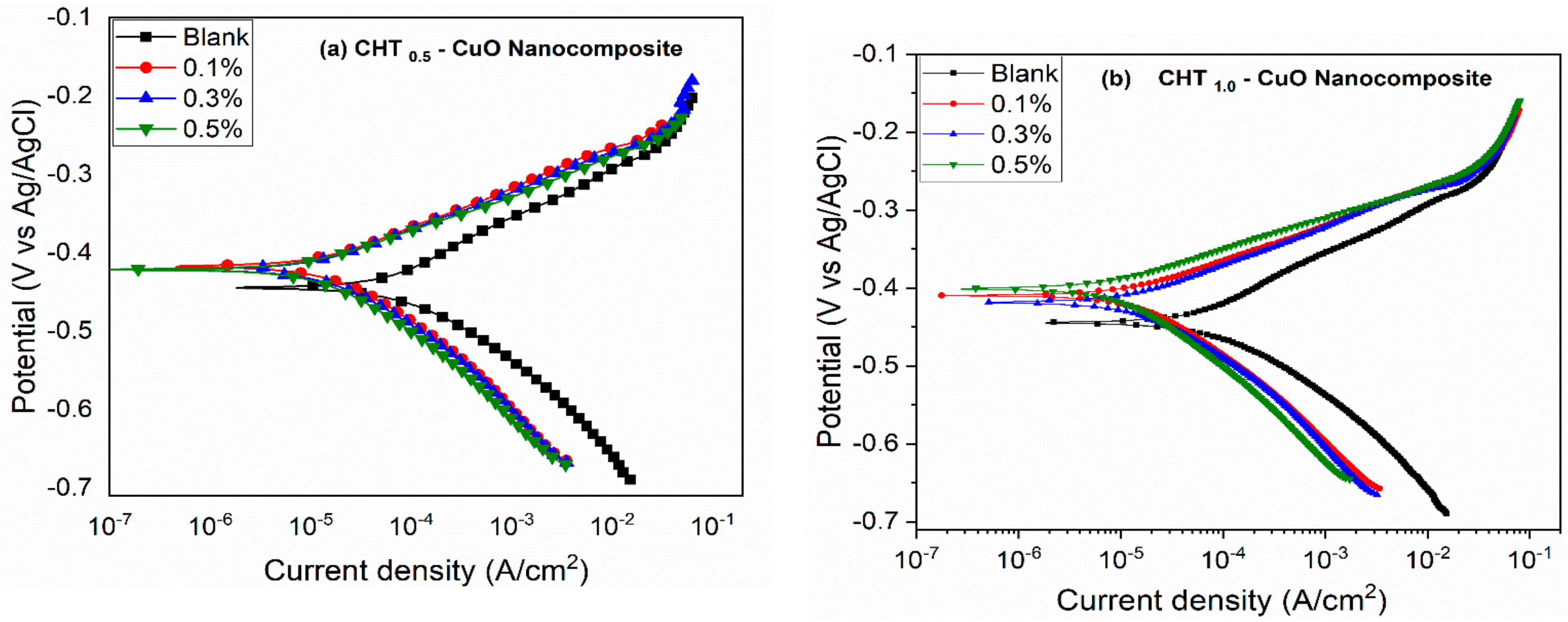
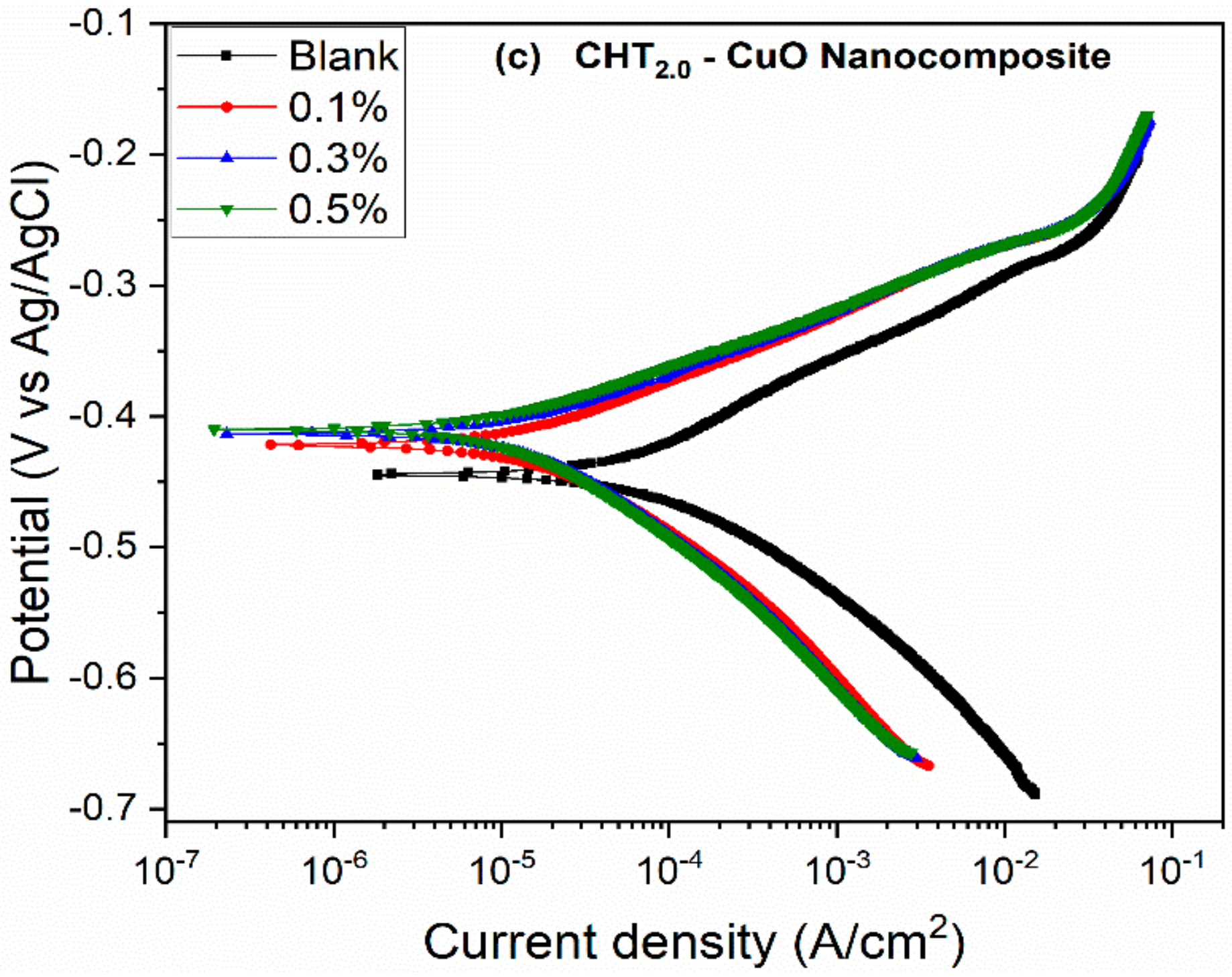
3.2. PDP and LPR Measurements
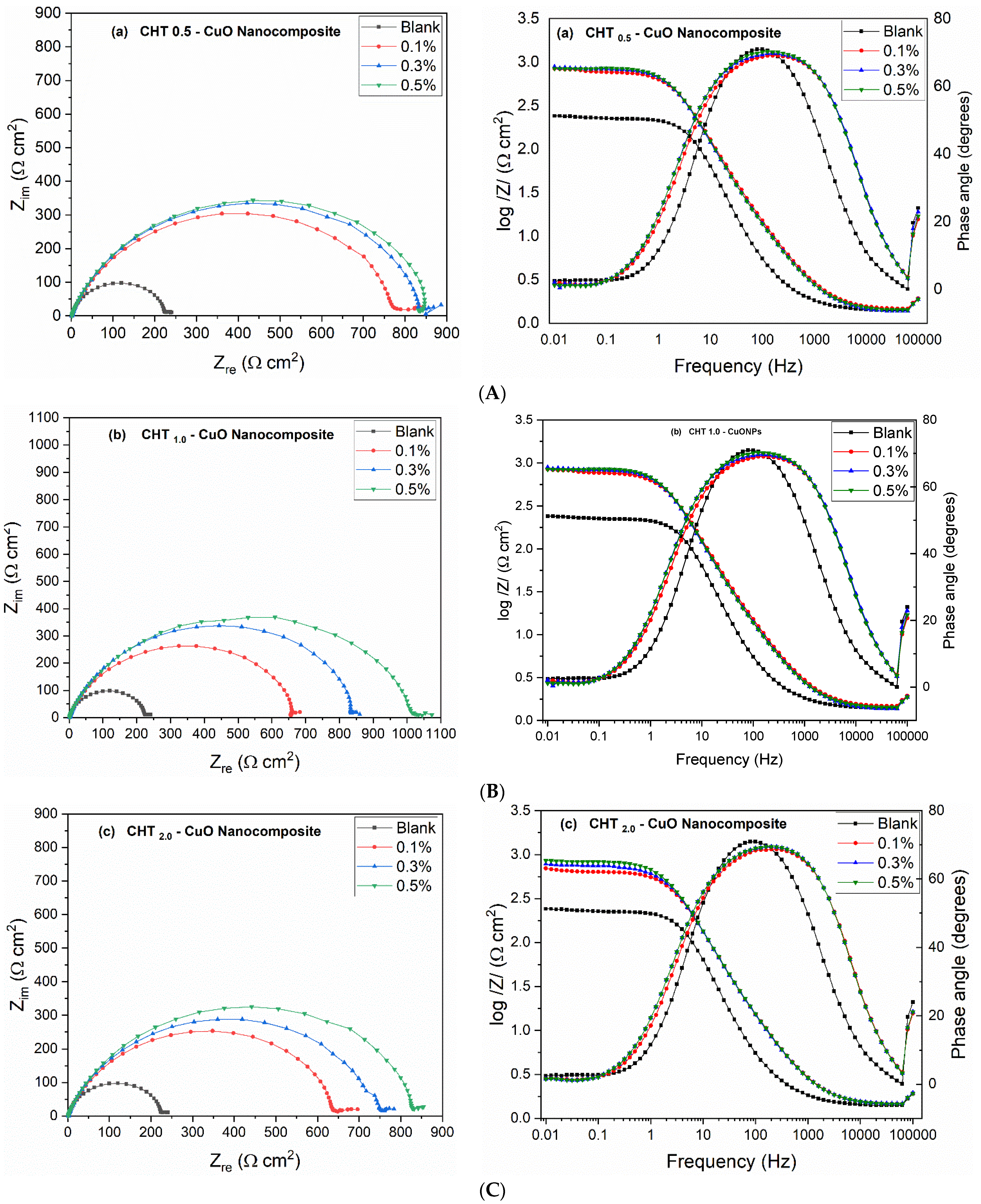
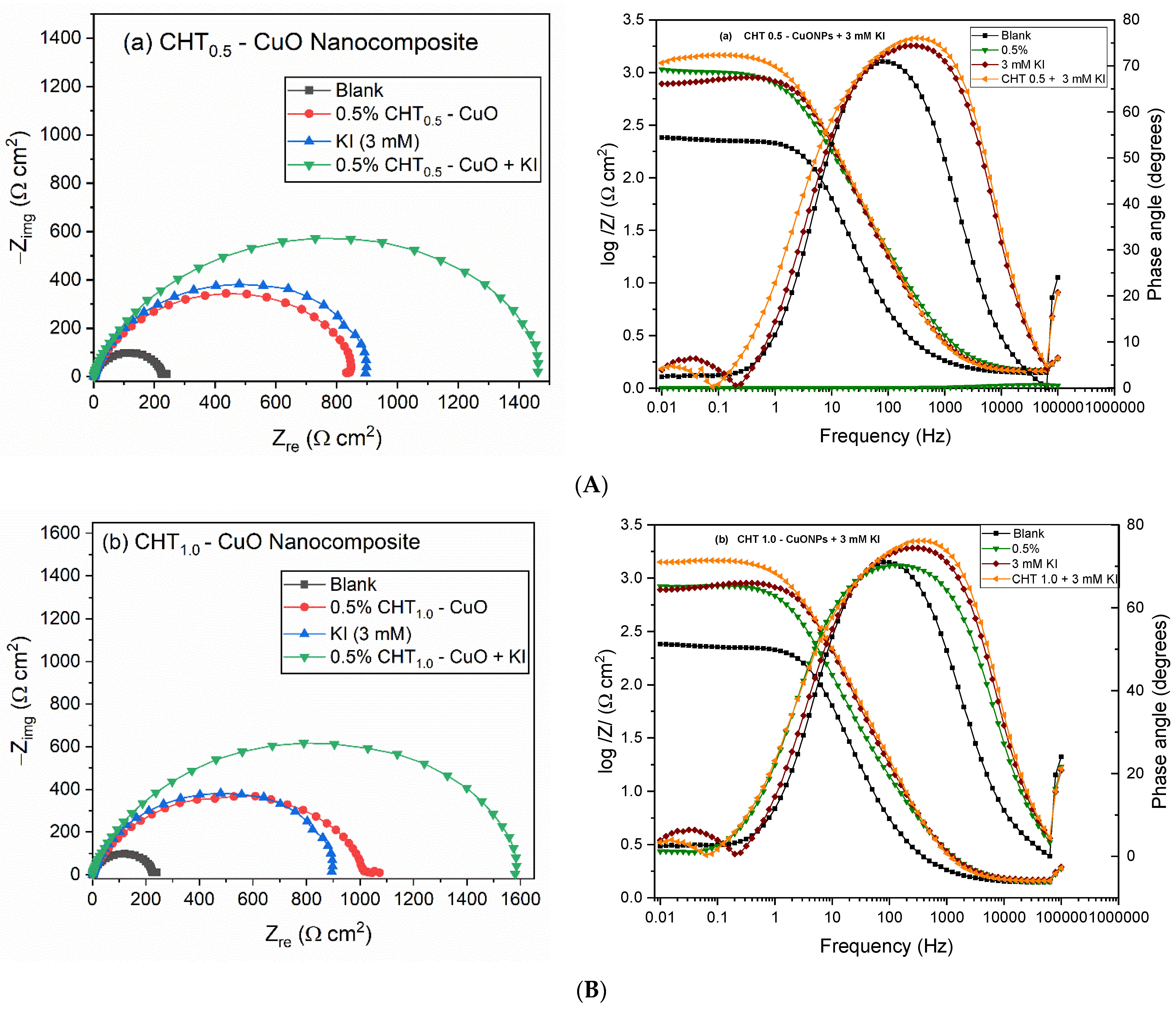
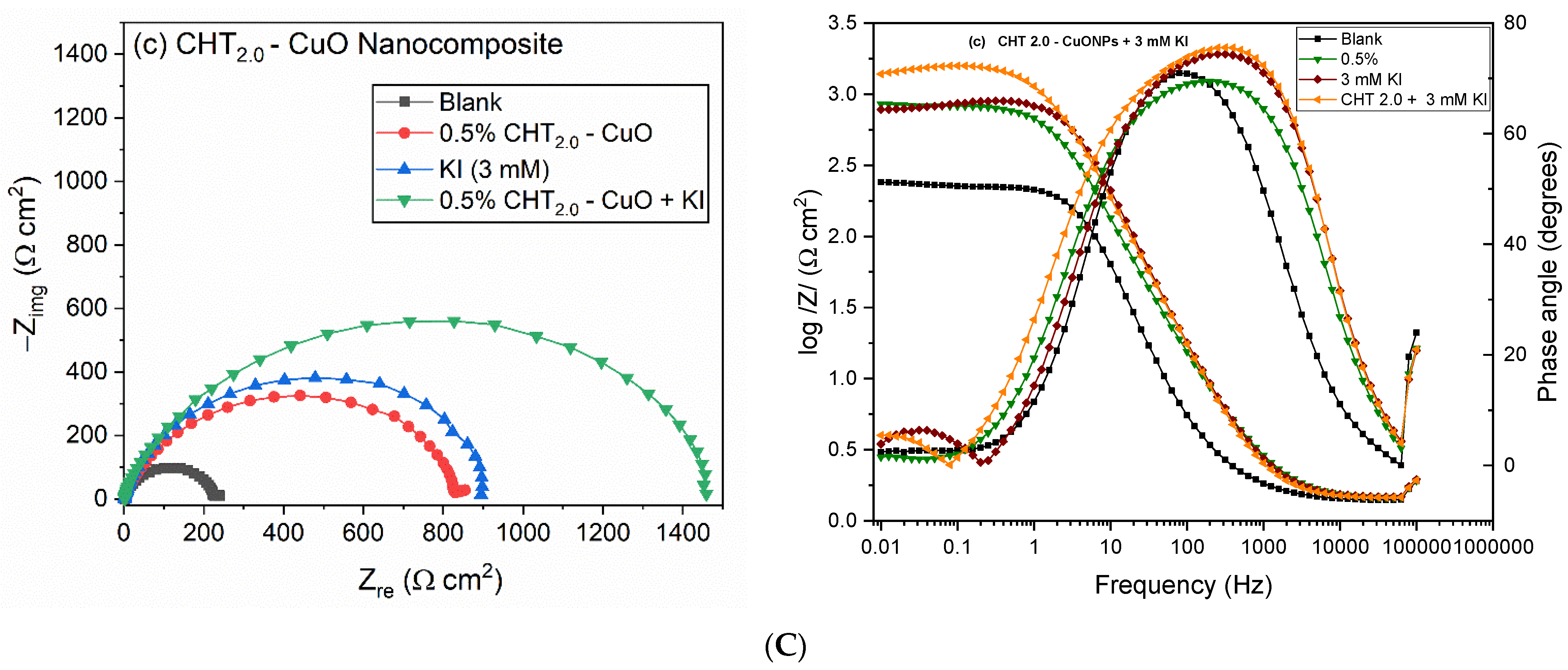
3.3. EIS Measurements
3.4. Temperature Effect
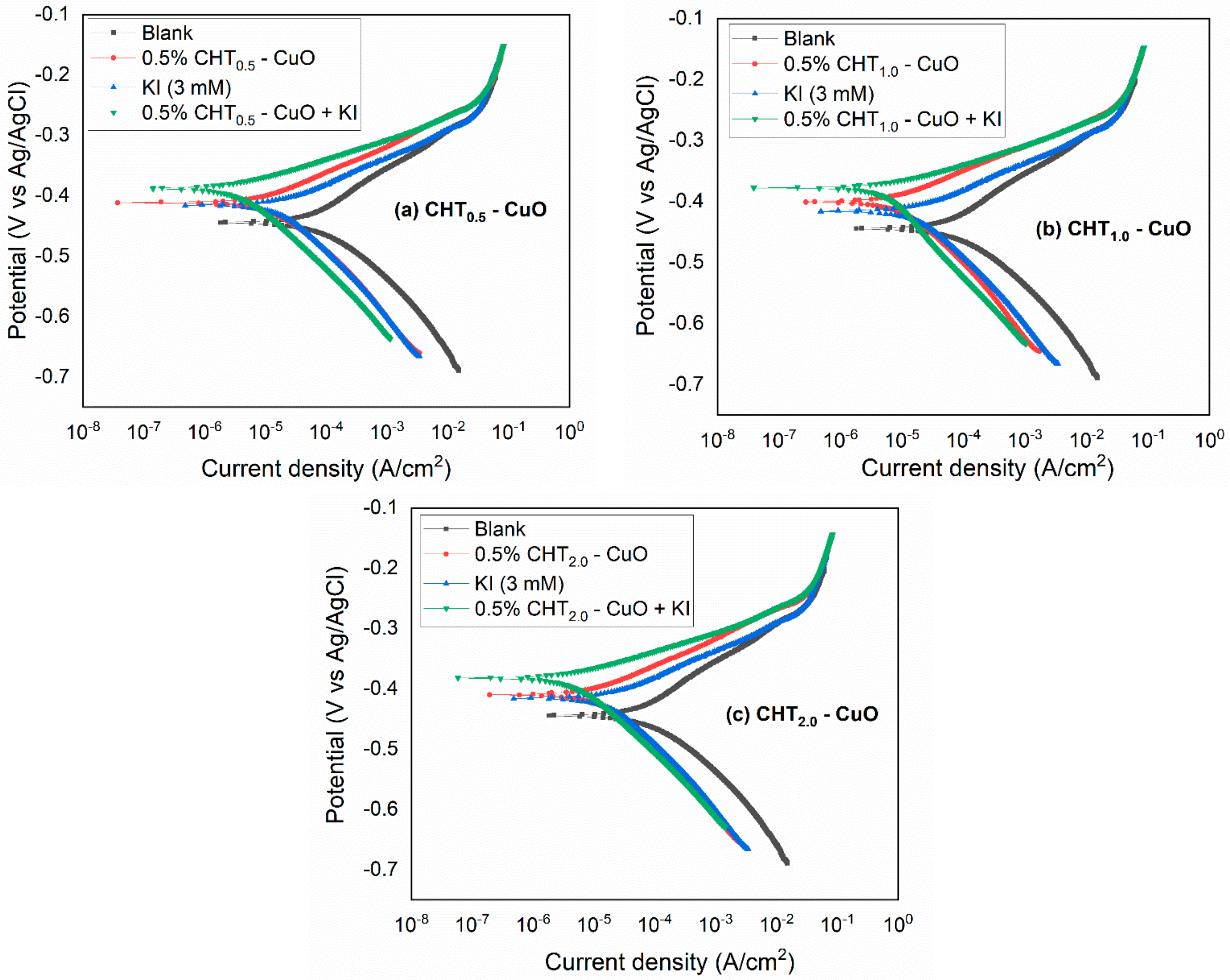
3.5. Effect of KI on CHT–CuO Nanocomposites Performance
3.6. Surface Assessments
4. Conclusions
- (a)
- The OLE-mediated CHT–CuO nanocomposites act as an effective corrosion inhibitor for X60 carbon steel in 5% HCl solution. IE increases with increase in concentration of the nanocomposite. The order of corrosion protection performance is found to follow the order CHT1.0–CuO (90.35%) > CHT0.5–CuO (90.16%) > CHT2.0–CuO (89.52%) nanocomposite from impedance measurements.
- (b)
- Increase in IE of the nanocomposites was observed with temperature rise from 25 to 40 °C. Thereafter, further increase in temperature to 50 and 60 °C causes a decrease in IE.
- (c)
- Addition of KI to the nanocomposites resulted in improved corrosion inhibition performance which could not be ascribed to synergistic effect. The non-synergistic inhibition effect was confirmed from the calculated synergism parameter which was found to be less than unity with values of 0.89, 0.74 and 0.75 for CHT0.5–CuO, CHT1.0–CuO and CHT2.0–CuO nanocomposites respectively at 60 °C.
- (d)
- Also, the addition of KI induced a change in corrosion inhibition mechanism from physisorption to chemisorption based on the increase in IE from 68.0, 72.8 and 62.4% at 25 °C to 72.5, 74.7 and 71.7% at 60 °C for CHT0.5–CuO, CHT1.0–CuO and CHT2.0–CuO nanocomposites respectively.
- (e)
- The potentiodynamic polarization results suggest that the nanocomposites alone and in combination with KI inhibited the corrosion of X60 carbon steel by active site blocking mechanism.
- (f)
- The results from all the experimental techniques viz weight loss, EIS, LPR and PDP are in good agreement.
- (g)
- Surface assessments of the corroded X60 carbon steel specimens in the blank corrodent, in the corrodent containing nanocomposites alone, and in the corrodent with KI addition, were undertaken using a 3D optical profilometer. These assessments confirm the additives’ corrosion inhibitive effect based on the reduction in surface roughness parameters in the additive’s presence in comparison to the blank.
- (h)
- As a future perspective, we propose the evaluation of the prepared nanocomposites as sweet (CO2) and sour (H2S) corrosion inhibitors as well as biocides for microbial influenced corrosion (MIC) mitigation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbasov, V.M.; Abd El-Lateef, H.M.; Aliyeva, L.I.; Ismayilov, I.T.; Qasimov, E.E.; Narmin, M.M. Efficient complex surfactants from the type of fatty acids as corrosion inhibitors for mild steel C1018 in CO2-environments. J. Korean Chem. Soc. 2013, 57, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Osokogwu, U.; Oghenekaro, E. Evaluation of corrosion inhibitors effectiveness in oilfield production operations. Int. J. Sci. Technol. Res. 2012, 1, 19–23. [Google Scholar]
- Kumar, H.; Yadav, V. Corrosion characteristics of mild steel under different atmospheric by vapour phase corrosion inhibitors. Am. J. Mater. Sci. Eng. 2013, 1, 34–39. [Google Scholar]
- Desimone, M.; Grundmeier, G.; Gordillo, G.; Simison, S. Amphiphilic amido-amine as an effective corrosion inhibitor for mild steel exposed to CO2 saturated solution: Polarization, EIS and PM-IRRAS studies. Electrochim. Acta 2011, 56, 2990–2998. [Google Scholar] [CrossRef]
- Gunavathy, N.; Murugavel, S. Corrosion inhibition studies of mild steel in acid medium using musa acuminata fruit peel extract. E-J. Chem. 2012, 9, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Lopez, D.A.; Simison, S.N.; de Sanchez, S.R. Inhibitors performance in CO2 corrosion: EIS studies on the interaction between their molecular structure and steel microstructure. Corros. Sci. 2005, 47, 735–755. [Google Scholar] [CrossRef]
- Abbasov, V.M.; Gadjiyeva, S.R.; Magerramov, R.S.; Akhmedov, N.S.; Rasulov, S. Influence of potassium salts of nitro derivative high α-olefins in 1% NaCl solution saturated with CO2 on steel corrosion. Azerbaijani Oil Ind. 2010, 8, 1–4. [Google Scholar]
- Benton, W.J.; Koskan, L.P. Inhibtion of Carbon Dioxide Corrosion of Metals. U.S. Patent 5607623 A 4 March 1997. [Google Scholar]
- Umoren, S.A.; Solomon, M.M. Recent Developments on the Use of Polymers as Corrosion Inhibitors -A Review. Open Mater. Sci. J. 2014, 8, 39–54. [Google Scholar] [CrossRef]
- Negm, N.A.; Yousef, M.A.; Tawfik, M. Impact of synthesized and natural compounds in corrosion inhibition of carbon steel and aluminium in acidic media. Recent Pat. Corros. Sci. 2013, 3, 58–68. [Google Scholar]
- Solomon, M.M.; Umoren, S.A.; Udosoro, I.I.; Udoh, A.P. Inhibitive and adsorption of carboxymethyl cellulose on mild steel corrosion in sulphuric acid. Solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical of glucose, gellan gum, and hydroxypropyl cellulose—Inhibition of cast iron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.M.; Maayta, A.K.; Al-Mustafa, J.A. Corrosion inhibition of iota-carrageenan natural polymer on aluminum in presence of zwitterions mediator in HCl media. Corros. Sci. 2012, 65, 223–230. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Inhibitory effect of xanthan gum and synergistic surfactant additives for mild steel corrosion in 1 M HCl. Carbohydr. Polym. 2016, 136, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Udosoro, I.I.; Udoh, A.P. Synergistic and antagonistic effects between halide ions and carboxymethyl cellulose for the corrosion inhibition of mild steel in sulphuric acid solution. Cellulose 2010, 17, 635–648. [Google Scholar] [CrossRef]
- Banerjee, S.; Srivastava, V.; Singh, M.M. Chemically modified natural polysaccharide as green corrosion inhibitor for mild steel in acidic medium. Corros. Sci. 2012, 59, 35–41. [Google Scholar] [CrossRef]
- Hefni, H.H.H.; Azzam, E.M.; Badr, E.A.; Hussein, M.; Tawfik, S.M. Synthesis, characterization and anticorrosion potentials of chitosan-g-PEG assembled on silver nanoparticles. Int. J. Biol. Macromol. 2016, 83, 297–305. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Kaya, T.; Umoren, S.A. Performance evaluation of a chitosan/silver nanoparticles composite on St37 steel corrosion in a 15% HCl solution. ACS Sustain. Chem. Eng. 2017, 5, 809–820. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Kaya, T.; Umoren, S.A. Enhanced corrosion inhibition of chitosan for St37 in 15% H2SO4 environment by silver nanoparticles. Int. J. Biol. Macromol. 2017, 104, 638–649. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, S.; Ji, G.; Prakash, R. Chitosan based new nanocomposites for corrosion protection of mild steel in aggressive chloride media. Int. J. Biol. Macromol. 2019, 140, 177–187. [Google Scholar] [CrossRef]
- Fetouh, H.; Hefnawy, A.; Attia, A.; Ali, E. Facile and low-cost green synthesis of eco-friendly chitosan-silver nanocomposite as novel and promising corrosion inhibitor for mild steel in chilled water circuits. J. Mol. Liq. 2020, 319, 114355. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl cellulose/silver nanoparticles composite: Synthesis, characterization and application as a benign corrosion inhibitor for st37 steel in 15% H2SO4 medium. ACS Appl. Mater. Interf. 2017, 9, 6376–6389. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Lateef, H.M.; Albokheet, W.; Gouda, M. Carboxymethyl cellulose/metal (Fe, Cu Ni) nanocomposites as non-precious inhibitors of C-steel corrosion in HCl solutions: Synthesis, characterization, electrochemical and surface morphology studies. Cellulose 2020, 27, 8039–8057. [Google Scholar] [CrossRef]
- El-Lateef, H.M.; Albokheet, W.A.; Gouda, M. Enhancement of corrosion protection of mild steel by chitosan/ZnO nanoparticle composite membranes. Prog. Organ. Coat. 2015, 84, 28–34. [Google Scholar]
- Li, X.; Deng, S.; Fu, H.; Mu, G. Synergism between rare earth cerium (IV) in and vanillin on the corrosion of cold rolled steel in 1 M HCl solution. Corros. Sci. 2008, 50, 3599–3609. [Google Scholar] [CrossRef]
- Telegdi, J.; Shaglouf, M.M.; Shaban, A.; Karman, F.H.; Betroti, I.; Mohai, M.; Kalman, E. Influence of cations on the corrosion inhibition efficiency of aminophosphoric acid. Electrochim. Acta 2001, 46, 3791–3799. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Cai, Q.R.; He, X.M.; Gao, L.X.; Kim, G.S. Corrosion inhibition and adsorption behavior of methionine on copper in HCl and synergistic effect of zinc ions. Mater. Chem. Phys. 2009, 114, 612–617. [Google Scholar] [CrossRef]
- Larabi, L.; Harek, Y.; Traisnel, M.; Mansri, A. Synergistic influence of poly (4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1 M HCl. J. Appl. Electrochem. 2004, 34, 833–839. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ebenso, E.E. The synergistic effect of polyacrylamide and iodide ions on the corrosion inhibition of mild steel in H2SO4. Mater. Chem. Phys. 2007, 106, 387–393. [Google Scholar] [CrossRef]
- Usman, B.J.; Umoren, S.A.; Gasem, Z.M. Inhibition of API 5L X60 steel corrosion in CO2 –saturated 3.5% NaCl solution by tannic acid and synergistic effect of KI additive. J. Mol. Liq. 2017, 237, 146–156. [Google Scholar] [CrossRef]
- Umoren, P.S.; Kavaz, D.; Nzila, A.; Sankaran, S.S.; Umoren, S.A. Biogenic synthesis and characterization of chitosan-CuO nanocomposite and evaluation of antibacterial activity against gram positive and negative bacteria. Polymers 2022, 14, 1832. [Google Scholar] [CrossRef] [PubMed]
- ASTM International. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test; ASTM International: West Conshohocken, PA, USA, 1999. [Google Scholar]
- Zeino, A.; Abdulazeez, I.; Khaled, M.; Jawish, M.W.; Obot, I.B.; Alhooshani, K. Mechanistic study of polyepoxy succinic acid (PESA) as green corrosion inhibitor on carbon steel in aerated nacl solution. Mater. Today Commun. 2021, 29, 102848. [Google Scholar] [CrossRef]
- Kousar, K.; Walczak, M.S.; Ljungdahl, T.; Wetzel, A.; Oskarsson, H.; Restuccia, P.; Ahmad, A.; Harrison, N.M.; Lindsay, R. Corrosion inhibition of carbon steel in hydrochloric acid: Elucidating the performance of an imidazoline-based surfactant. Corros. Sci. 2021, 180, 109195. [Google Scholar] [CrossRef]
- Arellanes-Lozada, P.; Díaz-Jiménez, V.; Hernández-Cocoletzi, H.; Nava, N.; Olivares Xometl, O.; Likhanova, N.V. Corrosion inhibition properties of iodide ionic liquids for API 5L X52 steel in acid medium. Corros. Sci. 2020, 175, 108888. [Google Scholar] [CrossRef]
- Cao, C. On electrochemical techniques for interface inhibitor research. Corros. Sci. 1996, 38, 2073–2082. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. Effect of intensifier additives on the performance of butanolic extract of date palm leaves against the corrosion of API 5L X60 carbon steel in 15 wt.% HCl solution. Sustainability 2021, 13, 5569. [Google Scholar] [CrossRef]
- Umoren, S.A. Polypropylene glycol: A novel corrosion inhibitor for X60 pipeline steel in 15% HCl solution. J. Mol. Liq. 2016, 219, 946–958. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Xie, X. Experimental and theoretical study on corrosion inhibition of oxime compounds for aluminium in HCl solution. Corros. Sci. 2014, 81, 162–175. [Google Scholar]
- Zhang, Z.; Tian, N.C.; Huang, X.D.; Shang, W.; Wu, L. Synergistic inhibition of carbon steel corrosion in 0.5 M HCl solution by indigo carmine and some cationic organic compounds: Experimental and theoretical studies. RSC Adv. 2016, 6, 22250–22268. [Google Scholar] [CrossRef]
- Appa Rao, B.V.; Srinivasa Rao, S. Electrochemical and surface analytical studies of synergistic effect of phosphonate, Zn2+ and ascorbate in corrosion control of carbon steel. Mater. Corros. 2010, 61, 285–301. [Google Scholar]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Du, Y.-T.; Wang, H.-L.; Chen, Y.-R.; Qi, H.-P.; Jiang, W.-F. Synthesis of baicalin derivatives as eco-friendly green corrosion inhibitors for aluminum in hydrochloric acid solution. Environ. Chem. Eng. 2017, 5, 5891–5901. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Koh, E.; Park, S. Self-anticorrosion performance efficiency of renewable dimer-acid-based polyol microcapsules containing corrosion inhibitors with two triazole groups. Prog. Organ. Coat. 2017, 109, 61–69. [Google Scholar] [CrossRef]
- Wazzan, N.; Obot, I.B.; Fagieh, T.M. The role of some triazoles on the corrosion inhibition of C1020 steel and copper in a desalination descaling solution. Desalination 2022, 527, 115551. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J. The corrosion inhibition and adsorption behavior of Uncaria gambir extract on mild steel in 1 M HCl. Mater. Chem. Phys. 2011, 125, 461–468. [Google Scholar] [CrossRef]
- de Assunção Araújo Pereira, S.S.; Pêgas, M.M.; Fernández, T.L.; Magalhães, M.; Schöntag, T.G.; Lago, D.C.; de Senna, L.F.; D’Elia, E. Inhibitory action of aqueous garlic peel extract on the corrosion of carbon steel in HCl solution. Corros. Sci. 2012, 65, 360–366. [Google Scholar] [CrossRef]
- Mobin, M.; Zehra, S.; Aslam, R. L-Phenylalaninemethyl ester hydrochloride as a green corrosion inhibitor for mild steel in hydrochloric acid solution and the effect of surfactant additive. RSC Adv. 2016, 6, 5890–5902. [Google Scholar] [CrossRef]
- Roy, P.; Pal, A.; Sukul, D. Origin of the synergistic effect between polysaccharide and thiourea towards adsorption and corrosion inhibition for mild steel in sulphuric acid. RSC Adv. 2014, 4, 10607–10613. [Google Scholar] [CrossRef]
- Aramaki, K.; Hackerman, N. Inhibition mechanism of medium-sized polymethyleneimine. J. Electrochem. Soc. 1969, 116, 568–574. [Google Scholar] [CrossRef]
- Jeyaprabha, C.; Sathiyanarayanan, S.; Venkatachari, G. Effect of cerium ions on corrosion inhibition of PANI for iron in 0.5 M H2SO4. Appl. Surf. Sci. 2006, 253, 432–438. [Google Scholar] [CrossRef]
- Umoren, S.A. Synergistic inhibition effect of polyethylene glycol-polyvinyl pyrrolidone blends for mild steel corrosion in sulphuric acid medium. J. Appl. Polym. Sci. 2011, 119, 2072–2084. [Google Scholar] [CrossRef]
- Okafor, P.C.; Zheng, Y. Synergistic inhibition behaviour of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros. Sci. 2009, 51, 850–859. [Google Scholar] [CrossRef]






| PDP Method | LPR Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| System/ Concentration | −Ecorr (mV/Ag/AgCl) | icorr (µA cm–2) | βa (mV dec–1) | −βc (mV dec–1) | fa | fc | IE (%) | Rp (Ω cm2) | IE (%) |
| Blank | 443.9 | 73.20 | 83.48 | 80.25 | - | - | - | 247.73 | - |
| CHT0.5–CuO | |||||||||
| 0.1% | 418.0 | 14.91 | 56.54 | 86.09 | 0.32 | 0.27 | 79.63 | 880.38 | 71.86 |
| 0.3% | 415.1 | 14.56 | 54.81 | 89.79 | 0.34 | 0.27 | 80.11 | 899.36 | 72.45 |
| 0.5% | 409.1 | 11.73 | 49.41 | 90.86 | 0.32 | 0.23 | 83.98 | 931.79 | 73.41 |
| CHT1.0–CuO | |||||||||
| 0.1% | 418.4 | 13.19 | 55.95 | 81.75 | 0.28 | 0.25 | 81.98 | 915.88 | 73.05 |
| 0.3% | 409.6 | 11.24 | 50.61 | 86.34 | 0.30 | 0.23 | 84.64 | 955.61 | 74.07 |
| 0.5% | 400.1 | 9.75 | 48.88 | 101.61 | 0.33 | 0.20 | 86.68 | 1174.00 | 78.89 |
| CHT2.0–CuO | |||||||||
| 0.1% | 419.2 | 14.99 | 55.20 | 90.41 | 0.32 | 0.27 | 79.52 | 795.92 | 68.87 |
| 0.3% | 412.3 | 14.25 | 51.90 | 93.58 | 0.36 | 0.27 | 80.53 | 865.96 | 71.39 |
| 0.5% | 409.1 | 11.80 | 49.87 | 92.55 | 0.32 | 0.23 | 83.88 | 934.43 | 73.49 |
| Conc. (ppm) | Rs (Ω cm2) | CPEf | Rf (Ω cm2) | CPEdl | Rct (Ω cm2) | (Rp = Rf + Rct) (Ω cm2) | Cdl (mFcm–2) | (×10–3) | IE (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yf (µFcm–2 sn–1) | nf | Ydl (µF cm–2 sn–1) | ndl | ||||||||
| Blank | 1.81 | 64.5 | 1.00 | 7.15 | 552.0 | 0.61 | 74.8 | 81.95 | 4.50 | 5.43 | – |
| CHT0.5–CuO | |||||||||||
| 0.1% | 1.54 | 541.0 | 0.99 | 1.91 | 157.0 | 0.87 | 277.9 | 279.81 | 0.36 | 10.64 | 70.71 |
| 0.3% | 1.65 | 192.0 | 0.93 | 14.76 | 105.0 | 0.91 | 733.7 | 748.46 | 0.17 | 9.65 | 89.05 |
| 0.5% | 1.57 | 175.0 | 0.94 | 15.17 | 105.0 | 0.93 | 766.8 | 781.97 | 0.15 | 10.05 | 89.52 |
| CHT1.0–CuO | |||||||||||
| 0.1% | 1.56 | 174.0 | 0.95 | 11.36 | 106.0 | 0.87 | 778.2 | 789.56 | 0.23 | 9.78 | 89.62 |
| 0.3% | 1.49 | 177.0 | 0.95 | 10.73 | 106.0 | 0.88 | 832.3 | 849.13 | 0.21 | 11.85 | 90.34 |
| 0.5% | 1.52 | 201.0 | 0.93 | 11.19 | 100.0 | 0.88 | 838.4 | 849.59 | 0.19 | 9.89 | 90.35 |
| CHT2.0–CuO | |||||||||||
| 0.1% | 1.57 | 158.0 | 0.93 | 17.18 | 105.0 | 0.87 | 733.3 | 750.48 | 0.22 | 9.20 | 89.08 |
| 0.3% | 1.57 | 175.0 | 0.94 | 15.17 | 100.0 | 0.88 | 806.6 | 821.77 | 0.19 | 10.01 | 90.03 |
| 0.5% | 1.55 | 169.0 | 0.94 | 16.90 | 94.0 | 0.88 | 815.7 | 832.60 | 0.18 | 9.59 | 90.16 |
| PDP Method | LPR Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| System/ Concentration | −Ecorr (mV/Ag/AgCl) | icorr (µA cm–2) | βa (mV dec–1) | –βc (mV dec–1) | fa | fc | IE (%) | Rp (Ω cm2) | IE (%) |
| Blank | 443.9 | 73.20 | 83.48 | 80.25 | - | - | - | 247.73 | - |
| CHT0.5-CuO | 409.1 | 11.73 | 49.41 | 90.86 | 0.32 | 0.23 | 83.98 | 931.79 | 73.41 |
| CHT1.0-CuO | 400.1 | 9.75 | 48.88 | 101.61 | 0.33 | 0.20 | 86.68 | 1174.00 | 78.89 |
| CHT2.0-CuO | 409.1 | 11.80 | 49.87 | 92.55 | 0.32 | 0.23 | 83.88 | 934.43 | 73.74 |
| 3 mM KI | 417.1 | 18.67 | 46.59 | 101.71 | 0.45 | 0.33 | 74.49 | 802.38 | 69.13 |
| CHT0.5-CuO + KI | 378.3 | 5.16 | 29.30 | 117.38 | 0.66 | 0.12 | 92.95 | 1519.00 | 83.69 |
| CHT1.0-CuO + KI | 387.8 | 3.32 | 32.75 | 89.59 | 0.25 | 0.08 | 95.46 | 1950.00 | 87.29 |
| CHT2.0-CuO + KI | 382.3 | 4.07 | 32.29 | 88.23 | 0.37 | 0.11 | 94.44 | 1834.00 | 86.49 |
| Conc. (ppm) | Rs (Ω cm2) | CPEf | Rf (Ω cm2) | CPEdl | Rct (Ω cm2) | (Rp = Rf + Rct) (Ω cm2) | Cdl (mFcm–2) | (×10–3) | IE (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yf (µFcm–2 sn–1) | nf | Ydl (µF cm–2 sn–1) | ndl | ||||||||
| Blank | 1.81 | 64.5 | 1.00 | 7.15 | 552.0 | 0.61 | 74.8 | 81.95 | 4.50 | 5.43 | – |
| CHT0.5-CuO | 1.57 | 175.0 | 0.94 | 15.17 | 105.0 | 0.93 | 766.8 | 781.97 | 0.15 | 10.05 | 89.52 |
| CHT1.0-CuO | 1.52 | 201.0 | 0.93 | 11.19 | 100.0 | 0.88 | 838.4 | 849.59 | 0.19 | 9.89 | 90.35 |
| CHT2.0-CuO | 1.55 | 169.0 | 0.94 | 16.90 | 94.0 | 0.88 | 815.7 | 832.60 | 0.18 | 9.59 | 90.16 |
| 3 mM KI | 1.59 | 58.0 | 0.93 | 28.16 | 104.0 | 0.95 | 874.6 | 902.76 | 0.14 | 12.34 | 90.92 |
| CHT0.5-CuO + KI | 1.55 | 61.4 | 0.92 | 103.8 | 74.6 | 0.94 | 1346.0 | 1449.80 | 0.10 | 12.20 | 94.35 |
| CHT1.0-CuO + KI | 1.55 | 67.8 | 0.91 | 88.91 | 88.6 | 0.94 | 1477.0 | 1565.91 | 0.12 | 12.15 | 94.76 |
| CHT2.0-CuO + KI | 1.54 | 63.7 | 0.92 | 107.6 | 68.3 | 0.95 | 1329.0 | 1436.60 | 0.09 | 12.42 | 94.29 |
| System/Concentration | Corrosion Rate (mm/yr) | Inhibition Efficiency (%) | ||
|---|---|---|---|---|
| 25 °C | 60 °C | 25 °C | 60 °C | |
| Blank | 0.44 | 4.02 | - | - |
| CHT0.5-CuO | 0.24 | 2.86 | 44.80 | 28.50 |
| CHT1.0-CuO | 0.19 | 2.13 | 57.60 | 46.99 |
| CHT2.0-CuO | 0.21 | 2.43 | 51.20 | 39.42 |
| 3 mM KI | 0.19 | 1.41 | 56.80 | 65.01 |
| CHT0.5-CuO + KI | 0.14 | 1.11 | 68.00 | 72.49 |
| CHT1.0-CuO + KI | 0.12 | 1.01 | 72.80 | 74.76 |
| CHT2.0-CuO + KI | 0.16 | 1.14 | 62.40 | 71.71 |
| System | Synergism Parameter (S1) | ||||
|---|---|---|---|---|---|
| PDP | LPR | EIS | WL 25 °C | WL 60 °C | |
| CHT0.5–CuO | 0.59 | 0.50 | 0.17 | 0.74 | 0.89 |
| CHT1.0–CuO | 0.68 | 0.54 | 0.20 | 0.67 | 0.74 |
| CHT2.0–CuO | 0.69 | 0.58 | 0.17 | 0.66 | 0.75 |
| Systems/Concentration | Surface Roughness | ||
|---|---|---|---|
| RA (µm) | RRMS (µm) | RT (µm) | |
| Polished X60 carbon steel | 0.071 | 0.110 | 1.558 |
| X60 steel in blank at 25 °C | 0.384 | 0.680 | 9.895 |
| X60 steel inhibited with CHT0.5-CuO | 0.128 | 0.167 | 3.918 |
| X60 steel inhibited with CHT1.0-CuO | 0.124 | 0.161 | 3.761 |
| X60 steel inhibited with CHT0.5-CuO + KI | 0.116 | 0.156 | 6.638 |
| X60 steel inhibited with CHT1.0-CuO + KI | 0.104 | 0.137 | 4.288 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umoren, P.S.; Kavaz, D.; Umoren, S.A. Corrosion Inhibition Evaluation of Chitosan–CuO Nanocomposite for Carbon Steel in 5% HCl Solution and Effect of KI Addition. Sustainability 2022, 14, 7981. https://doi.org/10.3390/su14137981
Umoren PS, Kavaz D, Umoren SA. Corrosion Inhibition Evaluation of Chitosan–CuO Nanocomposite for Carbon Steel in 5% HCl Solution and Effect of KI Addition. Sustainability. 2022; 14(13):7981. https://doi.org/10.3390/su14137981
Chicago/Turabian StyleUmoren, Peace S., Doga Kavaz, and Saviour A. Umoren. 2022. "Corrosion Inhibition Evaluation of Chitosan–CuO Nanocomposite for Carbon Steel in 5% HCl Solution and Effect of KI Addition" Sustainability 14, no. 13: 7981. https://doi.org/10.3390/su14137981







