Study on Water-Heat-Solution Transport Law in Cr(VI)-Contaminated Soil during Electric Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Pretreatment
2.2. Materials
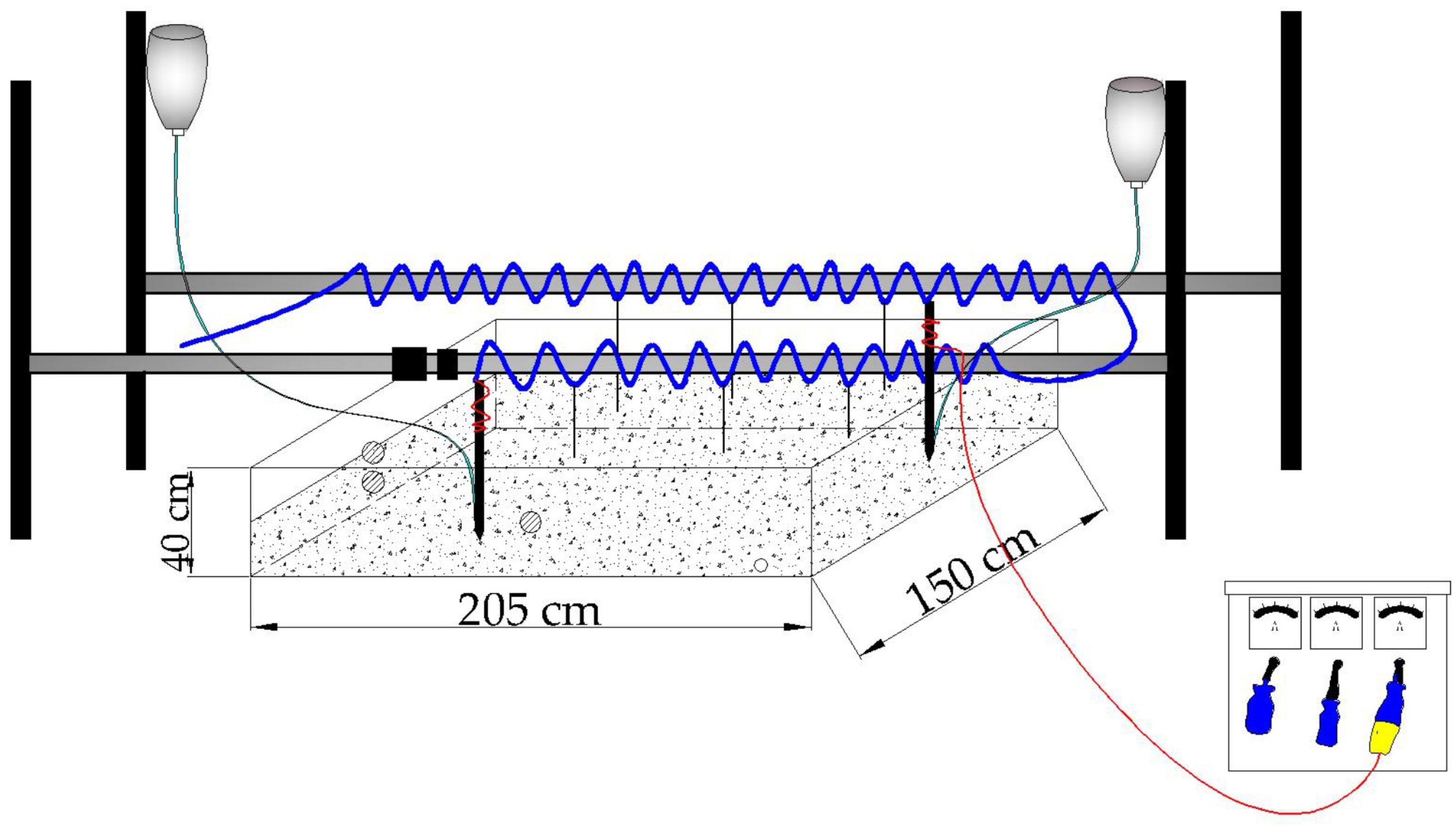
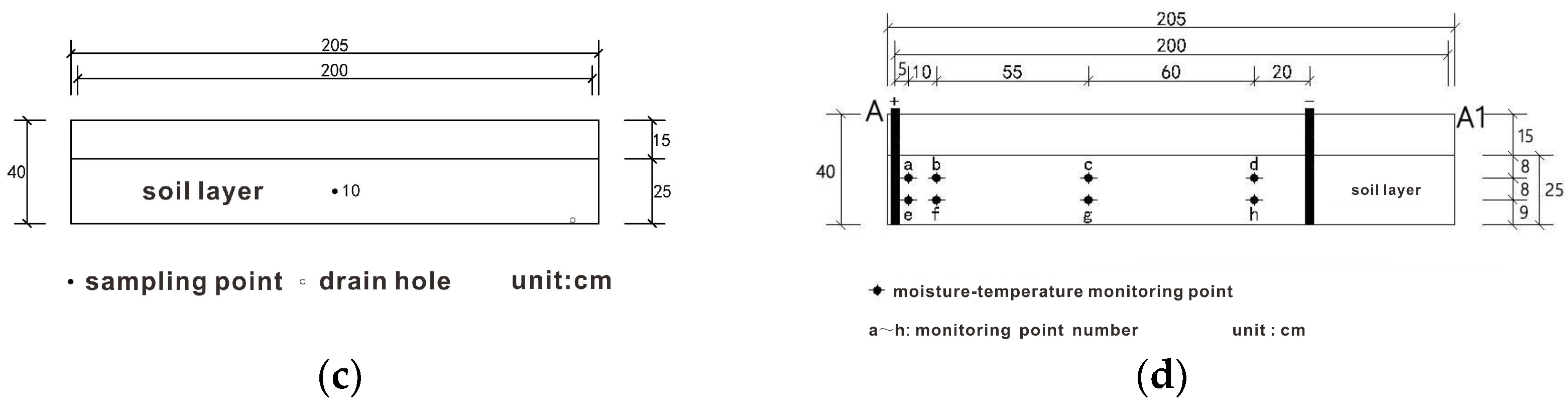
2.3. Experimental Setup and Procedure
2.4. Determination of Cr(VI) in Soil
2.5. Electric Repair Model
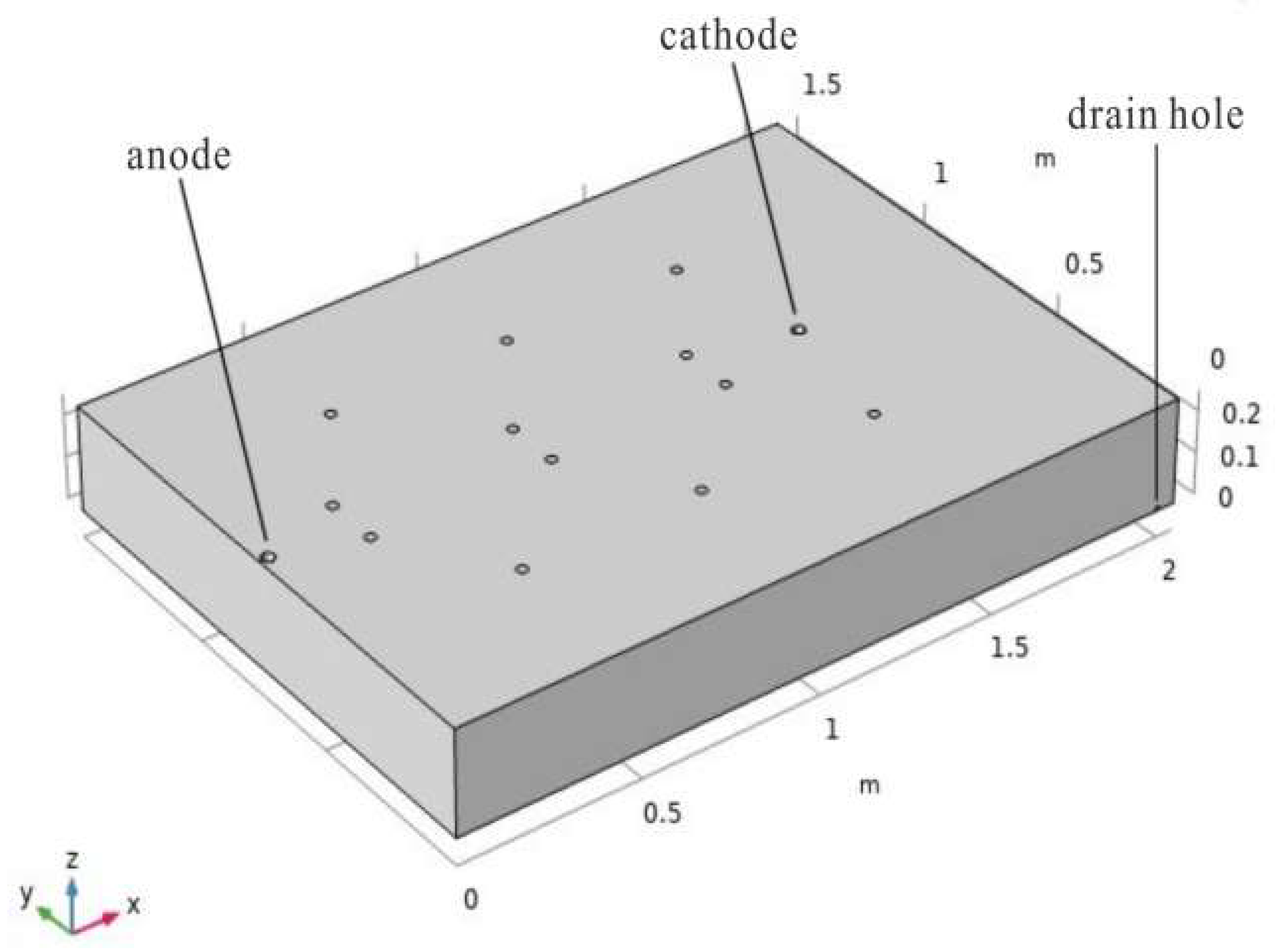
2.5.1. Model Establishment
2.5.2. Setting Observation Points and Time Steps
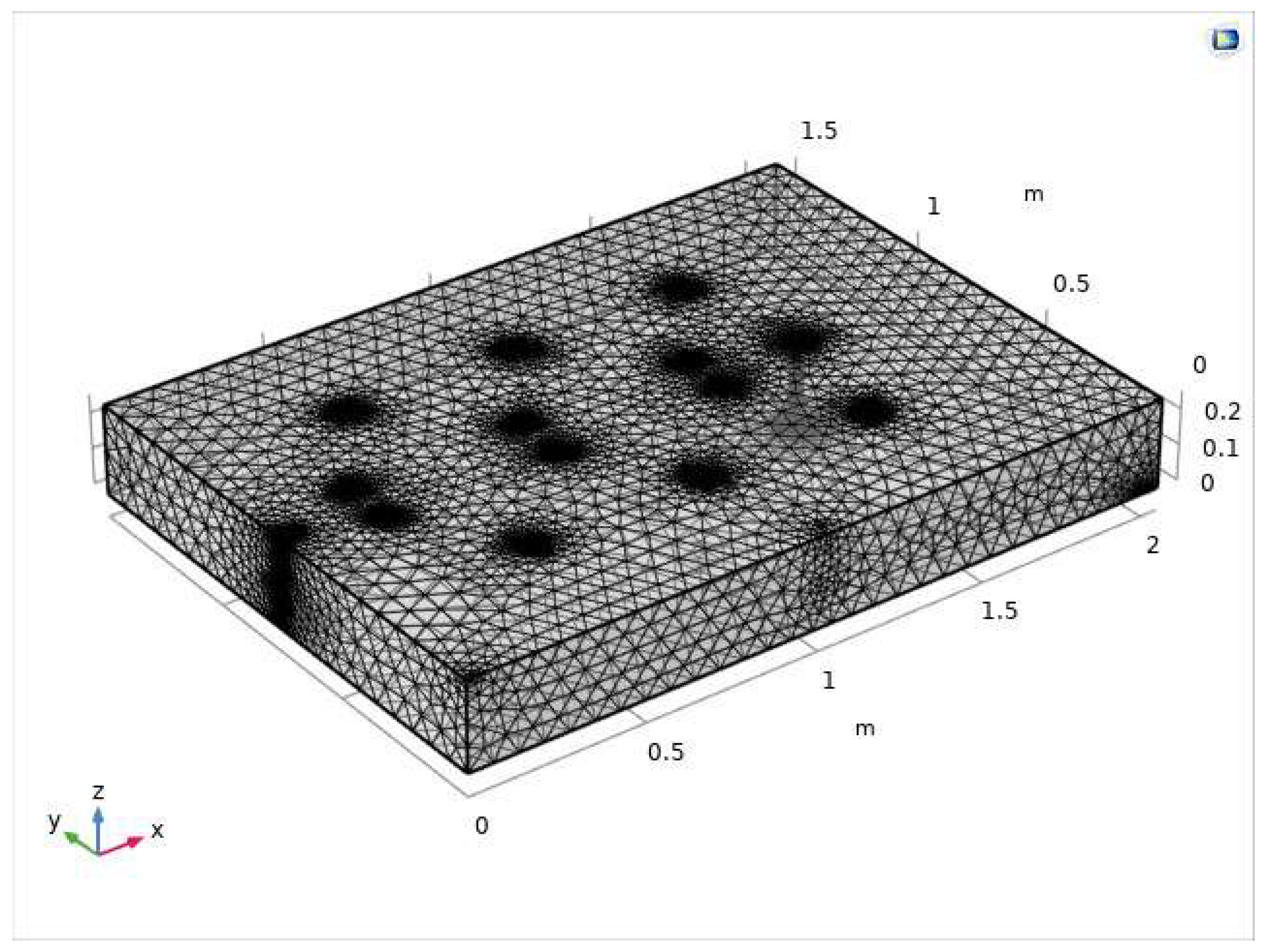
2.5.3. Meshing
2.5.4. Model Boundary Conditions
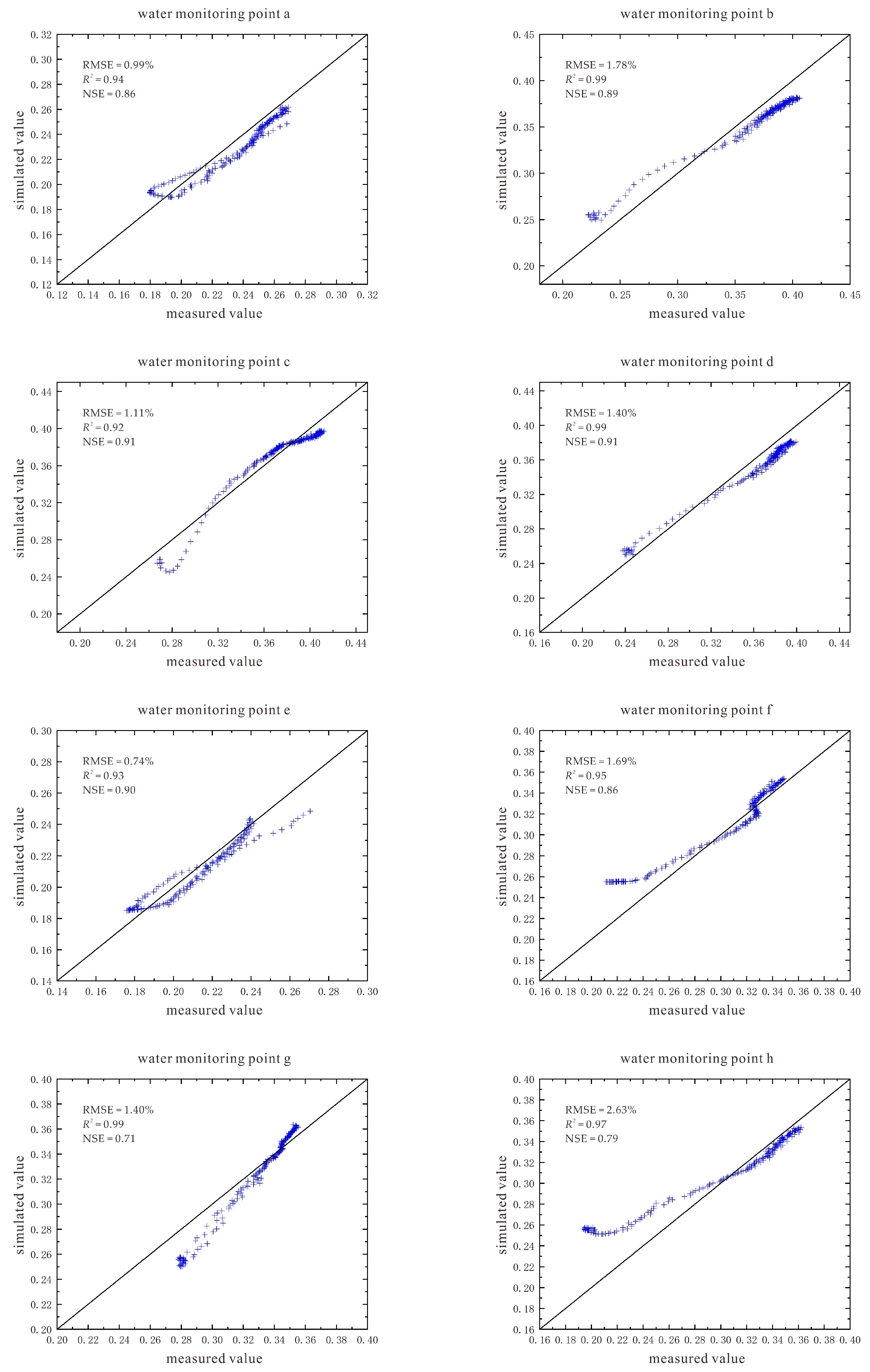
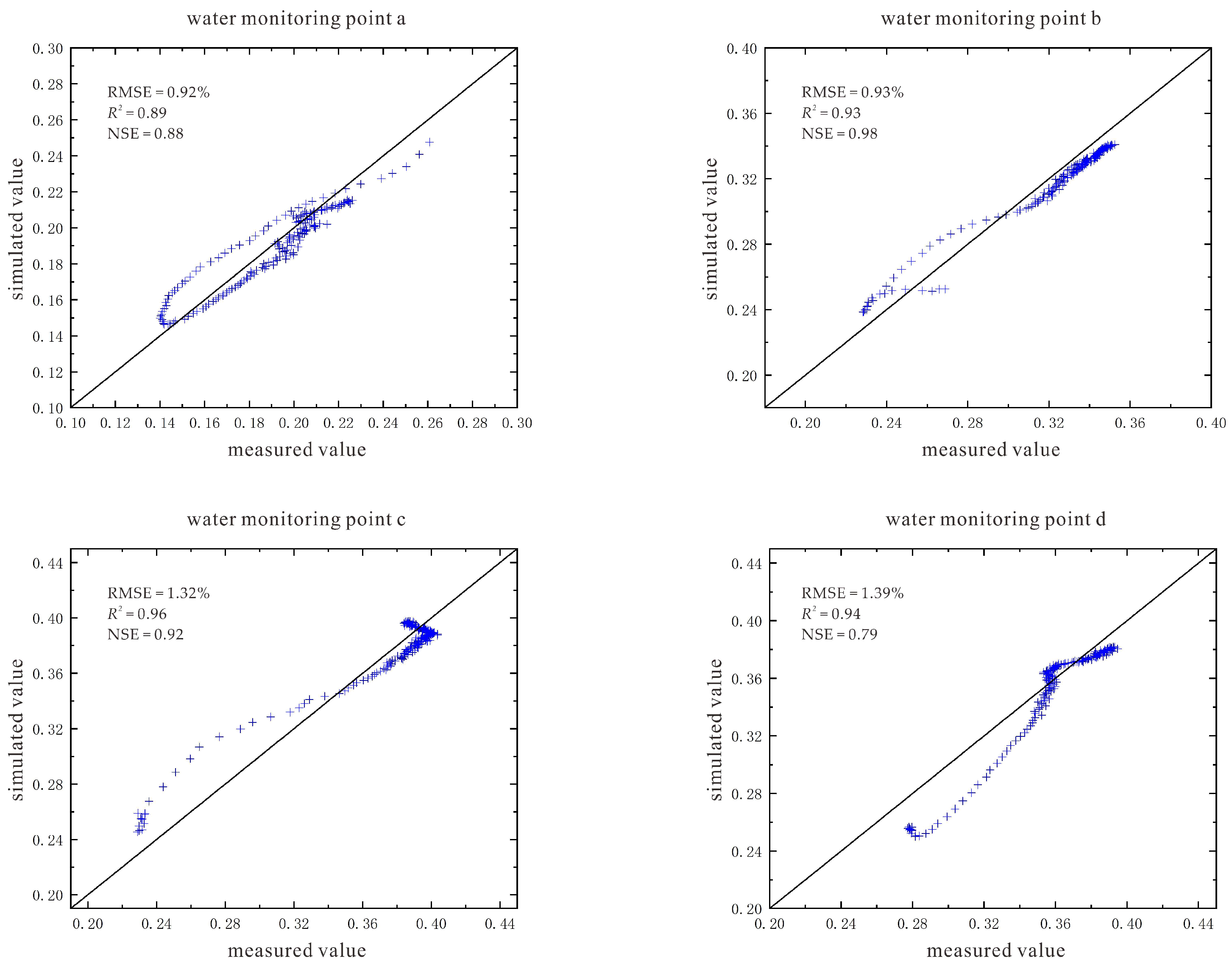
2.5.5. Model Evaluation Indicators
3. Results and Discussion
3.1. Experimental Analysis
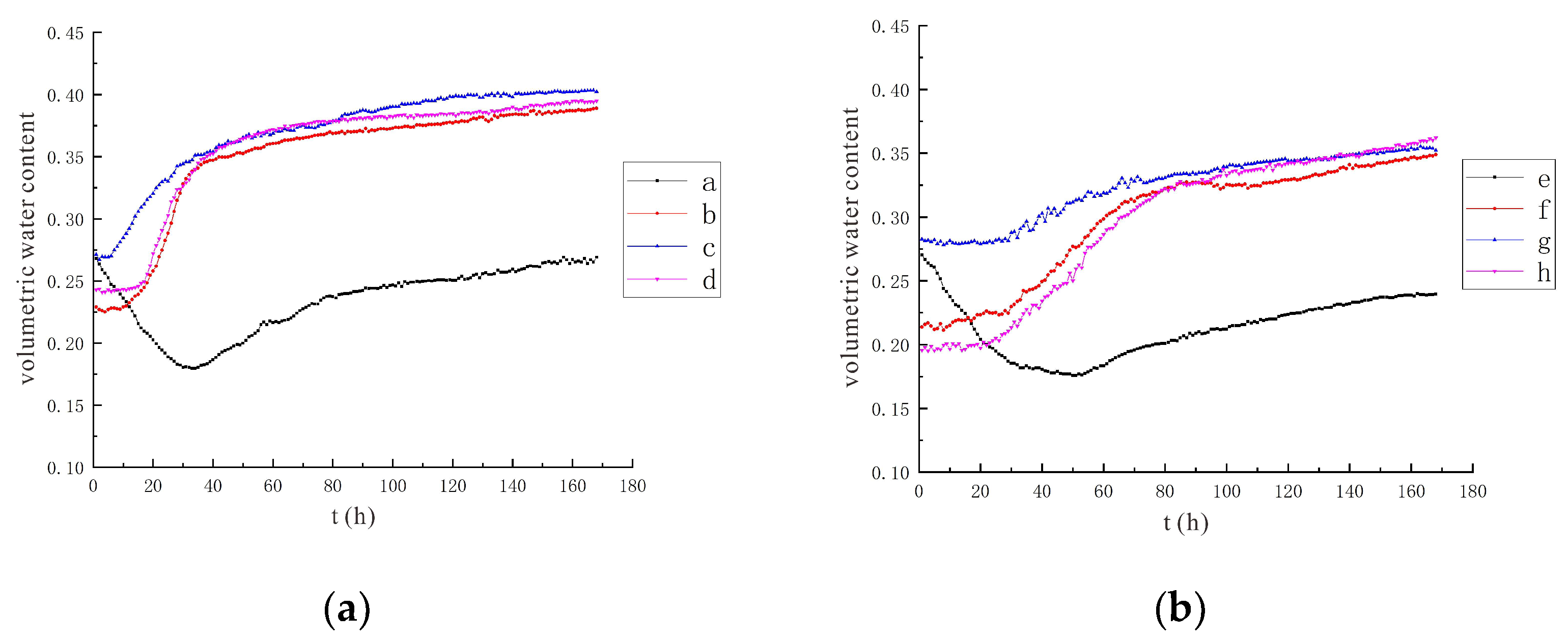
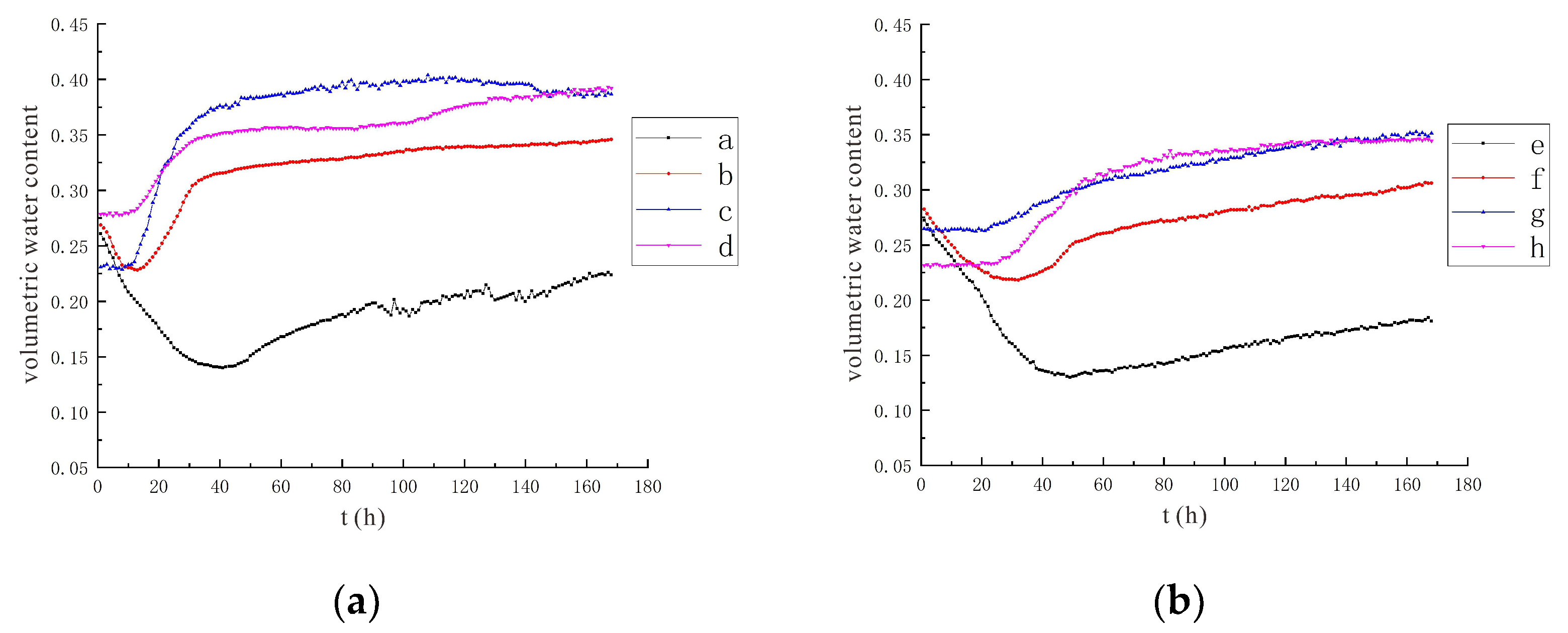
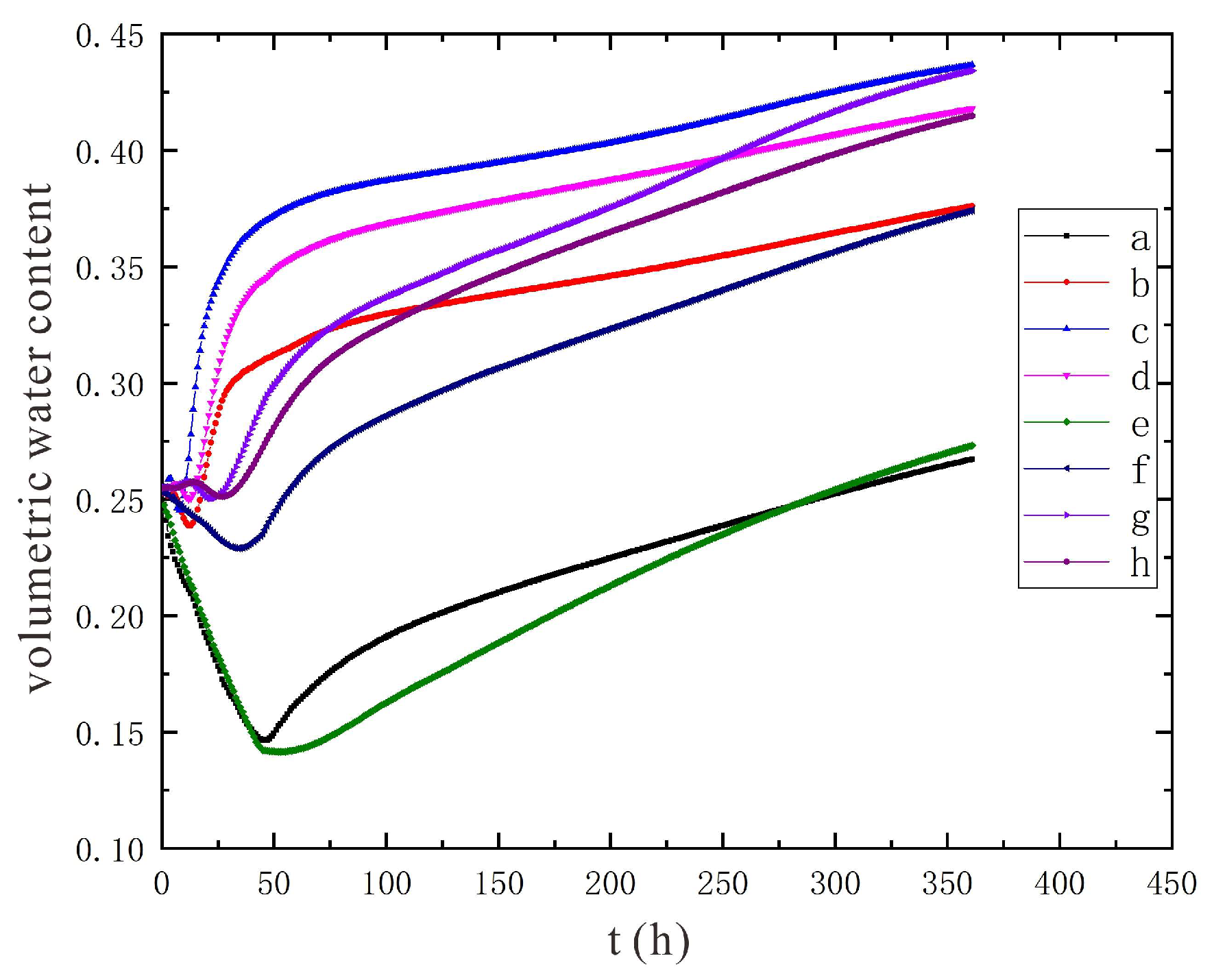
3.1.1. Characteristics of Soil Moisture Distribution
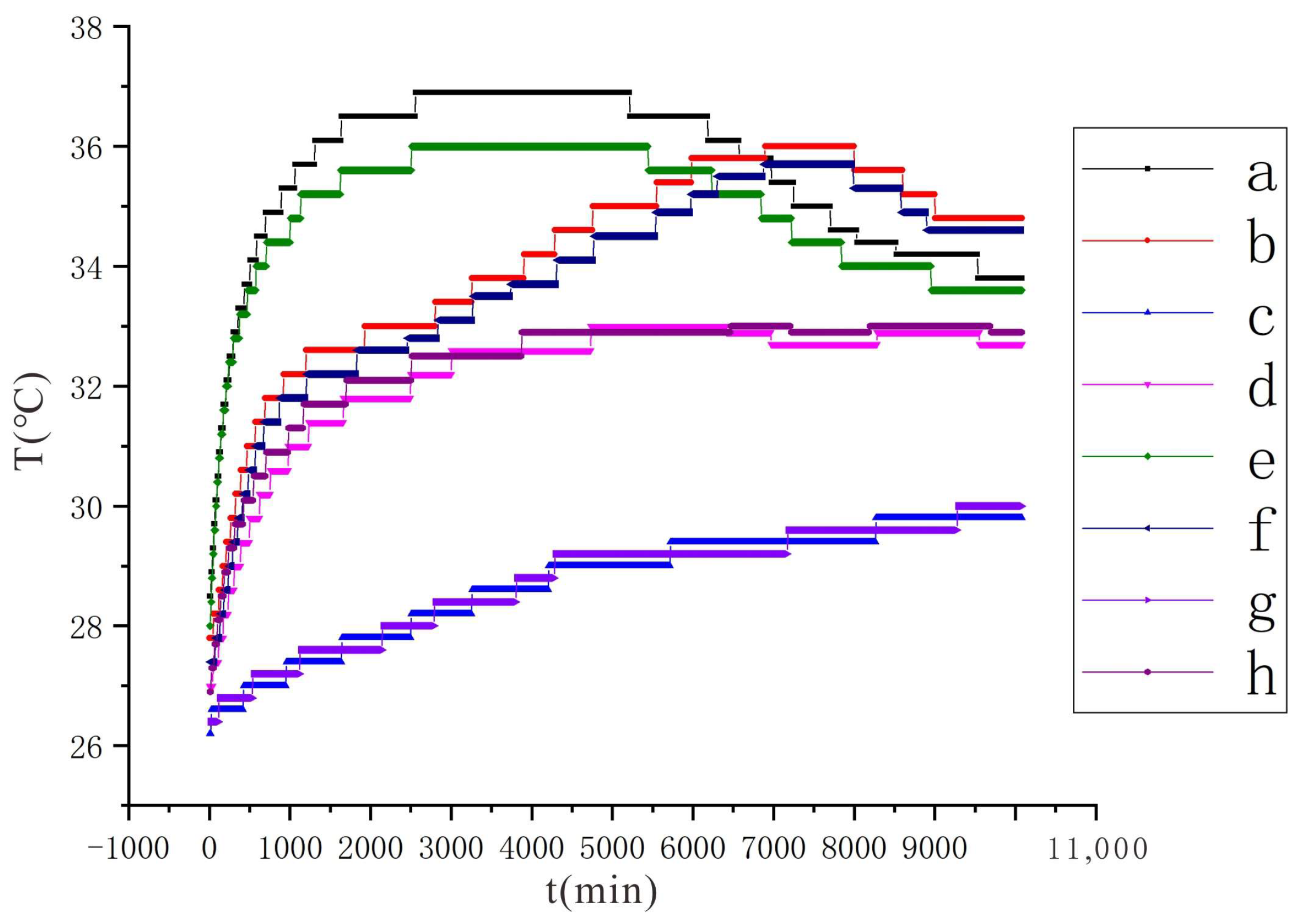
3.1.2. Characteristics of Soil Temperature Change
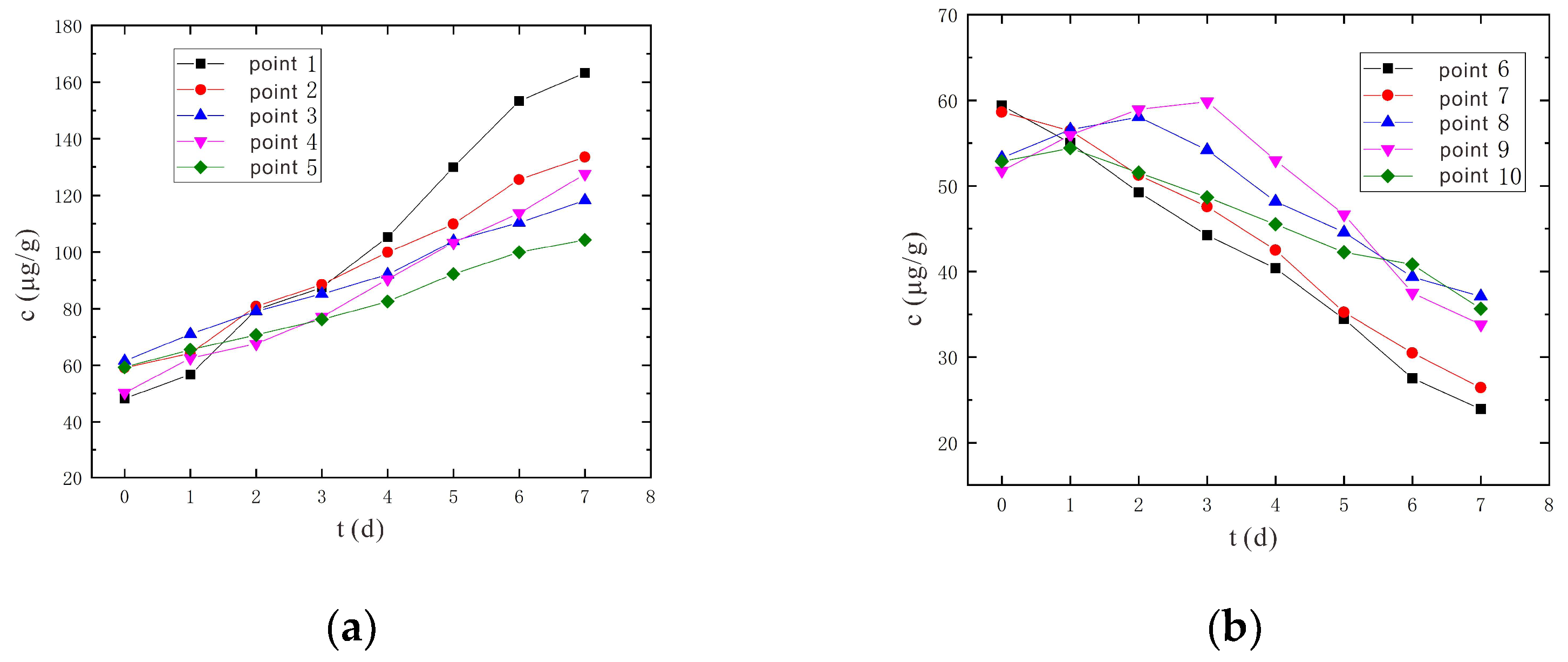
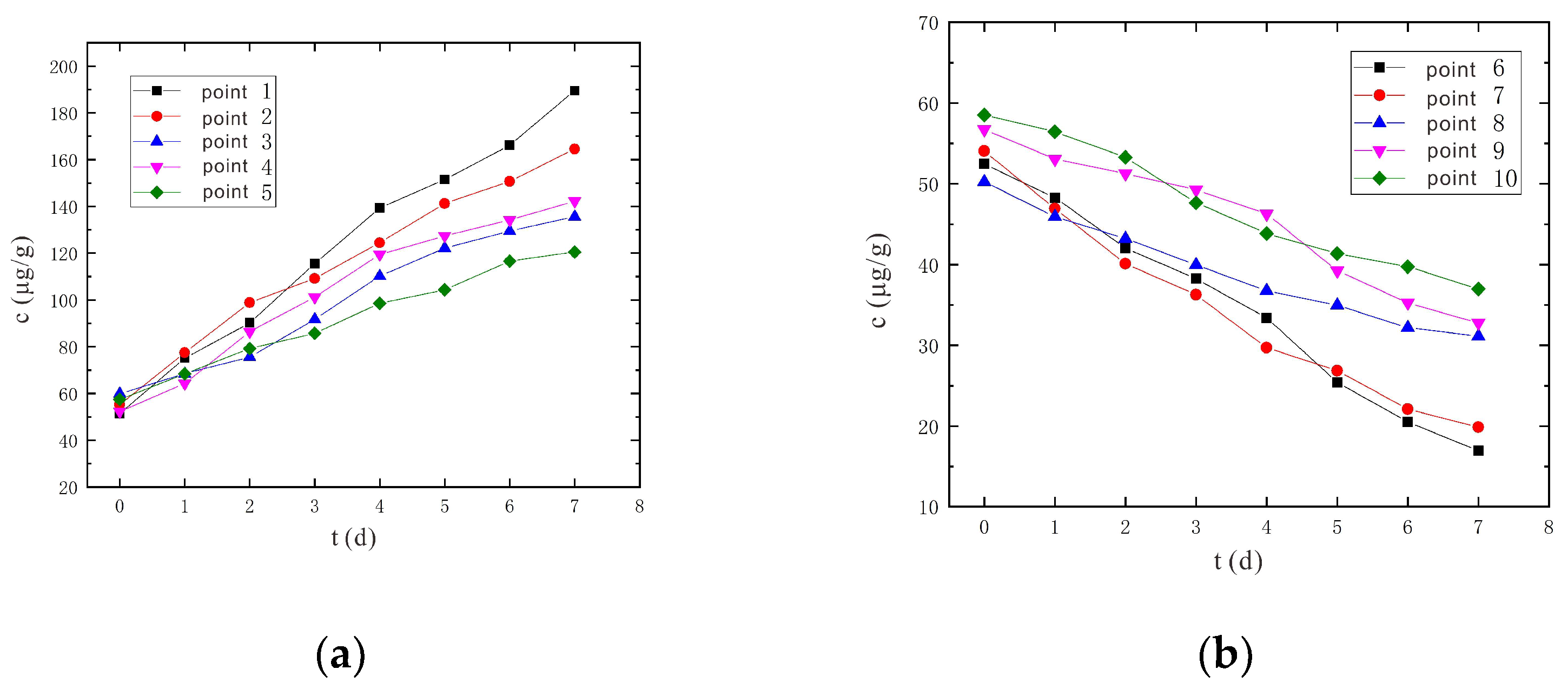
3.1.3. Variation Characteristics of Hexavalent Chromium Concentration
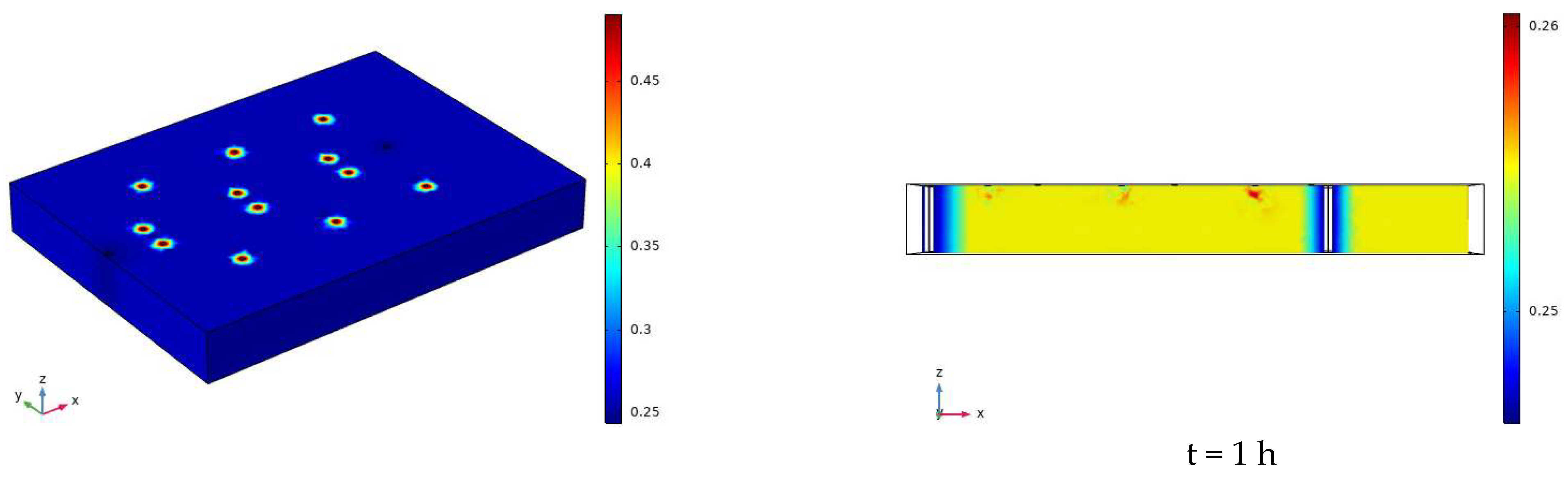
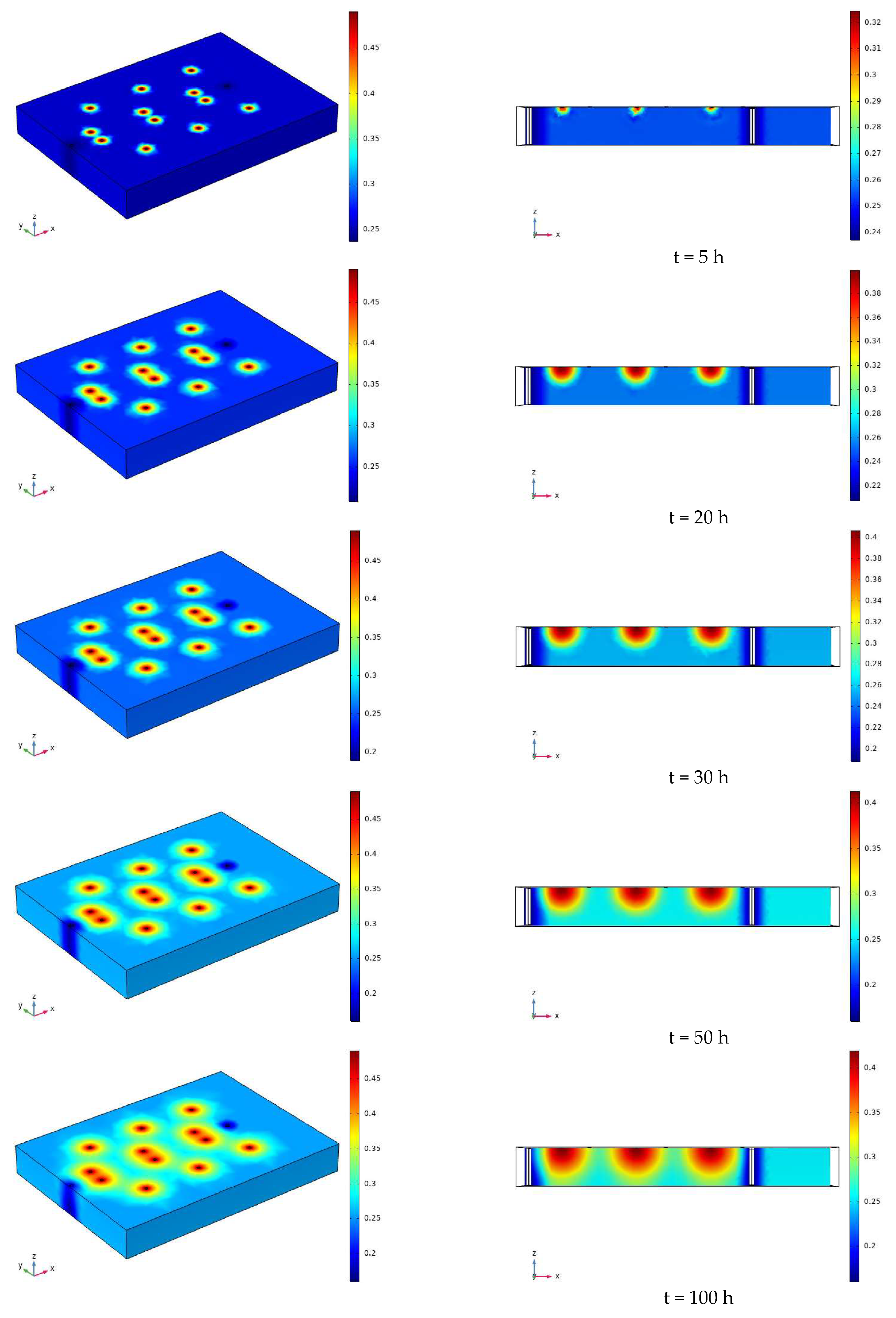
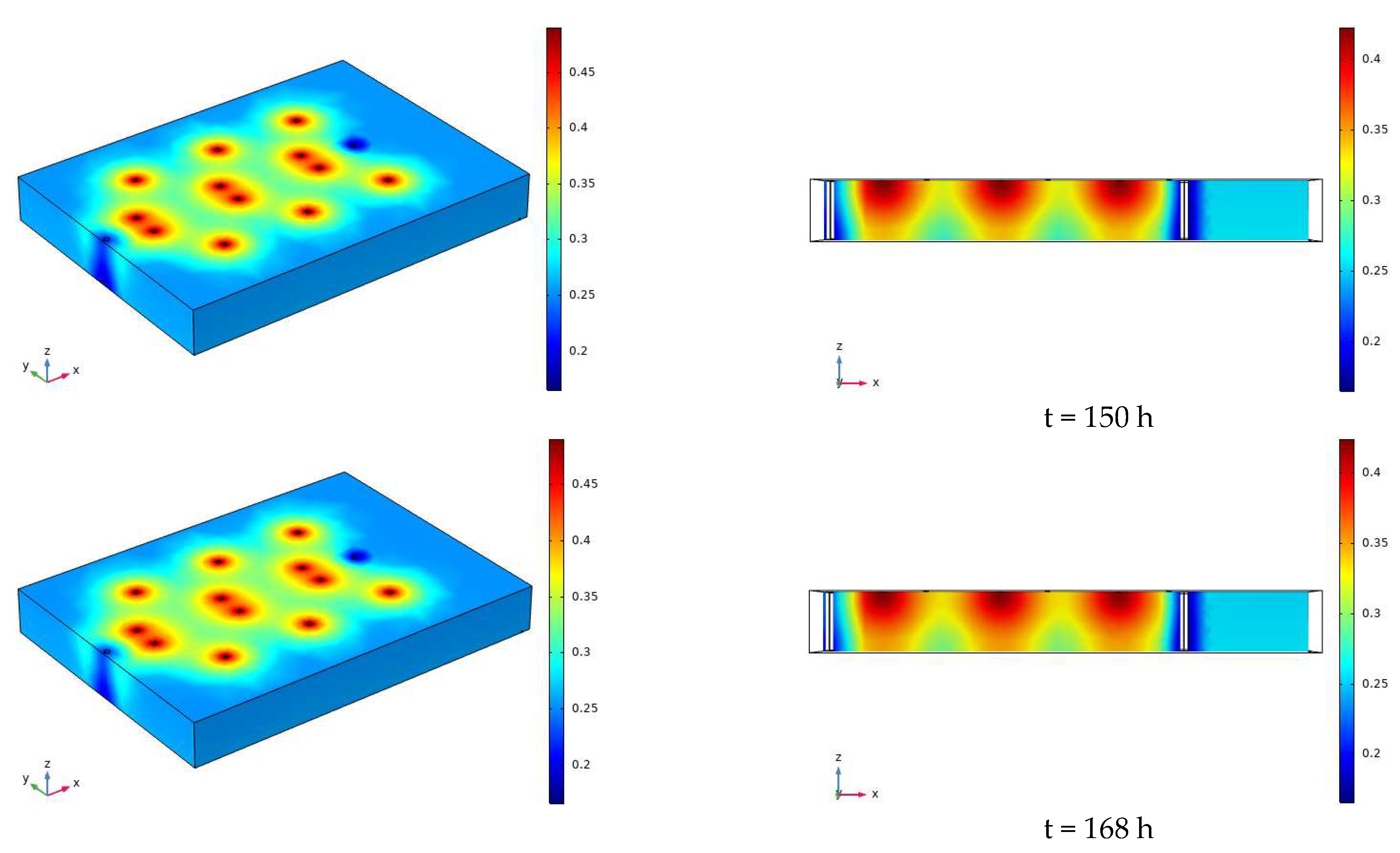
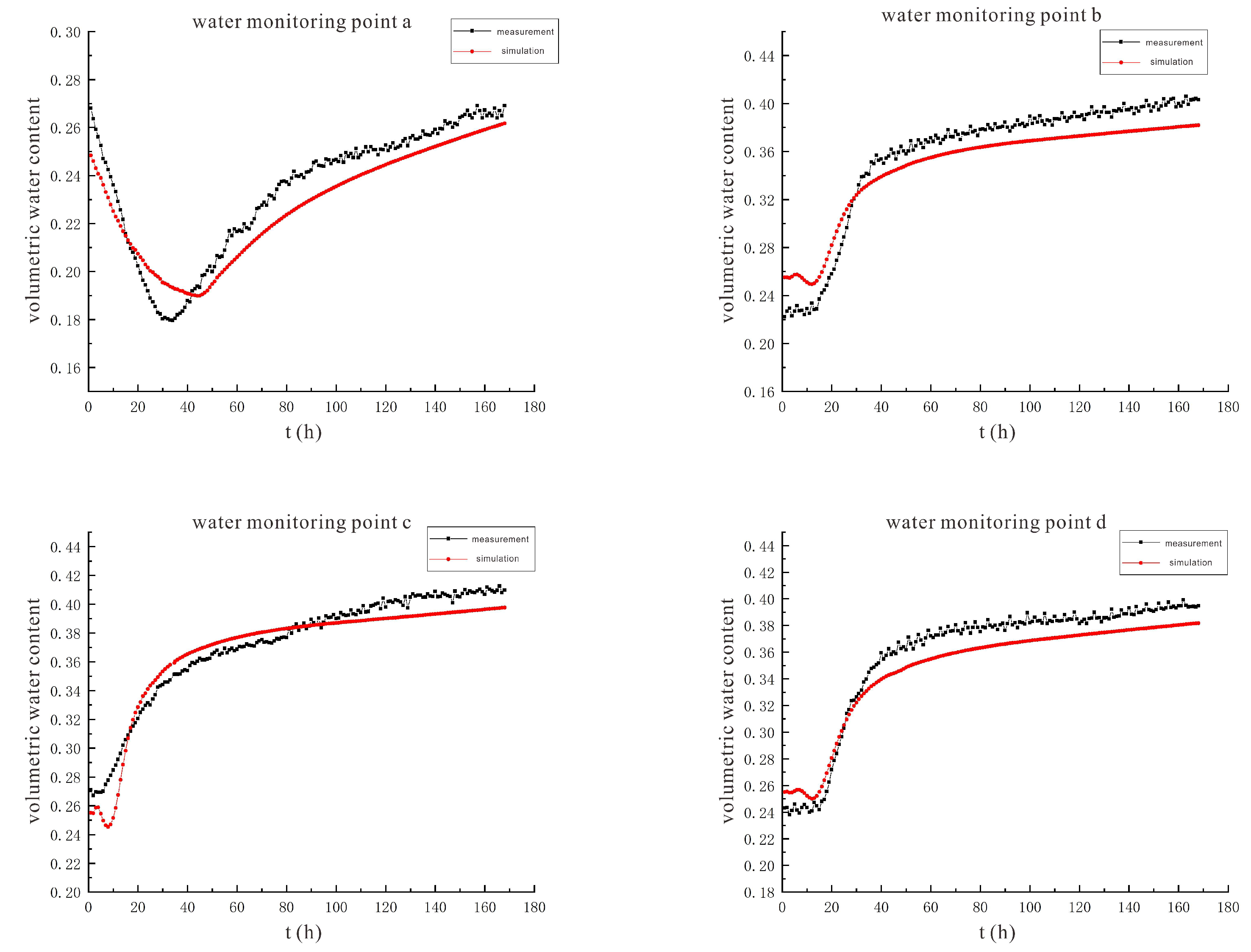
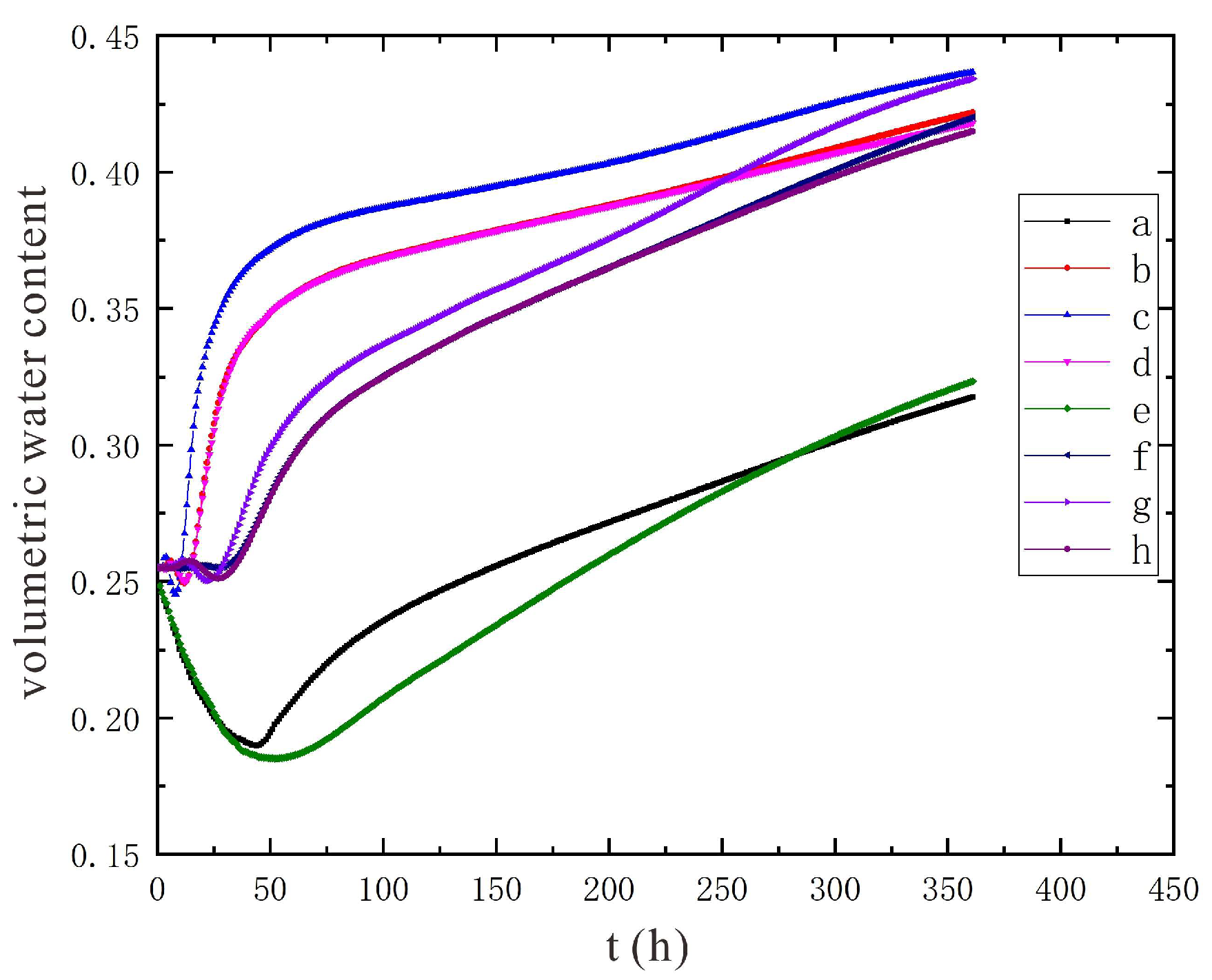
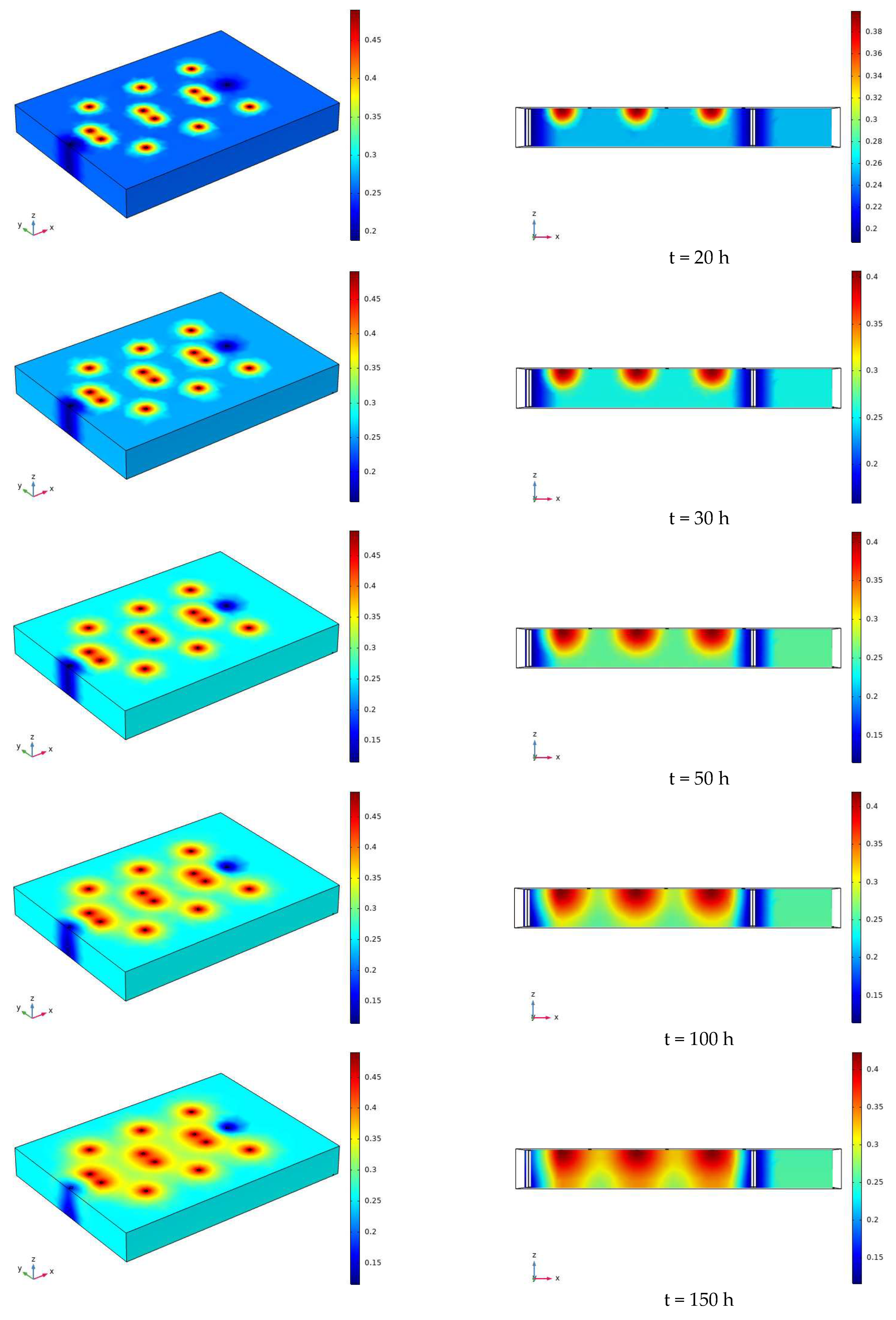
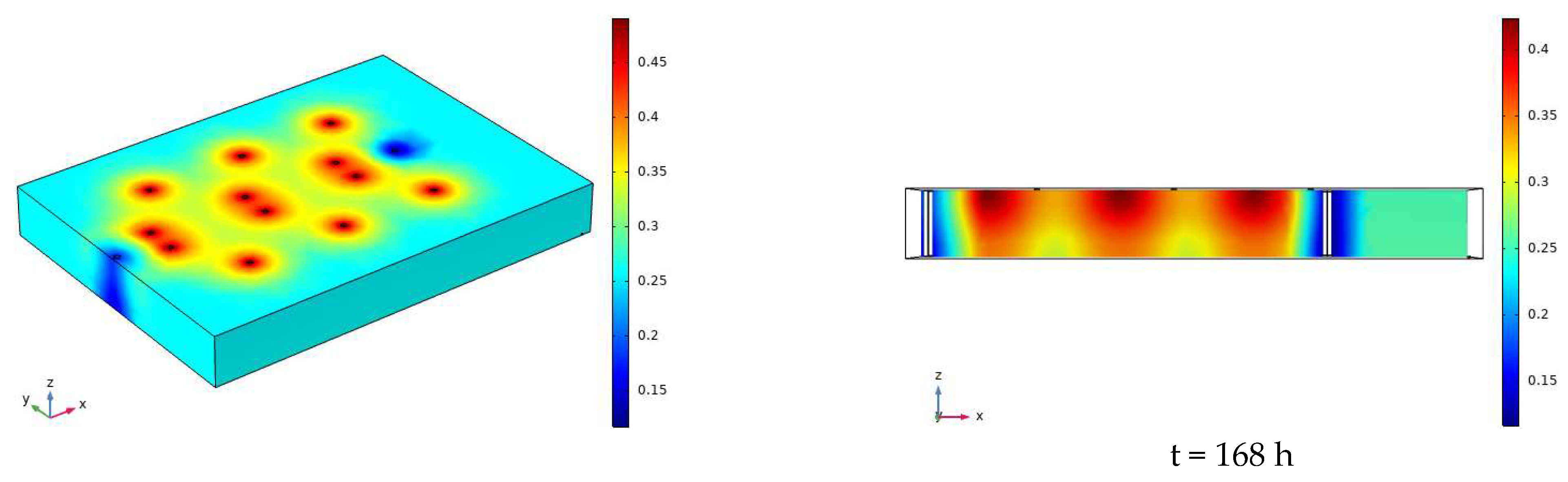
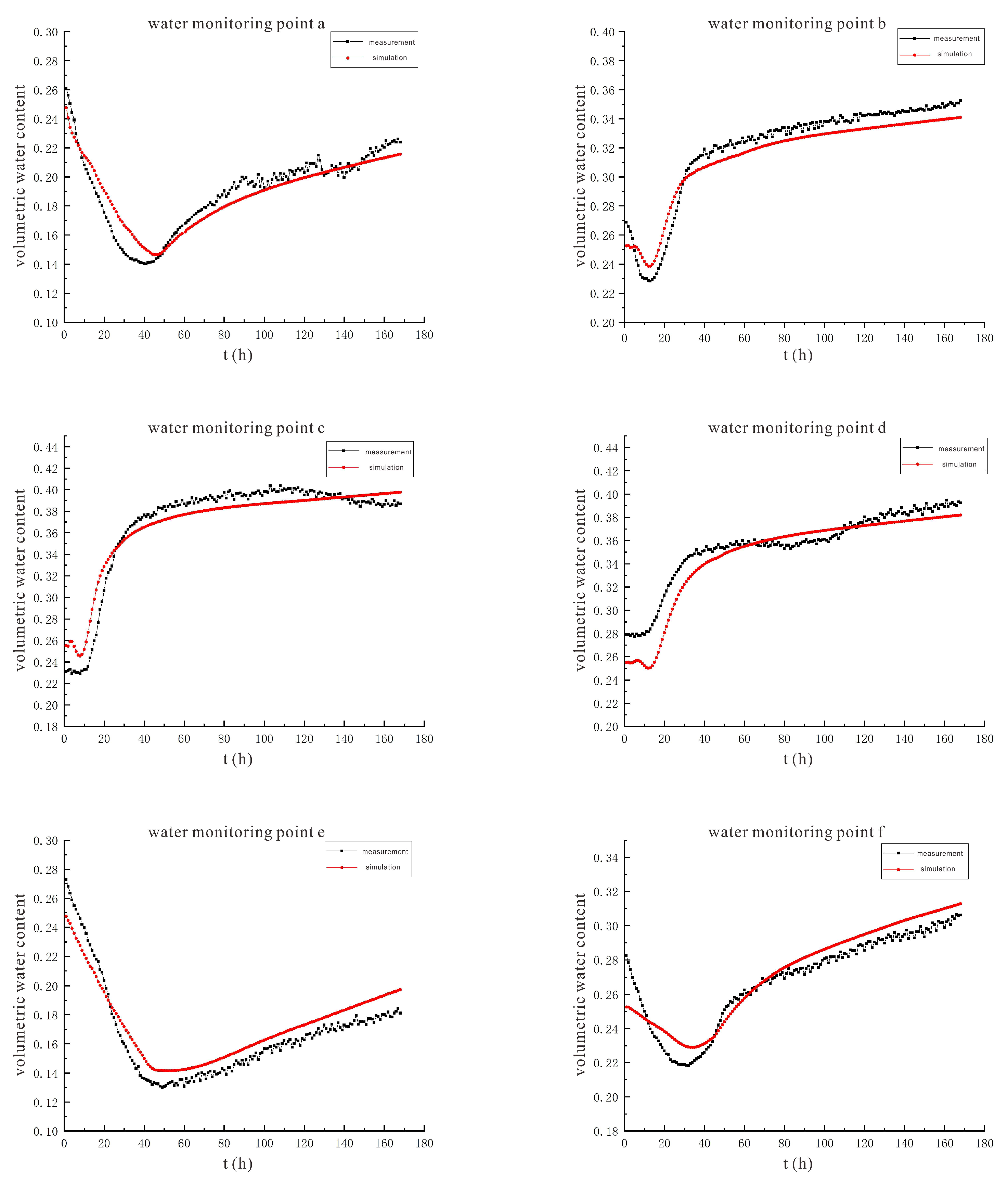
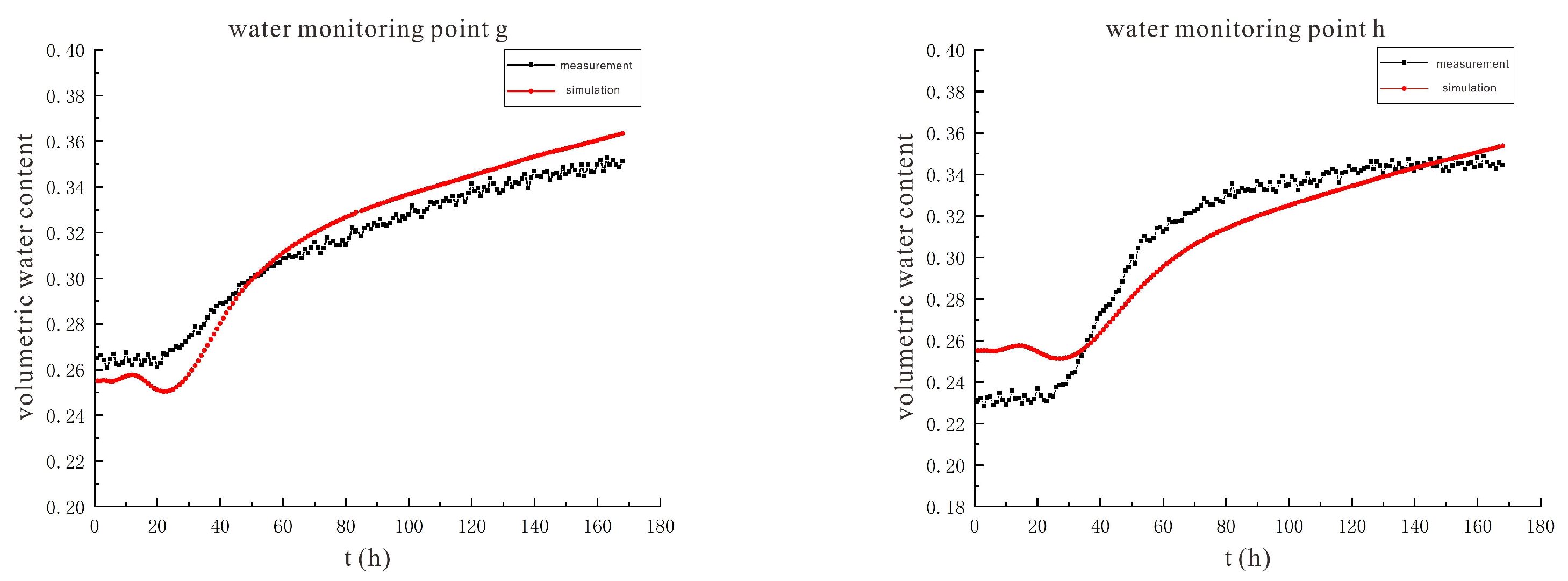
3.2. Characteristics of Model Soil Moisture Distribution
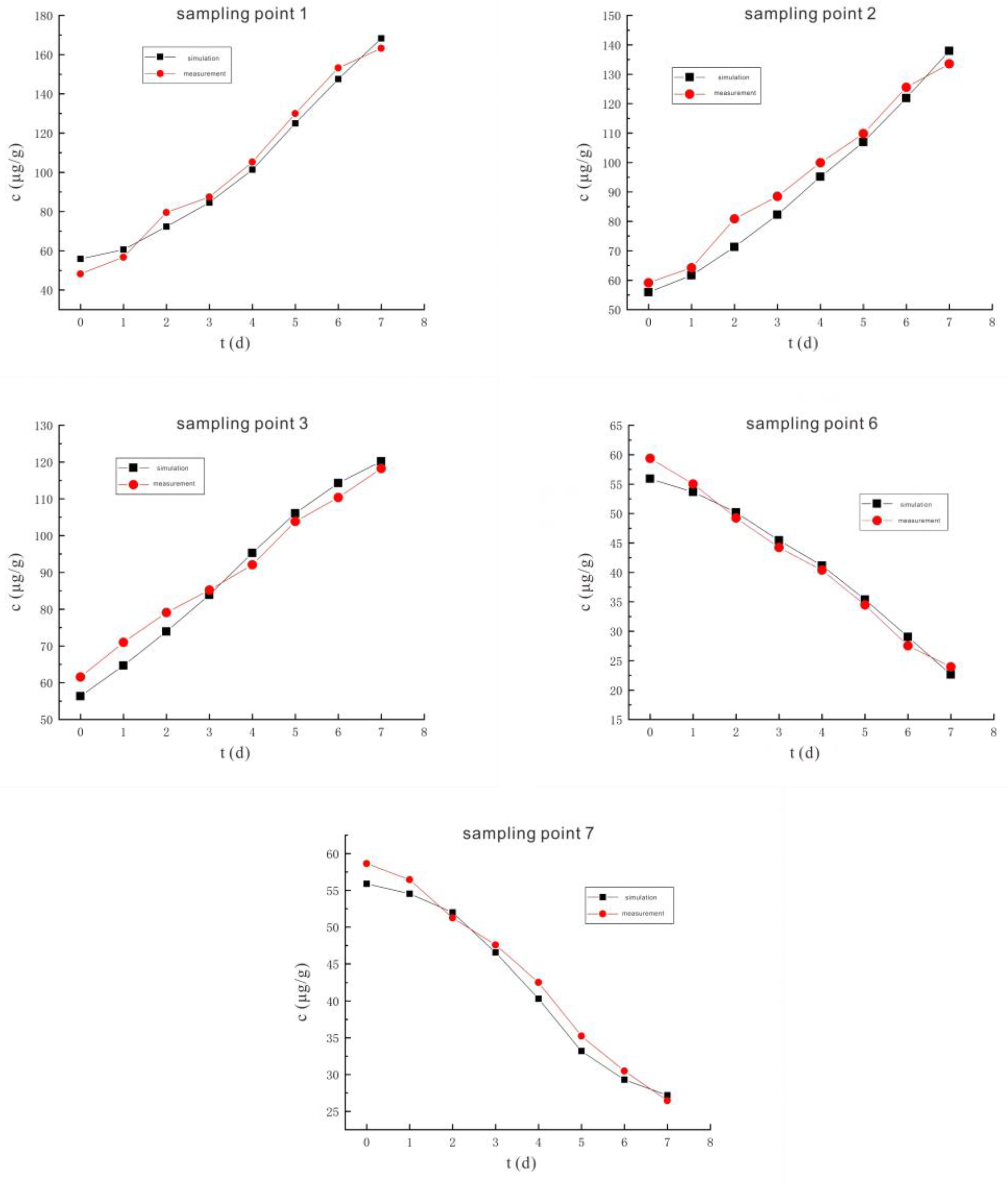
3.2.1. Voltage 90 V, Electrode Distance 1.5 m
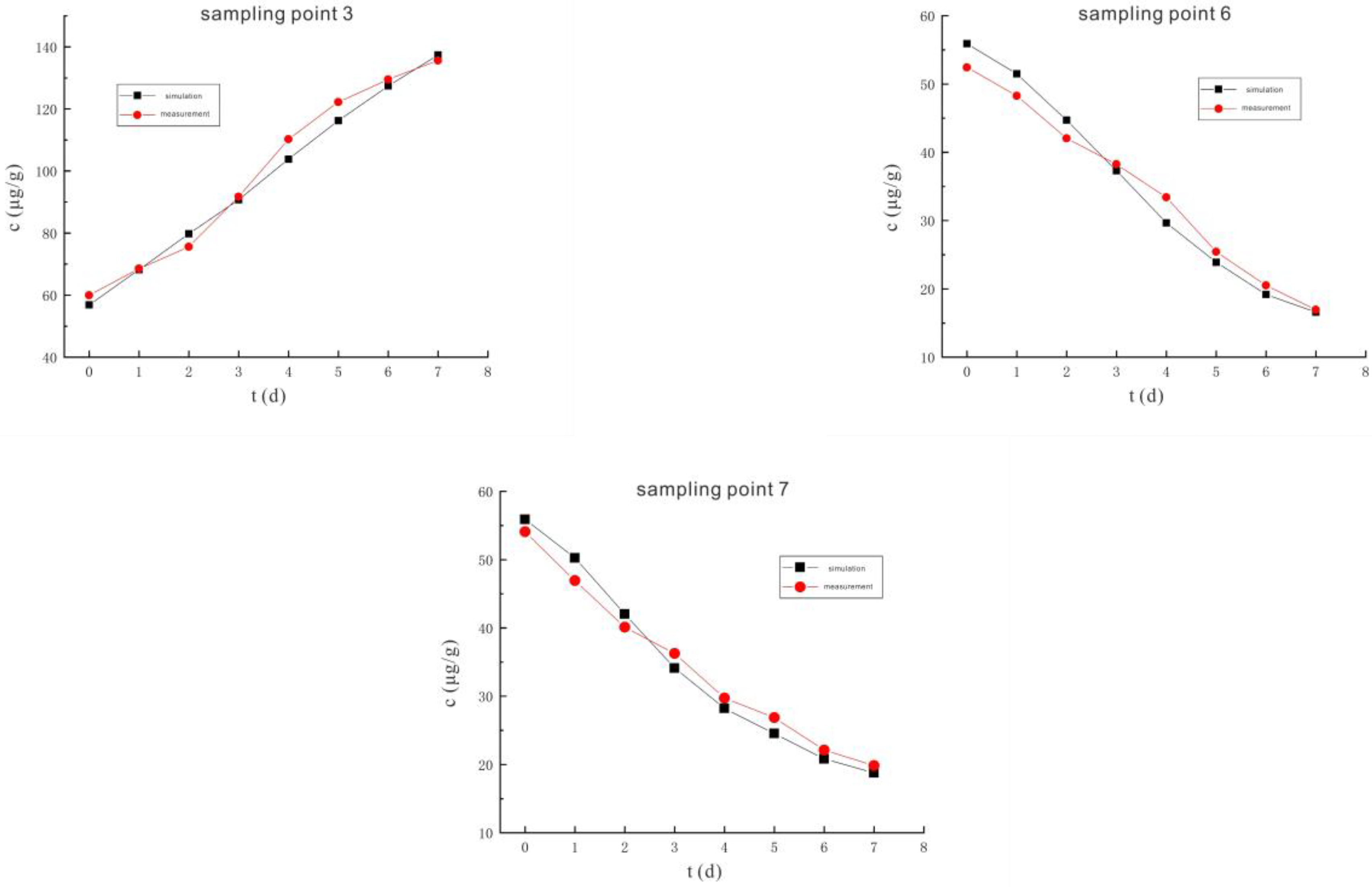
3.2.2. Voltage 110 V, Electrode Distance 1.5 m
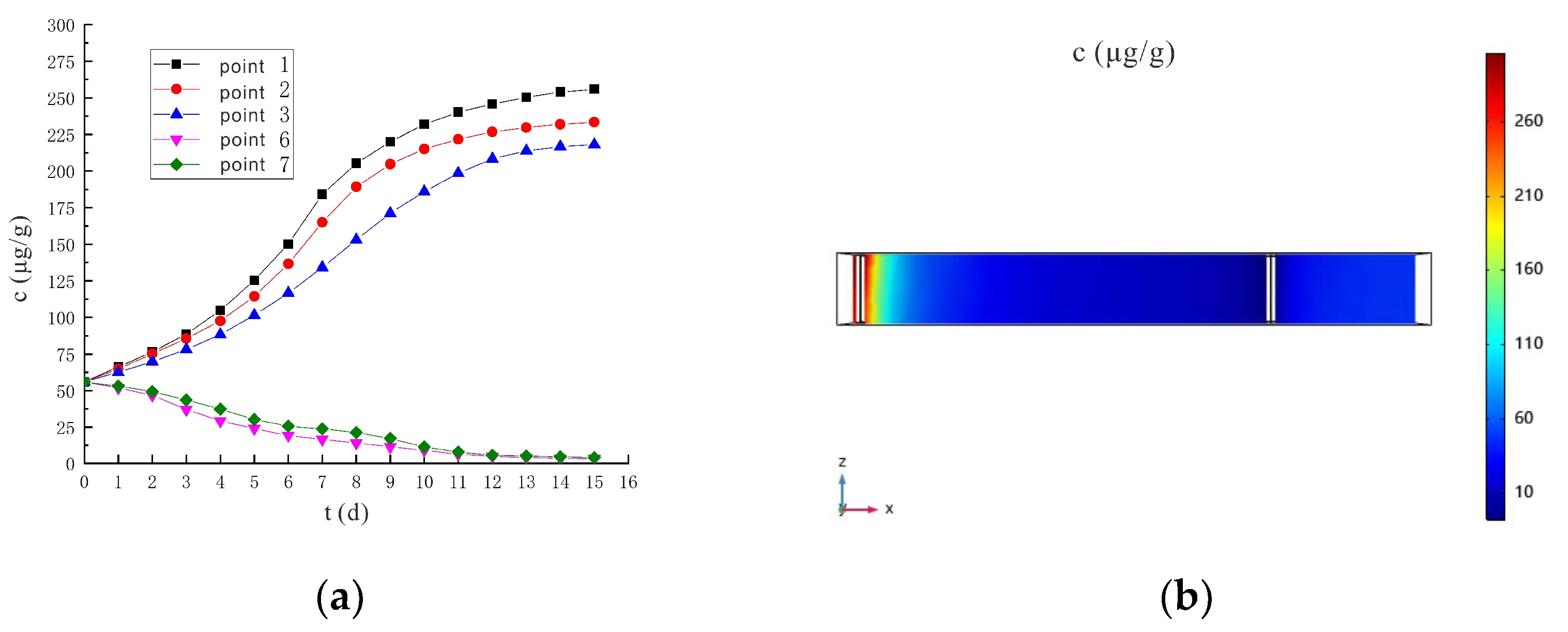
3.3. Variation Characteristics of Cr(VI) Concentration
3.3.1. Voltage 90 V, Electrode Distance 1.5 m
3.3.2. Voltage 110 V, Electrode Distance 1.5 m
4. Conclusions
- (1)
- During the electrification process of the indoor experiment of electric remediation, the volumetric water content of the soil near the anode began to decline. After the external supplementary water arrived, the volumetric water content of the soil near the anode gradually increased. As the voltage increases, the soil volumetric water content decreases to a greater extent. The electrolysis of water occurs in a certain range near the anode and cathode electrodes, and this range will expand when the voltage increases. Beyond this range, the influence of the electric field on the water transport can be ignored.
- (2)
- The closer the location to the cathode or anode, the faster the rate of soil temperature rise and the higher the maximum temperature that can be reached. Furthermore, the higher the voltage, the faster the soil temperature rises at the same location and the higher the peak temperature that can be reached. At a voltage of 90 V and an electrode distance of 1.5 m, the maximum temperature can reach 36.9 °C at a distance of 5 cm from the anode. At 110 V and an electrode distance of 1.5 m, the maximum temperature can reach 52.4 °C at a distance of 5 cm from the anode. The higher temperature also contributes to soil moisture transport.
- (3)
- An increase in voltage would increase the removal rate of Cr(VI), and the removal rate of Cr(VI) was higher in shallow soil than in deep soil. After 7 days of electrokinetic remediation, the Cr(VI) removal rates reached 66.03% and 60.80% for sampling points 6 and 7, respectively, at a voltage of 90 V and an electrode distance of 1.5 m. In particular, at 110 V and 1.5 m electrode distance, the removal rates of Cr(VI) at sampling points 6 and 7 reached 75.96% and 70.74%, respectively. The removal rate was improved by nearly 10% at higher voltage conditions.
- (4)
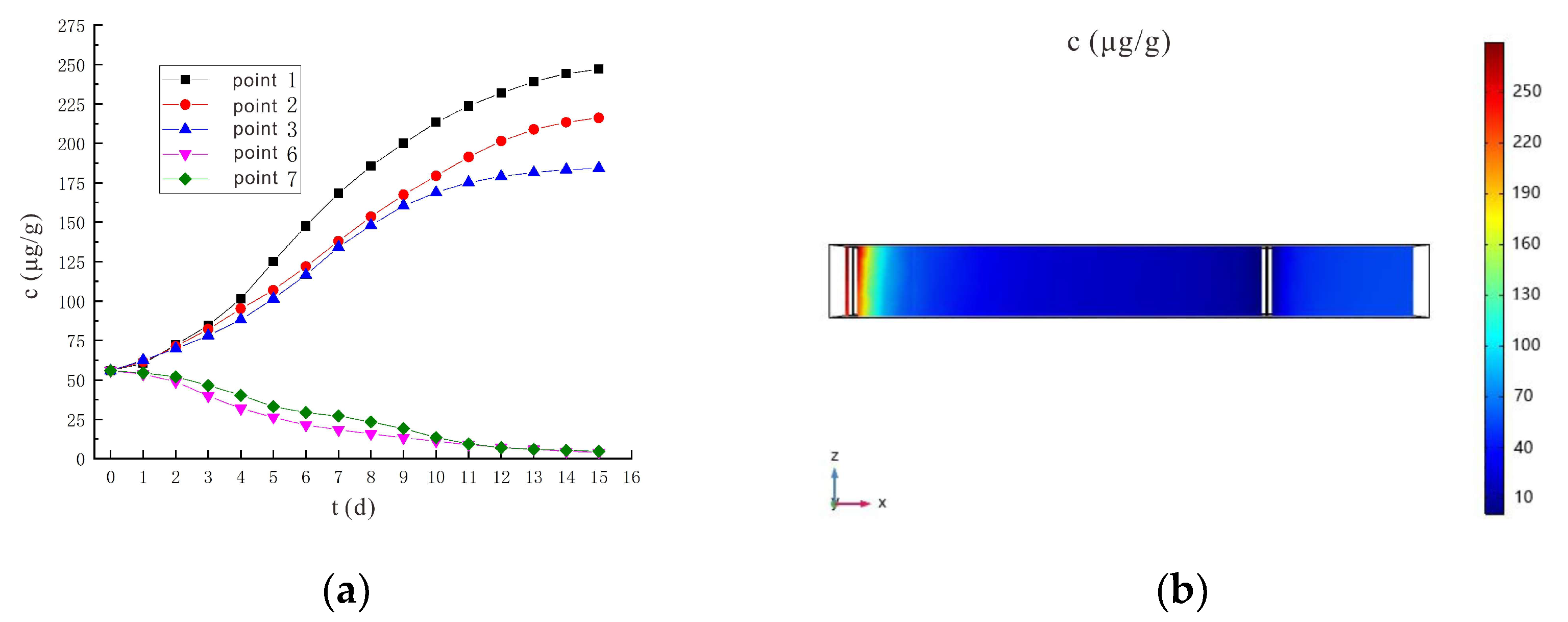
- Through the numerical simulation prediction results of the soil volumetric water content at different depths in the same location, it is found that the soil volumetric water content at the shallower depths is initially greater than that at the deeper depths. However, with the increase of time, the gap between the two will gradually narrow, and finally the volumetric water content of the soil at the deeper depths will in turn exceed the volumetric water content of the soil at the shallower depths.
- (5)
- It is predicted by numerical simulation that under the conditions of a voltage of 90 V and an electrode distance of 1.5 m, the content of hexavalent chromium in the soil at the sampling point on the 14th day is lower than the risk control value of hexavalent chromium in the soil 5.7 μg/g, which meets the remediation requirements. Under the conditions of a voltage of 110 V and an electrode distance of 1.5 m, the soil at the sampling point met the remediation requirements after 11 days. The increased voltage shortens the time it takes for the soil remediation to succeed.
- (6)
- This study did not consider the conversion of hexavalent chromium to trivalent chromium, which is also an important factor in the change of hexavalent chromium content in soil, so future work could be improved; at the same time, the influence of evaporation conditions was missing in the modeling process, and there was a relatively large increase in soil temperature under the action of the electric field, which would cause the strengthening of evaporation, and this factor would have some influence on the volumetric water content of soil. Additionally, the possible influence of electric remediation on the soil ecosystem of contaminated sites also needs to be included in future consideration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, Z.; Zhang, J.C.; Liao, S.L.; Ma, Q.; Wang, Q.G.; Zhang, J.F. Research and Application of Remediation Technology for Chromium Contaminated Sites. Environ. Eng. 2015, 33, 159–162. [Google Scholar]
- Kotas, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Hu, L.G.; Cai, Y.; Jiang, G.B. Occurrence and speciation of polymeric chromium(III), monomeric chromium(III) and chromium(VI) in environmental samples. Chemosphere 2016, 156, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Wang, H.; Sun, Y.; Gao, Y.; Li, S.; Wang, Y.; Guo, L. Remediation of Cr(VI) from contaminated soil with the combination of biogas residue and ferrous sulfate. Acta Sci. Circumstantiae 2021, 41, 4161–4169. [Google Scholar]
- Sarangi, A.; Krishnan, C. Comparison of in vitro Cr(VI) reduction by CFEs of chromate resistant bacteria isolated from chromate contaminated soil. Bioresour. Technol. 2008, 99, 4130–4137. [Google Scholar] [CrossRef]
- Deng, H.; Chen, G. Progress in Research on Microbial Remediation Technologies of Chromium-Contaminated Soil. Earth Environ. 2012, 40, 466–472. [Google Scholar]
- Li, J.; Huang, J. Studies and prospect on the speciation analysis of chromium. Metall. Anal. 2006, 26, 38–43. [Google Scholar]
- Wang, W.; Liu, Y.; Huang, S.; Li, Y.; Zhou, L.; Cheng, J. Stabilization of Cr(VI)-contaminated soil with calcium polysulfide. Chin. J. Environ. Eng. 2017, 11, 3853–3860. [Google Scholar]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Setshedi, K.Z.; Bhaumik, M.; Onyango, M.S.; Maity, A. Breakthrough studies for Cr(VI) sorption from aqueous solution using exfoliated polypyrrole-organically modified montmorillonite clay nanocomposite. J. Ind. Eng. Chem. 2014, 20, 2208–2216. [Google Scholar] [CrossRef]
- Hutchison, J.M.; Seaman, J.C.; Aburime, S.A.; Radcliffe, D.E. Chromate Transport and Retention in Variably Saturated Soil Columns. Vadose Zone J. 2003, 2, 702–714. [Google Scholar] [CrossRef]
- Wang, X.R.; Li, L.; Yan, X.H.; Tian, Y.Q. Progress in remediation of chromium-contaminated sites. Environ. Eng. 2020, 38, 1–8. [Google Scholar]
- Zhang, X.Y.; Zhong, T.Y.; Liu, L.; Zhang, X.M.; Cheng, M.; Li, X.H.; Jin, J.X. Chromium occurrences in arable soil and its influence on food production in China. Environ. Earth Sci. 2016, 75, 257. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Wang, C.C. Natural and Human Factors Affect the Distribution of Soil Heavy Metal Pollution: A Review. Water Air Soil Pollut. 2020, 231, 350. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in Agricultural Soils and Crops: A Review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Liu, Y.; Su, C.; Zhang, H.; Li, X.T.; Pei, J.F. Interaction of Soil Heavy Metal Pollution with Industrialisation and the Landscape Pattern in Taiyuan City, China. PLoS ONE 2014, 9, e105798. [Google Scholar] [CrossRef]
- Li, C.; Li, F.B.; Wu, Z.F.; Cheng, J. Impacts of landscape patterns on heavy metal contamination of agricultural top soils in the Pearl River Delta, South China. Chin. J. Appl. Ecol. 2015, 26, 1137–1144. [Google Scholar]
- Jeon, E.K.; Ryu, S.R.; Baek, K. Application of solar-cells in the electrokinetic remediation of As-contaminated soil. Electrochim. Acta 2015, 181, 160–166. [Google Scholar] [CrossRef]
- Ribeiro, A.; Mota, A.; Soares, M.; Castro, C.; Araújo, J.; Carvalho, J. Lead (II) Removal from Contaminated Soils by Electrokinetic Remediation Coupled with Modified Eggshell Waste. Key Eng. Mater. 2018, 777, 256–261. [Google Scholar] [CrossRef]
- Ortiz-Soto, R.; Leal, D.; Gutierrez, C.; Aracena, A.; Rojo, A.; Hansen, H.K. Electrokinetic remediation of manganese and zinc in copper mine tailings. J. Hazard. Mater. 2019, 365, 905–911. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Ma, C.; Yao, X. Electrochemical combined remediation of chromium contaminated soil based on strengthening by anode consumption. Environ. Eng. 2020, 38, 224–230. [Google Scholar]
- Tang, J.; Qiu, Z.P.; Tang, H.J.; Wang, H.Y.; Sima, W.P.; Liang, C.; Liao, Y.; Li, Z.H.; Wan, S.; Dong, J.W. Coupled with EDDS and approaching anode technique enhanced electrokinetic remediation removal heavy metal from sludge. Environ. Pollut. 2021, 272, 115975. [Google Scholar] [CrossRef] [PubMed]
- Telepanich, A.; Marshall, T.; Gregori, S.; Marangoni, A.G.; Pensini, E. Graphene-Alginate Fluids as Unconventional Electrodes for the Electrokinetic Remediation of Cr(VI). Water Air Soil Pollut. 2021, 232, 334. [Google Scholar] [CrossRef]
- Xu, H.C.; Bai, J.; Yang, X.R.; Zhang, C.P.; Yao, M.; Zhao, Y.S. Lab scale-study on the efficiency and distribution of energy consumption in chromium contaminated aquifer electrokinetic remediation. Environ. Technol. Innov. 2022, 25, 102194. [Google Scholar] [CrossRef]
- Wang, Y.C.; Han, Z.J.; Li, A.; Cui, C.W. Enhanced electrokinetic remediation of heavy metals contaminated soil by biodegradable complexing agents. Environ. Pollut. 2021, 283, 117111. [Google Scholar] [CrossRef]
- Rezaee, M.; Asadollahfardi, G. An Implicit Finite Difference Model for Electrokinetic Remediation of Cd-Spiked Kaolinite Under Acid-Enhanced and Unenhanced Conditions. Environ. Model. Assess. 2019, 24, 235–248. [Google Scholar] [CrossRef]
- Lopez-Vizcaino, R.; Yustres, A.; Leon, M.J.; Saez, C.; Canizares, P.; Rodrigo, M.A.; Navarro, V. Multiphysics Implementation of Electrokinetic Remediation Models for Natural Soils and Porewaters. Electrochim. Acta 2017, 225, 93–104. [Google Scholar] [CrossRef]
- Alshwabkeh, A.N.; Acar, Y.B. Electrokinetic Remediation II: Theoretical Mode. J. Geotech. Eng. 1996, 122, 186–196. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, X.; Xie, T.; Xu, X. Simulation of Heavy Metal Pollutants Migration in Soil Based on Finite Volume Method. J. Northeast. Univ. Nat. Sci. 2018, 39, 867–871. [Google Scholar]
- Liu, X.M.; Ma, C.; Tao, J.X.; Tian, B.R. Diphenylcarbohydrazide spectrophotometry and its improved methods for the determination of chromium(Ⅵ). Appl. Chem. Ind. 2018, 47, 1308–1311. [Google Scholar]
- Tang, T.; Wang, Y.; Tang, H.; Ren, J. Determination of hexavalent chromium in soil. Sichuan Environ. 2017, 36, 123–126. [Google Scholar]
- Xu, Y.B.; Fan, X.J.; Gong, X. Improvement of Spectrophotometric Determination of Hexavalent Chromium in Water with Diphenylcarbazide. China Water Wastewater 2015, 31, 106–108. [Google Scholar]
- Pourmohammad, M.; Faraji, M.; Jafarinejad, S. Extraction of chromium (VI) in water samples by dispersive liquid-liquid microextraction based on deep eutectic solvent and determination by UV-Vis spectrophotometry. Int. J. Environ. Anal. Chem. 2020, 100, 1146–1159. [Google Scholar] [CrossRef]
- Leng, Y.P.; Xue, X.K.; Zhang, M.H. Chromium reduction of hexavalent chromium detected by soil alkaline digestion and analysis of quality control results. J. Anhui Agric. Sci. 2019, 47, 206–208. [Google Scholar]
- Ma, W.; Zhou, Q.; Zhu, L.Z.; Wang, L.; Ji, Y.J. Study on Determination of Hexavalent Chromium in Solid Waste by Alkaline Digestion. Environ. Prot. Sci. 2012, 38, 41–43. [Google Scholar]
- Yi, M.; Ou, F.P. Rapid pretreatment method for alkali digestion of solid waste hexavalent chromium analytical samples. Environ. Eng. 2010, 28, 87–88. [Google Scholar]
- Qin, T.; Dong, Z.F.; Lv, X.H.; Zhang, X.L. Determination of Hexavalent Chromium in Soil by Alkaline Digestion-Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). Chin. J. Inorg. Anal. Chem. 2019, 9, 10–13. [Google Scholar]
- Huang, J.; Huang, Y.; Yao, J.; Wang, C.X. Determination of Hexavalent Chromium in PM10 by Alkaline Digestion-Graphite Furnace Atomic Absorption Spectrometry. Chem. Anal. Meterage 2015, 24, 52–54. [Google Scholar]
- Chen, X.L.; Wang, Y.M.; Zhao, Y.K.; Li, Z.Y.; Yi, B.Z.; Xu, L.; Sha, J.; Huang, Y. Comparison and Research of Acid Digestion Technique for Pt, Pd and Rh in Catalysts. Rare Met. Mater. Eng. 2011, 40, 1867–1870. [Google Scholar]
- Li, Q.; Cao, Y.; Gao, C.; Liu, Y.; Zhou, M.; Liu, X. Comparative study of acidification systems for determination of heavy metals in soils from different areas in China. Environ. Chem. 2020, 39, 1153–1157. [Google Scholar]
- Li, R.; Wu, B.; Wang, S.; Li, G. Analysis of key parameters for electrokinetic remediation of contaminated soil under high electric field strength. Environ. Eng. 2018, 36, 149–153. [Google Scholar]








| Sampling Point Number | σ1 (μg/g) | σ2 (μg/g) | σ0 (μg/g) | ρ (%) |
|---|---|---|---|---|
| Sampling point 6 (90 V, 1.5 m) | 59.38 | 23.94 | 5.7 | 66.03 |
| Sampling point 7 (90 V, 1.5 m) | 58.64 | 26.45 | 5.7 | 60.80 |
| Sampling point 8 (90 V, 1.5 m) | 53.28 | 37.10 | 5.7 | 34.01 |
| Sampling point 9 (90 V, 1.5 m) | 51.78 | 33.81 | 5.7 | 40.00 |
| Sampling point 10 (90 V, 1.5 m) | 52.86 | 35.67 | 5.7 | 36.44 |
| Sampling point 6 (110 V, 1.5 m) | 52.45 | 16.94 | 5.7 | 75.96 |
| Sampling point 7 (110 V, 1.5 m) | 54.08 | 19.86 | 5.7 | 70.74 |
| Sampling point 8 (110 V, 1.5 m) | 50.26 | 31.14 | 5.7 | 42.90 |
| Sampling point 9 (110 V, 1.5 m) | 56.72 | 32.78 | 5.7 | 46.93 |
| Sampling point 10 (110 V, 1.5 m) | 58.53 | 36.97 | 5.7 | 40.81 |
| Experimental Conditions | Evaluation Indicators | Sampling Point Number | ||||
|---|---|---|---|---|---|---|
| Sampling Point 1 | Sampling Point 2 | Sampling Point 3 | Sampling Point 6 | Sampling Point 7 | ||
| 90 V, 1.5 m | RMSE | 5.38 | 5.15 | 4.02 | 1.65 | 1.73 |
| R2 | 0.94 | 0.96 | 0.98 | 0.99 | 0.99 | |
| NSE | 0.94 | 0.90 | 0.80 | 0.63 | 0.98 | |
| Experimental Conditions | Evaluation Indicators | Sampling Point Number | ||||
|---|---|---|---|---|---|---|
| Sampling Point 1 | Sampling Point 2 | Sampling Point 3 | Sampling Point 6 | Sampling Point 7 | ||
| 110 V, 1.5 m | RMSE | 5.97 | 4.78 | 3.76 | 2.46 | 2.03 |
| R2 | 0.90 | 0.95 | 0.93 | 0.98 | 0.96 | |
| NSE | 0.79 | 0.65 | 0.77 | 0.94 | 0.82 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Wei, Y.; Ren, J.; Zhang, H.; Yang, Y. Study on Water-Heat-Solution Transport Law in Cr(VI)-Contaminated Soil during Electric Remediation. Sustainability 2022, 14, 8136. https://doi.org/10.3390/su14138136
Lu X, Wei Y, Ren J, Zhang H, Yang Y. Study on Water-Heat-Solution Transport Law in Cr(VI)-Contaminated Soil during Electric Remediation. Sustainability. 2022; 14(13):8136. https://doi.org/10.3390/su14138136
Chicago/Turabian StyleLu, Xiaohui, Yantong Wei, Jianglin Ren, Haitao Zhang, and Yang Yang. 2022. "Study on Water-Heat-Solution Transport Law in Cr(VI)-Contaminated Soil during Electric Remediation" Sustainability 14, no. 13: 8136. https://doi.org/10.3390/su14138136
APA StyleLu, X., Wei, Y., Ren, J., Zhang, H., & Yang, Y. (2022). Study on Water-Heat-Solution Transport Law in Cr(VI)-Contaminated Soil during Electric Remediation. Sustainability, 14(13), 8136. https://doi.org/10.3390/su14138136





