Effects of Synthesis Gas Concentration, Composition, and Operational Time on Tubular Solid Oxide Fuel Cell Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fuel Cell Manufacturing
2.2. Performance Testing
3. Results
3.1. Performance Tests with Varying Temperature, Fuel Composition, and Fuel Dilution
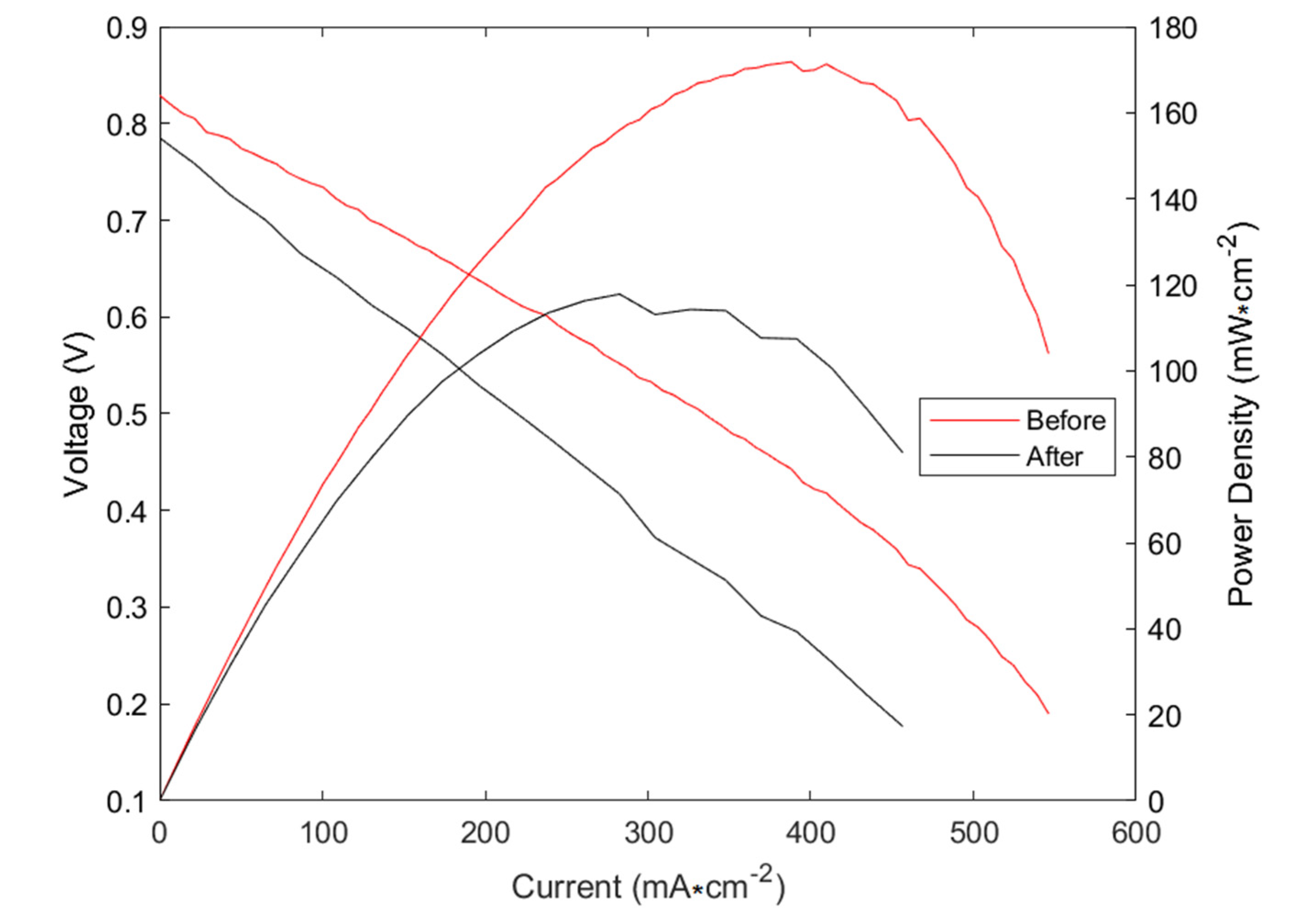
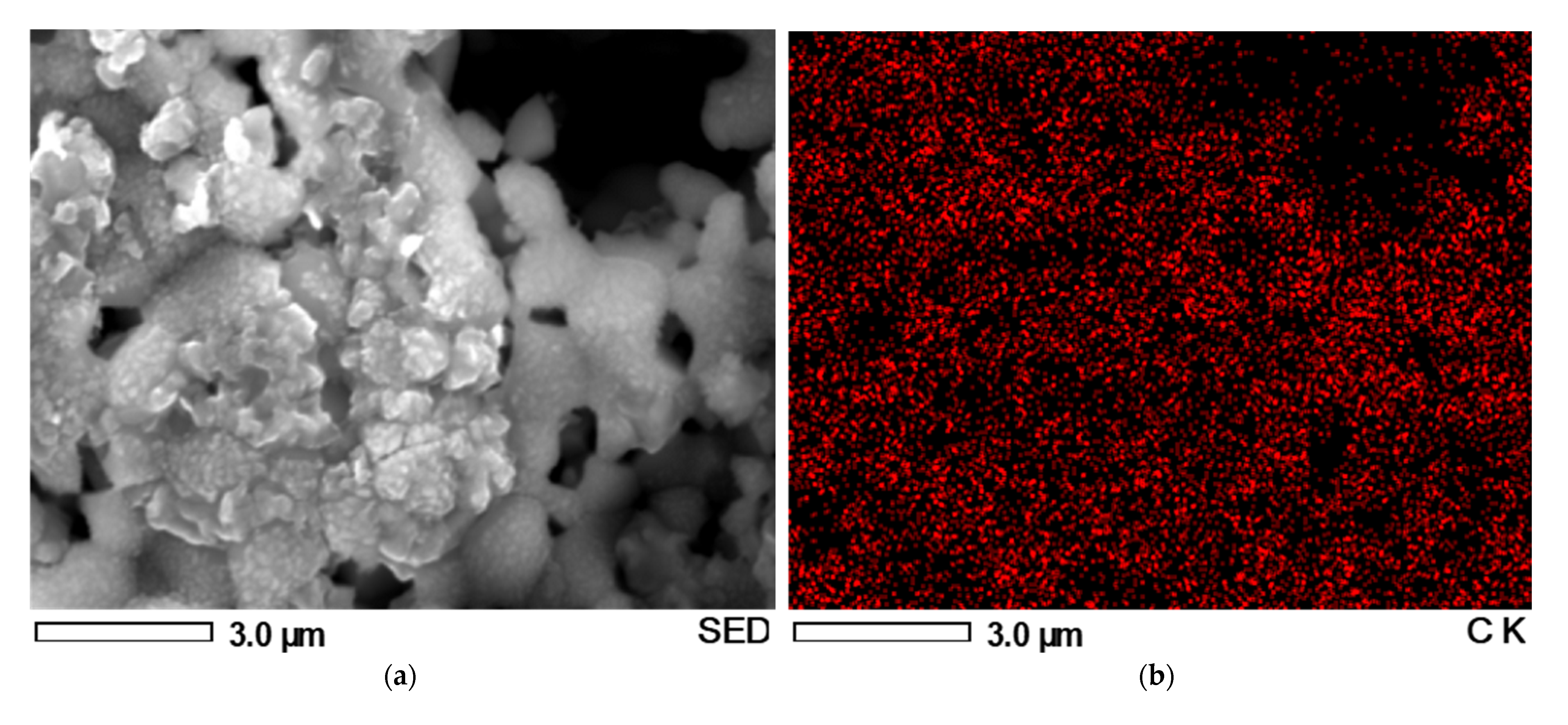
3.2. Longevity and Carbon Deposition Examinations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Shi, Y.; Yu, X.; Cai, N.; Qian, J.; Wang, S. Experimental Characterization of a Direct Methane Flame Solid Oxide Fuel Cell Power Generation Unit. J. Electrochem. Soc. 2014, 161, F1348–F1353. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, P.; Ahn, J. High Performance Direct Flame Fuel Cell Using a Propane Flame. Proc. Combust. Inst. 2011, 33, 3431–3437. [Google Scholar] [CrossRef]
- Wang, K.; Milcarek, R.J.; Zeng, P.; Ahn, J. Flame-Assisted Fuel Cells Running Methane. Int. J. Hydrogen Energy 2015, 40, 4659–4665. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zeng, H.; Shi, Y.; Cao, T.; Cai, N.; Ye, X.; Wang, S. Power and Heat Co-Generation by Micro-Tubular Flame Fuel Cell on a Porous Media Burner. Energy 2016, 109, 117–123. [Google Scholar] [CrossRef]
- Hartwell, A.R.; Welles, T.S.; Ahn, J. The Anode Supported Internal Cathode Tubular Solid Oxide Fuel Cell: Novel Production of a Cell Geometry for Combined Heat and Power Applications. Int. J. Hydrogen Energy 2021, 46, 37429–37439. [Google Scholar] [CrossRef]
- Milcarek, R.J.; Wang, K.; Falkenstein-Smith, R.L.; Ahn, J. Micro-Tubular Flame-Assisted Fuel Cells for Micro-Combined Heat and Power Systems. J. Power Sources 2016, 306, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, Y.; Cai, N.; Ye, X.; Wang, S. Performance Characteristics of a Micro-Tubular Solid Oxide Fuel Cell Operated with a Fuel-Rich Methane Flame. ECS Trans. 2015, 68, 2237–2243. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, H.; Cao, T.; Shi, Y.; Cai, N.; Ye, X.; Wang, S. Start-up and Operation Characteristics of a Flame Fuel Cell Unit. Appl. Energy 2016, 178, 415–421. [Google Scholar] [CrossRef]
- Zeng, H.; Gong, S.; Shi, Y.; Wang, Y.; Cai, N. Micro-Tubular Solid Oxide Fuel Cell Stack Operated with Catalytically Enhanced Porous Media Fuel-Rich Combustor. Energy 2019, 179, 154–162. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Shi, Y.X.; Yu, X.K.; Cai, N.S.; Li, S.Q. Integration of Solid Oxide Fuel Cells with Multi-Element Diffusion Flame Burners. J. Electrochem. Soc. 2013, 160, F1241–F1244. [Google Scholar] [CrossRef]
- Yari, M.; Mehr, A.S.; Mahmoudi, S.M.S.; Santarelli, M. A Comparative Study of Two SOFC Based Cogeneration Systems Fed by Municipal Solid Waste by Means of Either the Gasifier or Digester. Energy 2016, 114, 586–602. [Google Scholar] [CrossRef]
- Gandiglio, M.; Lanzini, A.; Santarelli, M.; Acri, M.; Hakala, T.; Rautanen, M. Results from an Industrial Size Biogas-Fed SOFC Plant (the DEMOSOFC Project). Int. J. Hydrogen Energy 2020, 45, 5449–5464. [Google Scholar] [CrossRef]
- Knight, I.; Ugursal, I. Residential Cogeneration Systems: A Review of the Current Technologies. 2005. Available online: https://www.osti.gov/etdeweb/biblio/20671036 (accessed on 16 May 2022).
- Dentice d’Accadia, M.; Sasso, M.; Sibilio, S.; Vanoli, L. Micro-Combined Heat and Power in Residential and Light Commercial Applications. Appl. Therm. Eng. 2003, 23, 1247–1259. [Google Scholar] [CrossRef]
- Naimaster, E.J.; Sleiti, A.K. Potential of SOFC CHP Systems for Energy-Efficient Commercial Buildings. Energy Build. 2013, 61, 153–160. [Google Scholar] [CrossRef]
- Farhad, S.; Hamdullahpur, F.; Yoo, Y. Performance Evaluation of Different Configurations of Biogas-Fuelled SOFC Micro-CHP Systems for Residential Applications. Int. J. Hydrogen Energy 2010, 35, 3758–3768. [Google Scholar] [CrossRef] [Green Version]
- Sorace, M.; Gandiglio, M.; Santarelli, M. Modeling and Techno-Economic Analysis of the Integration of a FC-Based Micro-CHP System for Residential Application with a Heat Pump. Energy 2017, 120, 262–275. [Google Scholar] [CrossRef]
- Milcarek, R.J.; Ahn, J. Rich-Burn, Flame-Assisted Fuel Cell, Quick-Mix, Lean-Burn (RFQL) Combustor and Power Generation. J. Power Sources 2018, 381, 18–25. [Google Scholar] [CrossRef]
- Milcarek, R.J.; Nakamura, H.; Tezuka, T.; Maruta, K.; Ahn, J. Microcombustion for Micro-Tubular Flame-Assisted Fuel Cell Power and Heat Cogeneration. J. Power Sources 2019, 413, 191–197. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Ni, M.; Cai, N. A Micro Tri-Generation System Based on Direct Flame Fuel Cells for Residential Applications. Int. J. Hydrogen Energy 2014, 39, 5996–6005. [Google Scholar] [CrossRef]
- Milcarek, R.J.; Ahn, J. Micro-Tubular Flame-Assisted Fuel Cells Running Methane, Propane and Butane: On Soot, Efficiency and Power Density. Energy 2018, 169, 776–782. [Google Scholar] [CrossRef]
- Milcarek, R.J.; Garrett, M.J.; Wang, K.; Ahn, J. Micro-Tubular Flame-Assisted Fuel Cells Running Methane. Int. J. Hydrogen Energy 2016, 41, 20670–20679. [Google Scholar] [CrossRef] [Green Version]
- Penchini, D.; Cinti, G.; Discepoli, G.; Sisani, E.; Desideri, U. Characterization of a 100 W SOFC Stack Fed by Carbon Monoxide Rich Fuels. Int. J. Hydrogen Energy 2013, 38, 525–531. [Google Scholar] [CrossRef]
- Fthenakis, V.M.; Trammell, S.R. Reference Guide for Hazard Analysis in PV Facilities. 2003. Available online: https://www.researchgate.net/profile/Vasilis-Fthenakis-2/publication/228611604_Reference_Guide_for_Hazard_Analysis_in_PV_Facilities/links/56b1188d08aed7ba3feaf653/Reference-Guide-for-Hazard-Analysis-in-PV-Facilities.pdf (accessed on 16 May 2022).
- Bioche, K.; Vervisch, L.; Ribert, G. Premixed Flame-Wall Interaction in a Narrow Channel: Impact of Wall Thermal Conductivity and Heat Losses. J. Fluid Mech. 2018, 856, 5–35. [Google Scholar] [CrossRef]
- Zlochower, I.A.; Green, G.M. The Limiting Oxygen Concentration and Flammability Limits of Gases and Gas Mixtures. J. Loss Prev. Process Ind. 2009, 22, 499–505. [Google Scholar] [CrossRef]
- Vanderstraeten, B.; Berghmans, J.; Tuerlinckx, D.; Smit, B.; Van’t Oost, E.; Vliegen, S. Experimental Study of the Pressure and Temperature Dependence on the Upper Flammability Limit of Methane/Air Mixtures. J. Hazard. Mater. 1997, 56, 237–246. [Google Scholar] [CrossRef]
- Chu, M.; Hartwell, A.R.; Ahn, J. Flame-Assisted Fuel Cell Boiler for Combined Heating and Micro-Power Generation. In Proceedings of the Nineteenth International Symposium on Advanced Fluid Information, Sendai, Japan, 6–8 November 2019; Volume 28. [Google Scholar]
- Haynes, B.S.; Wagner, H.G.G. Soot Formation. Prog. Energy Combust. Sci. 1981, 7, 229–273. [Google Scholar] [CrossRef]
- Sumi, H.; Lee, Y.H.; Muroyama, H.; Matsui, T.; Kamijo, M.; Mimuro, S.; Yamanaka, M.; Nakajima, Y.; Eguchi, K. Effect of Carbon Deposition by Carbon Monoxide Disproportionation on Electrochemical Characteristics at Low Temperature Operation for Solid Oxide Fuel Cells. J. Power Sources 2011, 196, 4451–4457. [Google Scholar] [CrossRef]
- Milcarek, R.J.; Garrett, M.J.; Welles, T.S.; Ahn, J. Performance Investigation of a Micro-Tubular Flame-Assisted Fuel Cell Stack with 3,000 Rapid Thermal Cycles. J. Power Sources 2018, 394, 86–93. [Google Scholar] [CrossRef]
- Fergus, J.W. Sealants for Solid Oxide Fuel Cells. J. Power Sources 2005, 147, 46–57. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Reddy, A.A.; Kharton, V.V.; Ferreira, J.M.F. Aluminosilicate-Based Sealants for SOFCs and Other Electrochemical Applications-A Brief Review. J. Power Sources 2013, 242, 486–502. [Google Scholar] [CrossRef]
- Brandon, N.P.; Ruiz-Trejo, E.; Boldrin, P. Solid Oxide Fuel Cell Lifetime and Reliability; Joe Hayton: London, UK, 2017. [Google Scholar]
- Hatae, T.; Sato, K.; Somekawa, T.; Matsuzaki, Y.; Amaha, S.; Yoshikawa, M.; Mugikura, Y.; Miyara, K.; Oshima, T.; Taniguchi, S.; et al. Durability Assessment of SOFC Stacks with Several Types of Structures for Thermal Cycles during Their Lifetimes on Residential Use. ECS Trans. 2015, 68, 2209–2216. [Google Scholar] [CrossRef]
- Sarkar, P.; Yamarte, L.; Rho, H.; Johanson, L. Anode-Supported Tubular Micro-Solid Oxide Fuel Cell. Int. J. Appl. Ceram. Technol. 2007, 4, 103–108. [Google Scholar] [CrossRef]
- Winkler, W.; Krüger, J. Design and Manufacturing of a Tubular Solid Oxide Fuel Cell Combustion System. J. Power Sources 1998, 71, 244–248. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, W.; Zhang, S.; Zhang, Q.; Su, S. Residual Stress Analysis of a Micro-Tubular Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2016, 41, 16173–16180. [Google Scholar] [CrossRef] [Green Version]
- Serincan, M.F.; Pasaogullari, U.; Sammes, N.M. Thermal Stresses in an Operating Micro-Tubular Solid Oxide Fuel Cell. J. Power Sources 2010, 195, 4905–4914. [Google Scholar] [CrossRef]
- Du, Y.; Sammes, N.M. Fabrication and Properties of Anode-Supported Tubular Solid Oxide Fuel Cells. J. Power Sources 2004, 136, 66–71. [Google Scholar] [CrossRef]
- Sin, Y.W.; Galloway, K.; Roy, B.; Sammes, N.M.; Song, J.H.; Suzuki, T.; Awano, M. The Properties and Performance of Micro-Tubular (Less than 2.0 Mm O.D.) Anode Suported Solid Oxide Fuel Cell (SOFC). Int. J. Hydrogen Energy 2011, 36, 1882–1889. [Google Scholar] [CrossRef]
- Hartwell, A.R.; Ahn, J. Complex Material Behavior Seen with Novel Internal Cathode Tubular Solid Oxide Fuel Cells. In ASME International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers: New York, NY, USA, 2021. [Google Scholar]
- O’Hayre, R.P.; Cha, S.-W.; Colella, W.G.; Prinz, F.B. Fuel Cell Fundamentals, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9781119191766. [Google Scholar]
- Ghotkar, R.; Milcarek, R.J. Modeling of the Kinetic Factors in Flame-Assisted Fuel Cells. Sustainability 2022, 14, 4121. [Google Scholar] [CrossRef]
- Fergus, J.W.; Hui, R.; Li, X.; Wilkinson, D.P.; Zhang, J. Solid Oxide Fuel Cells Materials Properties and Performance; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Shi, Y.; Li, C.; Cai, N. Experimental Characterization and Mechanistic Modeling of Carbon Monoxide Fueled Solid Oxide Fuel Cell. J. Power Sources 2011, 196, 5526–5537. [Google Scholar] [CrossRef]
- Yao, W. Hydrogen and Carbon Monoxide Electrochemical Oxidation Reaction Kinetics on Solid Oxide Fuel Cell Anodes. 2013. Available online: http://hdl.handle.net/10012/7772 (accessed on 16 May 2022).
- Li, C.; Shi, Y.; Cai, N. Carbon Deposition on Nickel Cermet Anodes of Solid Oxide Fuel Cells Operating on Carbon Monoxide Fuel. J. Power Sources 2013, 225, 1–8. [Google Scholar] [CrossRef]
- Lawlor, V. Review of the Micro-Tubular Solid Oxide Fuel Cell (Part II: Cell Design Issues and Research Activities). J. Power Sources 2013, 240, 421–441. [Google Scholar] [CrossRef]
- Pihlatie, M.; Kaiser, A.; Mogensen, M. Mechanical Properties of NiO/Ni-YSZ Composites Depending on Temperature, Porosity and Redox Cycling. J. Eur. Ceram. Soc. 2009, 29, 1657–1664. [Google Scholar] [CrossRef]







| Flow Rate (mL·min−1) | ||||||
|---|---|---|---|---|---|---|
| Simulated Combustion Exhaust | Dilute Hydrogen | |||||
| Equivalence Ratio | H2 | CO | CO2 | N2 | H2 | N2 |
| 1.1 | 32 | 68 | 321 | 1855 | 100 | 2177 |
| 1.2 | 37 | 63 | 145 | 982 | 100 | 1128 |
| 1.3 | 42 | 58 | 86 | 670 | 100 | 757 |
| 1.4 | 46 | 54 | 59 | 513 | 100 | 572 |
| 1.5 | 50 | 50 | 44 | 420 | 100 | 462 |
| 1.6 | 52 | 48 | 34 | 356 | 100 | 390 |
| 1.7 | 55 | 45 | 28 | 311 | 100 | 339 |
| 1.8 | 57 | 43 | 24 | 277 | 100 | 301 |
| 1.9 | 58 | 42 | 21 | 250 | 100 | 271 |
| No Stability Test | Stability Test | |||
|---|---|---|---|---|
| Element | Mass% | Atom% | Mass% | Atom% |
| C | 4.94 | 15.81 | 6.23 | 17.73 |
| O | 19.73 | 47.38 | 22.78 | 48.71 |
| Ni | 21.46 | 14.04 | 33.14 | 19.31 |
| Y | 7.7 | 3.33 | 5.54 | 2.13 |
| Zr | 46.16 | 19.44 | 32.31 | 12.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartwell, A.R.; Wilhelm, C.A.; Welles, T.S.; Milcarek, R.J.; Ahn, J. Effects of Synthesis Gas Concentration, Composition, and Operational Time on Tubular Solid Oxide Fuel Cell Performance. Sustainability 2022, 14, 7983. https://doi.org/10.3390/su14137983
Hartwell AR, Wilhelm CA, Welles TS, Milcarek RJ, Ahn J. Effects of Synthesis Gas Concentration, Composition, and Operational Time on Tubular Solid Oxide Fuel Cell Performance. Sustainability. 2022; 14(13):7983. https://doi.org/10.3390/su14137983
Chicago/Turabian StyleHartwell, Alexander R., Cole A. Wilhelm, Thomas S. Welles, Ryan J. Milcarek, and Jeongmin Ahn. 2022. "Effects of Synthesis Gas Concentration, Composition, and Operational Time on Tubular Solid Oxide Fuel Cell Performance" Sustainability 14, no. 13: 7983. https://doi.org/10.3390/su14137983







