Adding UVA and Far-Red Light to White LED Affects Growth, Morphology, and Phytochemicals of Indoor-Grown Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture
2.2. Light Treatments
2.3. Measurements
2.4. Experimental Design and Statistical Analysis
3. Results
3.1. Plant Biomass
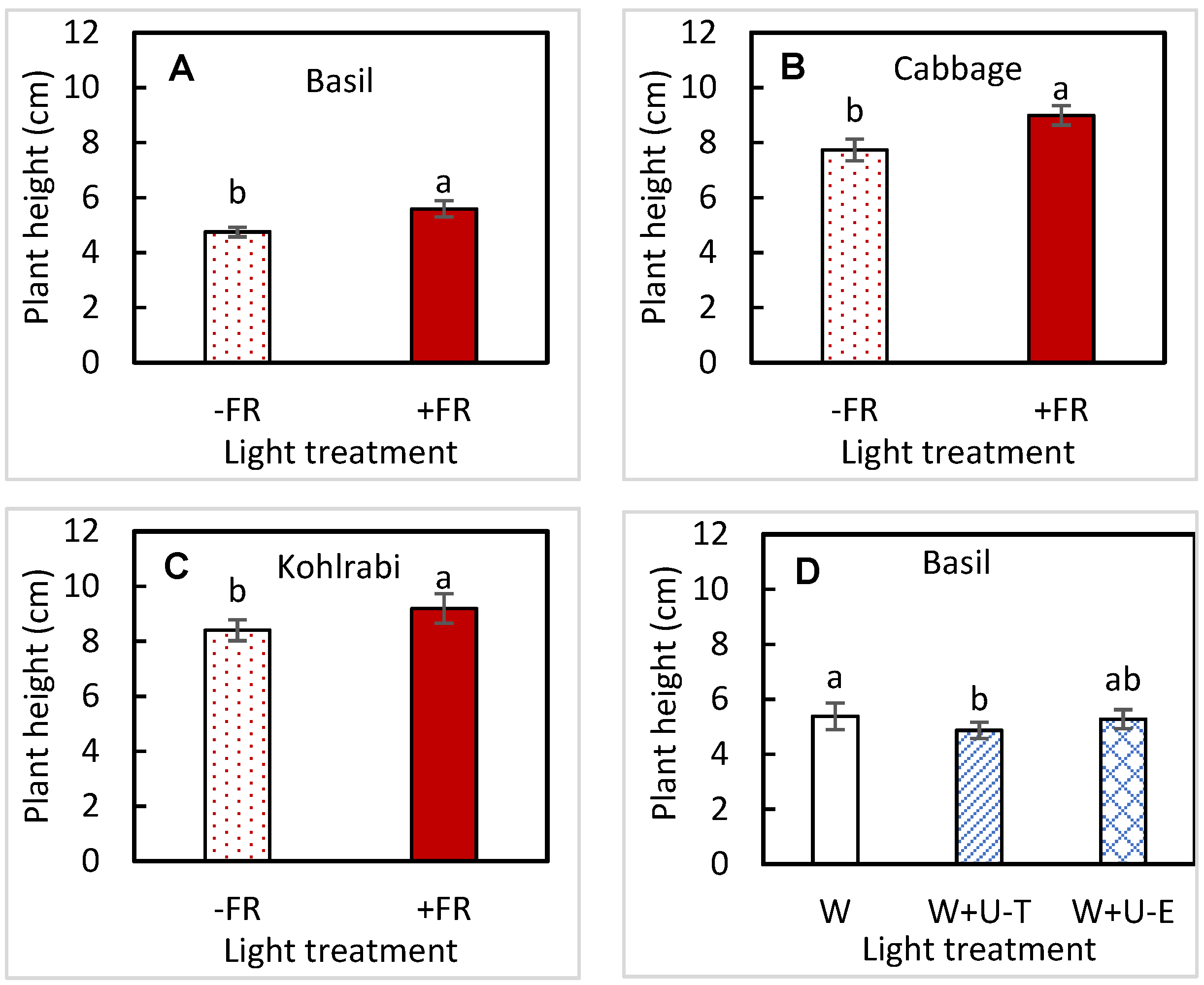
3.2. Plant Size
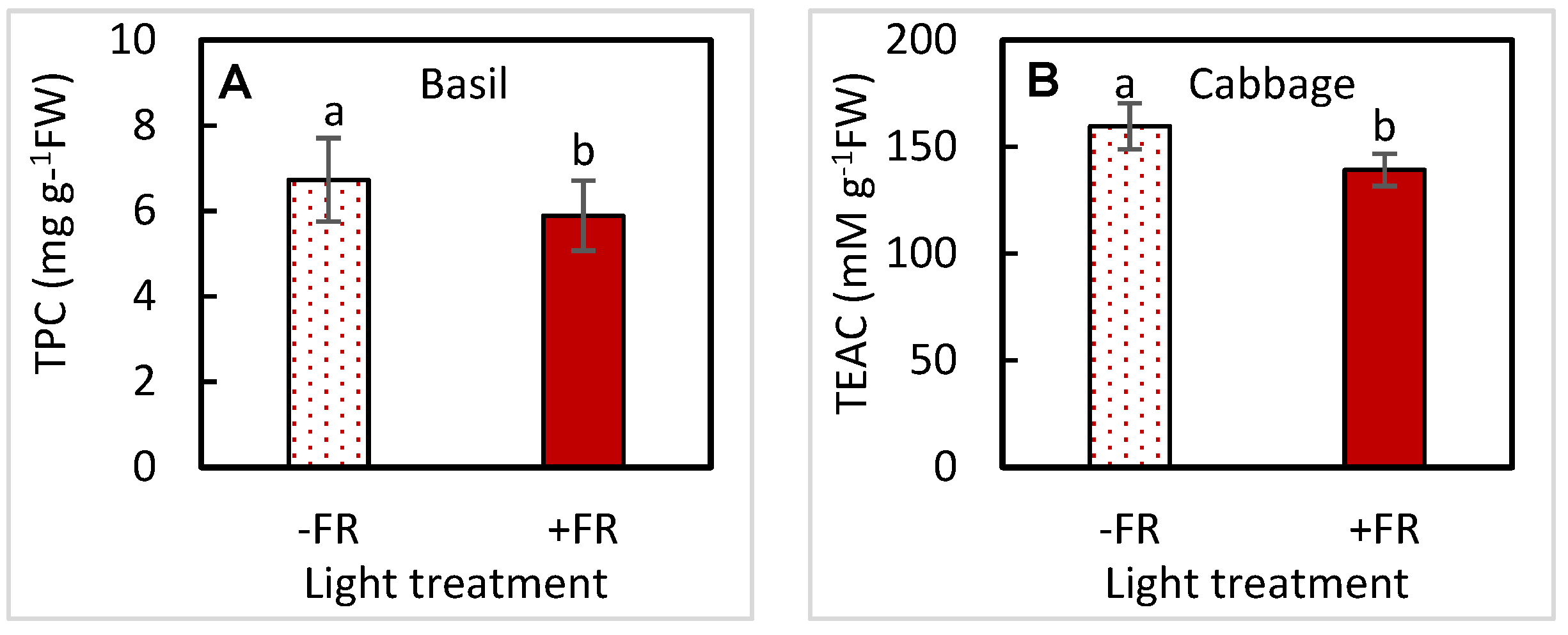
3.3. Nutritional Traits-Phytochemicals
4. Discussion
4.1. Adding UVA to White LED Has Limited Effects on Microgreens
4.2. Adding FR Light to White LED Affects Microgreen Biomass, Height, and Phytochemicals
4.3. Interactive Effect of UVA and FR Light on Microgreen Phytochemicals Is Not Significant
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Rajan, P.; Lada, R.R.; MacDonald, M.T. Advancement in indoor vertical farming for microgreen production. Am. J. Plant Sci. 2019, 10, 1397. [Google Scholar] [CrossRef] [Green Version]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Jones, M.A. Using light to improve commercial value. Hortic. Res. 2018, 5, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Runkle, E. LEDs on Lettuce: White Light Versus Red + Blue Light. Produce Grower. 2021. Available online: https://www.producegrower.com/article/production-leds-on-lettuce-white-light-versus-red-blue-light/#:~:text=%C2%B7s%E2%80%931.-Results,red%20%2B%2056%25%20blue%20light (accessed on 1 June 2022).
- Nozue, H.; Gomi, M. Usefulness of broad-spectrum white LEDs to envision future plant factory. In Smart Plant Factory; Kozai, T., Ed.; Springer: Singapore, 2018; pp. 197–210. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Virsile, A.; Jankauskiene, J.; Sakalauskiene, S.; Samuoliene, G.; Sirtautas, R.; Novickovas, A.; Dabasinskas, L.; Miliauskiene, J.; Vastakaite, V. Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int. Agrophys. 2015, 29, 13–22. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Samuolienė, G.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Miliauskienė, J.; Novičkovas, A.; Duchovskis, P. Response of mustard microgreens to different wavelengths and durations of UV-A LEDs. Front. Plant Sci. 2019, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; He, R.; Shi, R.; Li, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Combination of selenium and UVA radiation affects growth and phytochemicals of broccoli microgreens. Molecules 2021, 26, 4646. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Schiestel, K.; Zheng, Y. Pure blue light effects on growth and morphology are slightly changed by adding low-level UVA or far-red light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2019, 157, 58–68. [Google Scholar] [CrossRef]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of pre-harvest supplemental UV-A/Blue and Red/Blue LED lighting on lettuce growth and nutritional quality. Horticulturae 2021, 7, 80. [Google Scholar] [CrossRef]
- Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality. Horticulturae 2022, 8, 435. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Growth and appearance quality of four microgreen species under light-emitting diode lights with different spectral combinations. HortScience 2020, 55, 1399–1405. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Applying blue light alone, or in combination with far-red light, during nighttime increases elongation without compromising yield and quality of indoor-grown microgreens. HortScience 2020, 55, 876–881. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.; Calhau, C.; Morais, R.; Pintado, M. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Maximum elongation growth promoted as a shade-avoidance response by blue light is related to deactivated phytochrome: A comparison with red light in four microgreen species. Can. J. Plant Sci. 2019, 100, 314–326. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue versus red light can promote elongation growth independent of photoperiod: A study in four Brassica microgreens species. HortScience 2019, 54, 1955–1961. [Google Scholar] [CrossRef] [Green Version]
- Franklin, K.A.; Whitelam, G.C. Phytochromes and shade-avoidance responses in plants. Ann. Bot. 2005, 96, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Zheng, Y. Early-stage dark treatment promotes hypocotyl elongation associated with varying effects on yield and quality in sunflower and arugula microgreens. Can. J. Plant Sci. 2021, 101, 954–961. [Google Scholar] [CrossRef]
- Oh, H.E.; Yoon, A.; Park, Y.G. Red Light Enhances the Antioxidant Properties and Growth of Rubus hongnoensis. Plants 2021, 10, 2589. [Google Scholar] [CrossRef]
- Alves, F.R.R.; Lira, B.S.; Pikart, F.C.; Monteiro, S.S.; Furlan, C.M.; Purgatto, E.; Pascoal, G.B.; Andrade, S.C.d.S.; Demarco, D.; Rossi, M. Beyond the limits of photoperception: Constitutively active PHYTOCHROME B2 overexpression as a means of improving fruit nutritional quality in tomato. Plant Biotechnol. J. 2020, 18, 2027–2041. [Google Scholar] [CrossRef] [Green Version]
- Rusaczonek, A.; Czarnocka, W.; Kacprzak, S.; Witoń, D.; Ślesak, I.; Szechyńska-Hebda, M.; Gawroński, P.; Karpiński, S. Role of phytochromes A and B in the regulation of cell death and acclimatory responses to UV stress in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 6679–6695. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]


| Common Name | Scientific Name | Variety Name | Seeding Rate (g m−2) |
|---|---|---|---|
| Basil | Ocimum basilicum | Dark Opal | 63 |
| Cabbage | Brassica oleracea var. capitata | Red Cabbage | 131 |
| Kale | Brassica napus | Red Russian | 125 |
| Kohlrabi | Brassica oleracea | Purple | 119 |
| Macroelement (mM) | Microelement (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | Fe | B | Mn | Zn | Cu | Mo |
| 7.3 | 0.75 | 3.6 | 2.2 | 0.96 | 0.96 | 35.8 | 18.5 | 4.0 | 1.2 | 0.73 | 0.42 |
| Treatment | UVA (340–399 nm) | Blue (400–499 nm) | Green (500–599 nm) | Red (600–699 nm) | Far-Red (700–799 nm) | PAR (400–700 nm) |
|---|---|---|---|---|---|---|
| W | 0 | 8 | 32 | 52 | 8 | 92 |
| W + U-T | 21 | 7 | 25 | 41 | 6 | 73 |
| W + U-E | 24 | 7 | 24 | 39 | 6 | 70 |
| W + FR | 0 | 5 | 23 | 39 | 33 | 67 |
| W + U-T + FR | 23 | 5 | 16 | 26 | 30 | 47 |
| W + U-E + FR | 23 | 5 | 16 | 27 | 30 | 48 |
| Plant Trait | Treatment | Species | |||
|---|---|---|---|---|---|
| Basil | Cabbage | Kale | Kohlrabi | ||
| Plant FW | UVA | 0.9911 | 0.3723 | 0.8962 | 0.5874 |
| FR | 0.1829 | 0.7935 | 0.5020 | 0.0146 | |
| UVA × FR | 0.4884 | 0.5147 | 0.7116 | 0.6399 | |
| Plant DW | UVA | 0.8765 | 0.2128 | 0.9351 | 0.9238 |
| FR | 0.1264 | 0.1224 | 0.0991 | 0.0023 | |
| UVA × FR | 0.2766 | 0.3542 | 0.9692 | 0.6060 | |
| Plant Trait | Treatment | Species | |||
|---|---|---|---|---|---|
| Basil | Cabbage | Kale | Kohlrabi | ||
| Plant height | UVA | 0.0407 | 0.3223 | 0.3937 | 0.3005 |
| FR | 0.0011 | 0.0327 | 0.4892 | 0.0104 | |
| UVA × FR | 0.2725 | 0.6809 | 0.9920 | 0.7790 | |
| Hypocotyl length | UVA | 0.1521 | 0.0551 | 0.3060 | 0.7812 |
| FR | 0.0775 | 0.1640 | 0.5158 | 0.9428 | |
| UVA × FR | 0.6213 | 0.1302 | 0.6289 | 0.8478 | |
| Plant Trait | Treatment | Species | |||
|---|---|---|---|---|---|
| Basil | Cabbage | Kale | Kohlrabi | ||
| Anthocyanin | UVA | 0.9538 | 0.9910 | 0.8517 | 0.9071 |
| FR | 0.8898 | 0.9911 | 0.0953 | 0.6601 | |
| UVA × FR | 0.9748 | 0.9640 | 0.8985 | 0.9016 | |
| TPC | UVA | 0.8070 | 0.7614 | 0.6635 | 0.4835 |
| FR | 0.0477 | 0.3173 | 0.1870 | 0.7896 | |
| UVA × FR | 0.2315 | 0.8158 | 0.4043 | 0.3997 | |
| TEAC | UVA | 0.8419 | 0.2033 | 0.5034 | 0.4993 |
| FR | 0.1760 | 0.0497 | 0.0642 | 0.5622 | |
| UVA × FR | 0.2354 | 0.1435 | 0.7642 | 0.7163 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Adding UVA and Far-Red Light to White LED Affects Growth, Morphology, and Phytochemicals of Indoor-Grown Microgreens. Sustainability 2022, 14, 8552. https://doi.org/10.3390/su14148552
Hooks T, Sun L, Kong Y, Masabni J, Niu G. Adding UVA and Far-Red Light to White LED Affects Growth, Morphology, and Phytochemicals of Indoor-Grown Microgreens. Sustainability. 2022; 14(14):8552. https://doi.org/10.3390/su14148552
Chicago/Turabian StyleHooks, Triston, Ling Sun, Yun Kong, Joseph Masabni, and Genhua Niu. 2022. "Adding UVA and Far-Red Light to White LED Affects Growth, Morphology, and Phytochemicals of Indoor-Grown Microgreens" Sustainability 14, no. 14: 8552. https://doi.org/10.3390/su14148552
APA StyleHooks, T., Sun, L., Kong, Y., Masabni, J., & Niu, G. (2022). Adding UVA and Far-Red Light to White LED Affects Growth, Morphology, and Phytochemicals of Indoor-Grown Microgreens. Sustainability, 14(14), 8552. https://doi.org/10.3390/su14148552







