Rainfall Variability and Tidal Inundation Influences on Mangrove Greenness in Karimunjawa National Park, Indonesia
Abstract
:1. Introduction
2. Materials and Methods
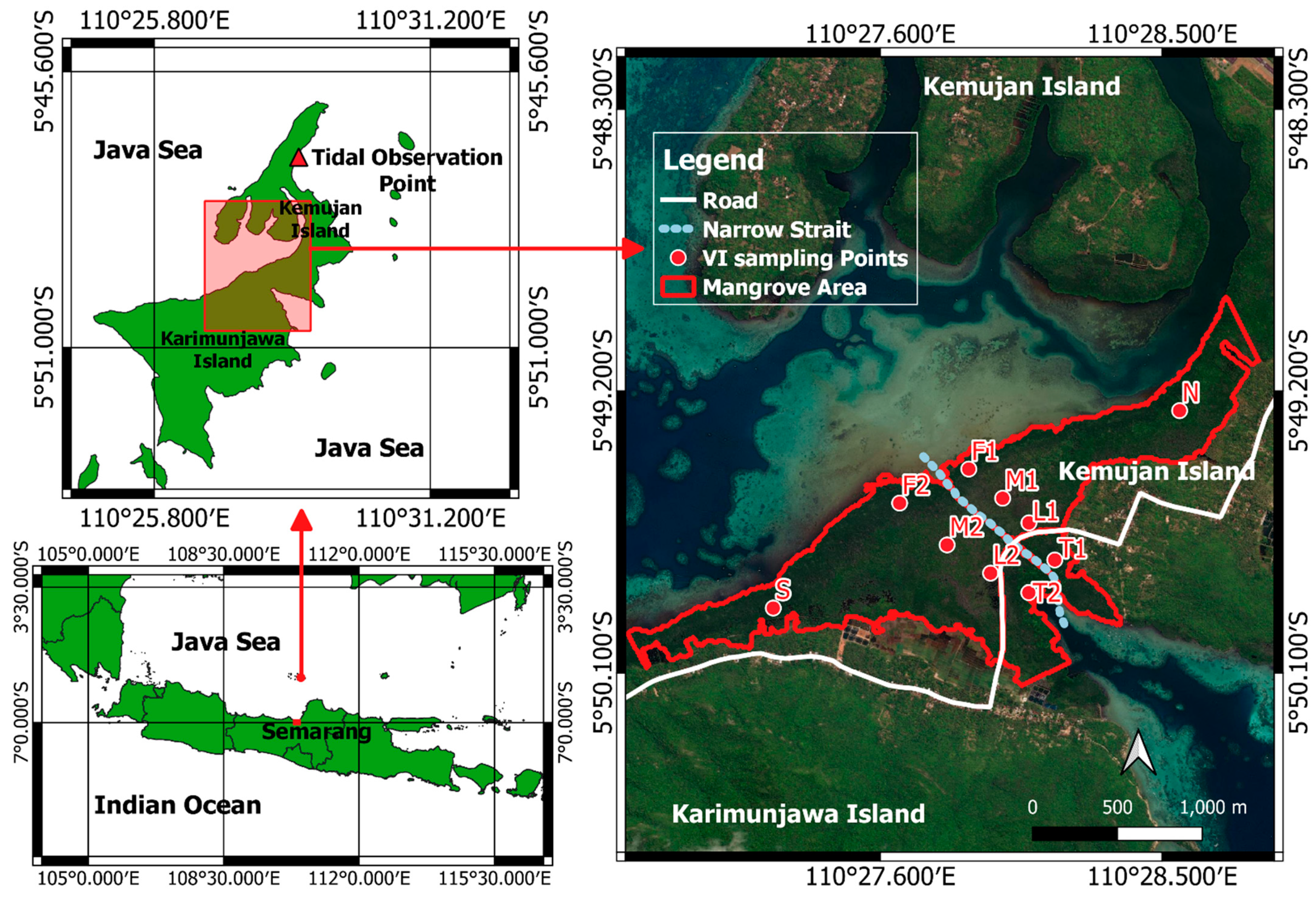
2.1. Study Site
2.2. Rainfall Data, Land Surface Temperature Data, and Vegetation Indices Calculation
2.3. Digital Terrain Model and Mangrove Canopy Height Calculation
2.4. Intertidal Area Estimation
2.5. Pixel Extraction in the Intertidal and Non-Intertidal Area
2.6. Phenology Metrics Calculation
3. Results
3.1. Vegetation Indices and Canopy Height in Intertidal and Non-Intertidal Area
3.2. Mangrove Greenness Phenology
3.3. Rainfall and LST Variability Relationship with NDVI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alongi, D.M. Carbon Sequestration in Mangrove Forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Alongi, D.M. Global Significance of Mangrove Blue Carbon in Climate Change Mitigation (Version 1). Science 2020, 2, 57. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the Most Carbon-Rich Forests in the Tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Bernardino, A.F.; Ferreira, T.O.; Giovannoni, L.R.; De Gomes, L.E.O.; Romero, D.J.; Jimenez, L.C.Z.; Ruiz, F. Carbon Stocks of Mangroves and Salt Marshes of the Amazon Region, Brazil. Biol. Lett. 2018, 14, 20180208. [Google Scholar] [CrossRef]
- Chiodi, A.M.; Dunne, J.P.; Harrison, D.E. Estimating Air-Sea Carbon Flux Uncertainty over the Tropical Pacific: Importance of Winds and Wind Analysis Uncertainty. Glob. Biogeochem. Cycles 2019, 33, 370–390. [Google Scholar] [CrossRef]
- Kartadikaria, A.R.; Watanabe, A.; Nadaoka, K.; Adi, N.S.; Prayitno, H.B.; Soemorumekso, S.; Muchtar, M.; Triyulianti, I.; Setiawan, A.; Suratno, S.; et al. CO2 Sink/Source Characteristics in the Tropical Indonesian Seas. J. Geophys. Res. Ocean. 2015, 120, 7842–7856. [Google Scholar] [CrossRef] [Green Version]
- Sidik, F.; Supriyanto, B.; Krisnawati, H.; Muttaqin, M.Z. Mangrove Conservation for Climate Change Mitigation in Indonesia. Wiley Interdiscip. Rev. Clim. Chang. 2018, 9, e529. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Purbopuspito, J.; Kauffman, J.B.; Warren, M.W.; Sasmito, S.D.; Donato, D.C.; Manuri, S.; Krisnawati, H.; Taberima, S.; Kurnianto, S. The Potential of Indonesian Mangrove Forests for Global Climate Change Mitigation. Nat. Clim. Chang. 2015, 5, 1089–1092. [Google Scholar] [CrossRef]
- Wirasatriya, A.; Sugianto, D.N.; Maslukah, L.; Ahkam, M.F.; Wulandari, S.Y.; Helmi, M. Carbon Dioxide Flux in the Java Sea Estimated from Satellite Measurements. Remote Sens. Appl. Soc. Environ. 2020, 20, 100376. [Google Scholar] [CrossRef]
- Latifah, N.; Febrianto, S.; Wirasatriya, A.; Endrawati, H.; Zainuri, M.; Suryanti, S.; Hidayat, A.N. Air-Sea Flux of CO2 In the Waters of Karimunjawa Island, Indonesia. Saintek Perikan. Indones. J. Fish. Sci. Technol. 2020, 16, 171–178. [Google Scholar] [CrossRef]
- Kamal, M.; Hartono, H.; Wicaksono, P.; Adi, N.S.; Arjasakusuma, S. Assessment of Mangrove Forest Degradation Through Canopy Fractional Cover in Karimunjawa Island, Central Java, Indonesia. Geoplan. J. Geomatics Plan. 2016, 3, 107. [Google Scholar] [CrossRef] [Green Version]
- Puryono, S.; Suryanti, S. Degradation of Mangrove Ecosystem in Karimunjawa Island Based on Public Perception and Management. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Moscow, Russia, 27 May–6 June 2019; Volume 246. [Google Scholar]
- Kanniah, K.D.; Kang, C.S.; Sharma, S.; Aldrie Amir, A. Remote Sensing to Study Mangrove Fragmentation and Its Impacts on Leaf Area Index and Gross Primary Productivity in the South of Peninsular Malaysia. Remote Sens. 2021, 13, 1427. [Google Scholar] [CrossRef]
- Alongi, D.M. The Impact of Climate Change on Mangrove Forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39. [Google Scholar] [CrossRef]
- Santini, N.S.; Reef, R.; Lockington, D.A.; Lovelock, C.E. The Use of Fresh and Saline Water Sources by the Mangrove Avicennia Marina. Hydrobiologia 2015, 745, 59–68. [Google Scholar] [CrossRef]
- Chamberlain, D.A.; Phinn, S.R.; Possingham, H.P. Mangrove Forest Cover and Phenology with Landsat Dense Time Series in Central Queensland, Australia. Remote Sens. 2021, 13, 3032. [Google Scholar] [CrossRef]
- Hayes, M.A.; Jesse, A.; Welti, N.; Tabet, B.; Lockington, D.; Lovelock, C.E. Groundwater Enhances Above-Ground Growth in Mangroves. J. Ecol. 2019, 107, 1120–1128. [Google Scholar] [CrossRef]
- Prihantono, J.; Adi, N.S.; Nakamura, T.; Nadaoka, K. The Impact of Groundwater Variability on Mangrove Greenness in Karimunjawa National Park Based on Remote Sensing Study. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Jakarta, Indonesia, 25–26 September 2021; Volume 925. [Google Scholar]
- Saleh, M.B.; Dewi, R.W.; Prasetyo, L.B.; Santi, N.A. Canopy Cover Estimation in Lowland Forest in South Sumatera, Using LiDAR and Landsat 8 OLI Imagery. J. Manaj. Hutan Trop. 2021, 27, 50–58. [Google Scholar] [CrossRef]
- Ansley, R.J.; Mirik, M.; Surber, B.W.; Park, S.C. Canopy Area and Aboveground Mass of Individual Redberry Juniper (Juniperus pinchotii) Trees. Rangel. Ecol. Manag. 2012, 65, 189–195. [Google Scholar] [CrossRef]
- Wang, L.; Jia, M.; Yin, D.; Tian, J. A Review of Remote Sensing for Mangrove Forests: 1956–2018. Remote Sens. Environ. 2019, 231, 111223. [Google Scholar] [CrossRef]
- Heumann, B.W. Satellite Remote Sensing of Mangrove Forests: Recent Advances and Future Opportunities. Prog. Phys. Geogr. 2011, 35, 87–108. [Google Scholar] [CrossRef]
- Pastor-Guzman, J.; Dash, J.; Atkinson, P.M. Remote Sensing of Mangrove Forest Phenology and Its Environmental Drivers. Remote Sens. Environ. 2018, 205, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Zhao, S.; Ke, Y. A Simple Phenology-Based Vegetation Index for Mapping Invasive Spartina Alterniflora Using Google Earth Engine. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 190–201. [Google Scholar] [CrossRef]
- Songsom, V.; Koedsin, W.; Ritchie, R.J.; Huete, A. Mangrove Phenology and Environmental Drivers Derived from Remote Sensing in Southern Thailand. Remote Sens. 2019, 11, 955. [Google Scholar] [CrossRef] [Green Version]
- Matongera, T.N.; Mutanga, O.; Sibanda, M.; Odindi, J. Estimating and Monitoring Land Surface Phenology in Rangelands: A Review of Progress and Challenges. Remote Sens. 2021, 13, 2060. [Google Scholar] [CrossRef]
- Reed, B.C.; Brown, J.F.; VanderZee, D.; Loveland, T.R.; Merchant, J.W.; Ohlen, D.O. Measuring Phenological Variability from Satellite Imagery. J. Veg. Sci. 1994, 5, 703–714. [Google Scholar] [CrossRef]
- Mandal, M.S.H.; Kamruzzaman, M.; Hosaka, T. Elucidating the Phenology of the Sundarbans Mangrove Forest Using 18-Year Time Series of MODIS Vegetation Indices. Tropics 2020, 29, 41–55. [Google Scholar] [CrossRef]
- Tang, J.; Körner, C.; Muraoka, H.; Piao, S.; Shen, M.; Thackeray, S.J.; Yang, X. Emerging Opportunities and Challenges in Phenology: A Review. Ecosphere 2016, 7, e01436. [Google Scholar] [CrossRef] [Green Version]
- Mulyadi, H.; Susanto, H.; Devi, Y.; Sahwan, F.F. Interpretation of Mangrove Trekking Karimunjawa National Park, 2nd ed.; Karimunjawa National Park Office (BTNKJ): Semarang, Indonesia, 2019. [Google Scholar]
- Balai Taman Nasional Karimunjawa (BTNKJ). Report of Mangrove Inventory at Karimunjawa Island; Balai Taman Nasional Karimunjawa (BTNKJ): Semarang, Indonesia, 2013. [Google Scholar]
- Balai Taman Nasional Karimunjawa (BTNKJ). Report of Mangrove Inventory at Kemujan Island; Balai Taman Nasional Karimunjawa (BTNKJ): Semarang, Indonesia, 2013. [Google Scholar]
- Wirasatriya, A.; Pribadi, R.; Iryanthony, S.B.; Maslukah, L.; Sugianto, D.N.; Helmi, M.; Ananta, R.R.; Adi, N.S.; Kepel, T.L.; Ati, R.N.A.; et al. Mangrove Above-Ground Biomass and Carbon Stock in the Karimunjawa-Kemujan Islands Estimated from Unmanned Aerial Vehicle-Imagery. Sustainability 2022, 14, 706. [Google Scholar] [CrossRef]
- Huffman, G.J.; Stocker, E.F.; Bolvin, D.T.; Nelkin, E.J.; Tan, J. GPM IMERG Final Precipitation L3 1 Month 0.1 Degree x 0.1 Degree V06, Greenbelt, MD. Available online: https://disc.gsfc.nasa.gov/datasets/GPM_3IMERGM_06/summary (accessed on 28 February 2022).
- Wan, Z. Collection-6 MODIS Land Surface Temperature Products Users’ Guide; ERI, University of California: Santa Barbara, CA, USA, 2013. [Google Scholar]
- Kriegler, F.; Malila, W.; Nalepka, R.; Richardson, W. Preprocessing Transformations and Their Effect on Multispectral Recognition. In Proceedings of the 6th International Symposium on Remote Sensing of Environment, Ann Arbor, MI, USA, 13–16 October 1969; University of Michigan: Ann Arbor, MI, USA, 1969; pp. 97–131. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1974; Greenbelt, NASA SP-351. pp. 48–62. [Google Scholar]
- Wilson, E.H.; Sader, S.A. Detection of Forest Harvest Type Using Multiple Dates of Landsat TM Imagery. Remote Sens. Environ. 2002, 80, 385–396. [Google Scholar] [CrossRef]
- Zhang, X.; Treitz, P.M.; Chen, D.; Quan, C.; Shi, L.; Li, X. Mapping Mangrove Forests Using Multi-Tidal Remotely-Sensed Data and a Decision-Tree-Based Procedure. Int. J. Appl. Earth Obs. Geoinf. 2017, 62, 201–214. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water from Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Alahacoon, N.; Edirisinghe, M. A Comprehensive Assessment of Remote Sensing and Traditional Based Drought Monitoring Indices at Global and Regional Scale. Geomat. Nat. Hazards Risk 2022, 13, 762–799. [Google Scholar] [CrossRef]
- Intergovernmental Committee on Surveying and Mapping (ICSM). Australian Tides Manual Special Publication No. 9; Intergovernmental Committee on Surveying and Mapping (ICSM): Canberra, Australia, 2021.
- Pawlowicz, R.; Beardsley, B.; Lentz, S. Classical Tidal Harmonic Analysis Including Error Estimates in MATLAB Using T_TIDE. Comput. Geosci. 2002, 28, 929–937. [Google Scholar] [CrossRef]
- Li, N.; Zhan, P.; Pan, Y.; Zhu, X.; Li, M.; Zhang, D. Comparison of Remote Sensing Time-Series Smoothing Methods for Grassland Spring Phenology Extraction on the Qinghai–Tibetan Plateau. Remote Sens. 2020, 12, 3383. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, L.; Menenti, M.; Gorte, B. On the Performance of Remote Sensing Time Series Reconstruction Methods—A Spatial Comparison. Remote Sens. Environ. 2016, 187, 367–384. [Google Scholar] [CrossRef]
- Li, H.; Jia, M.; Zhang, R.; Ren, Y.; Wen, X. Incorporating the Plant Phenological Trajectory into Mangrove Species Mapping with Dense Time Series Sentinel-2 Imagery and the Google Earth Engine Platform. Remote Sens. 2019, 11, 2479. [Google Scholar] [CrossRef] [Green Version]
- Malamiri, H.R.G.; Zare, H.; Rousta, I.; Olafsson, H.; Verdiguier, E.I.; Zhang, H.; Mushore, T.D. Comparison of Harmonic Analysis of Time Series (HANTS) and Multi-Singular Spectrum Analysis (M-SSA) in Reconstruction of Long-Gap Missing Data in NDVI Time Series. Remote Sens. 2020, 12, 2747. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, L.; Menenti, M.; Liu, X. Optimal Estimate of Global Biome—Specific Parameter Settings to Reconstruct Ndvi Time Series with the Harmonic Analysis of Time Series (Hants) Method. Remote Sens. 2021, 13, 4251. [Google Scholar] [CrossRef]
- Verhoef, W. Application of Harmonic Analysis of NDVI Time Series (HANTS). Fourier Anal. Temporal NDVI S. Afr. Am. Cont. 1996, 108, 19–24. [Google Scholar]
- Adams, B.; Iverson, L.; Matthews, S.; Peters, M.; Prasad, A.; Hix, D.M. Mapping Forest Composition with Landsat Time Series: An Evaluation of Seasonal Composites and Harmonic Regression. Remote Sens. 2020, 12, 610. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.B.; Crews, K.A.; Neuenschwander, A.L. Sensitivity of EVI-Based Harmonic Regression to Temporal Resolution in the Lower Okavango Delta. Int. J. Remote Sens. 2012, 33, 7703–7726. [Google Scholar] [CrossRef]
- Wilson, B.T.; Knight, J.F.; McRoberts, R.E. Harmonic Regression of Landsat Time Series for Modeling Attributes from National Forest Inventory Data. ISPRS J. Photogramm. Remote Sens. 2018, 137, 29–46. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, Z.; Xian, G.; Li, C. A Novel Regression Method for Harmonic Analysis of Time Series. ISPRS J. Photogramm. Remote Sens. 2022, 185, 48–61. [Google Scholar] [CrossRef]
- Zhou, Q. Harmonic Adaptive Penalty Operator (HAPO): U.S. Geological Survey Software Release. Available online: https://code.usgs.gov/lcmap/research/Harmonic-Adaptive-Penalty-Operator-HAPO (accessed on 7 February 2022).
- White, M.A.; Thornton, P.E.; Running, S.W. A Continental Phenology Model for Monitoring Vegetation Responses to Interannual Climatic Variability. Glob. Biogeochem. Cycles 1997, 11, 217–234. [Google Scholar] [CrossRef]
- Garonna, I.; de Jong, R.; de Wit, A.J.W.; Mücher, C.A.; Schmid, B.; Schaepman, M.E. Strong Contribution of Autumn Phenology to Changes in Satellite-Derived Growing Season Length Estimates across Europe (1982–2011). Glob. Chang. Biol. 2014, 20, 3457–3470. [Google Scholar] [CrossRef]
- Hudoyo, F.; Widada, S.; Maslukah, L.; Rochaddi, B.; Wirasatriya, A.; Adi, N.S. Study of Tidal Analysis, Distribution of Brackish Groundwater and Sediments and Their Effect on Mangrove Distribution Patterns in the Karimunjawa Islands. Indones. J. Oceanogr. 2021, 3, 409–418. [Google Scholar] [CrossRef]
- Abram, N.J.; Gagan, M.K.; Cole, J.E.; Hantoro, W.S.; Mudelsee, M. Recent Intensification of Tropical Climate Variability in the Indian Ocean. Nat. Geosci. 2008, 1, 849–853. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; D’Arrigo, R.D.; Anchukaitis, K.J.; Buckley, B.M.; Cook, E.R. Links between Indo-Pacific Climate Variability and Drought in the Monsoon Asia Drought Atlas. Clim. Dyn. 2013, 40, 1319–1334. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Wu, W.; Zheng, Y.; Sun, J.; Hu, T.; Wang, P. Correlation Analysis of Land Surface Temperature and Topographic Elements in Hangzhou, China. Sci. Rep. 2020, 10, 10451. [Google Scholar] [CrossRef]
- Tong, R.; Cao, Y.; Zhu, Z.; Lou, C.; Zhou, B.; Wu, T. Solar Radiation Effects on Leaf Nitrogen and Phosphorus Stoichiometry of Chinese Fir across Subtropical China. For. Ecosyst. 2021, 8, 62. [Google Scholar] [CrossRef]
- Latifah, N.; Febrianto, S.; Endrawati, H.; Zainuri, M. Mapping of Classification and Analysis of Changes in Mangrove Ecosystem Using Multi-Temporal Satellite Images in Karimunjawa, Jepara, Indonesia. J. Kelaut. Trop. 2018, 21, 97. [Google Scholar] [CrossRef]
- Küng, O.; Strecha, C.; Beyeler, A.; Zufferey, J.-C.; Floreano, D.; Fua, P.; Gervaix, F. The accuracy of automatic photogrammetric techniques on ultra-light UAV imagery. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2012, XXXVIII-1, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Lu, A.; Jia, S. Estimation of Daily Maximum and Minimum Air Temperature Using MODIS Land Surface Temperature Products. Remote Sens. Environ. 2013, 130, 62–73. [Google Scholar] [CrossRef]
- Feller, I.C.; Lovelock, C.E.; Berger, U.; McKee, K.L.; Joye, S.B.; Ball, M.C. Biocomplexity in Mangrove Ecosystems. Ann. Rev. Mar. Sci. 2010, 2, 395–417. [Google Scholar] [CrossRef] [Green Version]
- Lugo, A.E. Mangrove Ecosystems: Successional or Steady State? Biotropica 1980, 12, 65. [Google Scholar] [CrossRef]
- Bathmann, J.; Peters, R.; Reef, R.; Berger, U.; Walther, M.; Lovelock, C.E. Modelling Mangrove Forest Structure and Species Composition over Tidal Inundation Gradients: The Feedback between Plant Water Use and Porewater Salinity in an Arid Mangrove Ecosystem: The Feedback between Plant Water Use and Porewater Salinity in an Arid. Agric. For. Meteorol. 2021, 308–309, 108547. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree Growth as Indicator of Tree Vitality and of Tree Reaction to Environmental Stress: A Review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Ball, J.M.; James, R.D. Proposed Experimental Tests of a Theory of Fine Microstructure and the Two-Well Problem. Philos. Trans. R. Soc. Lond. Ser. A Phys. Eng. Sci. 1992, 338, 389–450. [Google Scholar] [CrossRef]
- Watson, J.G. Mangrove Forests of the Malay Peninsula; Malayan Forest Records; Fraser & Neave: Kuala Lumpur, Malaysia, 1928. [Google Scholar]
- Feller, I.C.; McKee, K.L.; Whigham, D.F.; O’Neill, J.P. Nitrogen vs. Phosphorus Limitation across an Ecotonal Gradient in a Mangrove Forest. Biogeochemistry 2003, 62, 145–175. [Google Scholar] [CrossRef]
- Ball, M.C. Ecophysiology of Mangroves. Trees 1988, 2, 129–142. [Google Scholar] [CrossRef]
- Cintron, G.; Lugo, A.E.; Pool, D.J.; Morris, G. Mangroves of Arid Environments in Puerto Rico and Adjacent Islands. Biotropica 1978, 10, 110. [Google Scholar] [CrossRef]
- Semeniuk, V. Mangrove Distribution in Northwestern Australia in Relationship to Regional and Local Freshwater Seepage. Vegetatio 1983, 53, 11–31. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer: Dordrecht, The Netherlands, 2009; ISBN 9781402042706. [Google Scholar]
- Naidoo, G. Ecophysiological Differences between Fringe and Dwarf Avicennia Marina Mangroves. Trees—Struct. Funct. 2010, 24, 667–673. [Google Scholar] [CrossRef]
- Passioura, J.B.; Ball, M.C.; Knight, J.H. Mangroves May Salinize the Soil and in so Doing Limit Their Transpiration Rate. Funct. Ecol. 1992, 6, 476. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Ahmed, M.T.; Alrumman, S.A.; Ahmed, D.A.; Eid, E.M. Evaluation of the Carbon Sequestration Capacity of Arid Mangroves along Nutrient Availability and Salinity Gradients along the Red Sea Coastline of Saudi Arabia. Oceanologia 2020, 62, 56–69. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Salt Tolerance Mechanisms in Mangroves: A Review. Trees—Struct. Funct. 2010, 24, 199–217. [Google Scholar] [CrossRef]
- Teh, S.Y.; Turtora, M.; DeAngelis, D.L.; Jiang, J.; Pearlstine, L.; Smith, T.J.; Koh, H.L. Application of a Coupled Vegetation Competition and Groundwater Simulation Model to Study Effects of Sea Level Rise and Storm Surges on Coastal Vegetation. J. Mar. Sci. Eng. 2015, 3, 1149–1177. [Google Scholar] [CrossRef]
- Ramadhan, F.; Kunarso, K.; Wirasatriya, A.; Maslukah, L.; Handoyo, G. Differences in Depth and Thickness of the Thermocline Layer on ENSO, IOD and Monsoon Variability in Southern Java Waters. Indones. J. Oceanogr. 2021, 3, 214–223. [Google Scholar] [CrossRef]
- Alahacoon, N.; Edirisinghe, M.; Ranagalage, M. Satellite-Based Meteorological and Agricultural Drought Monitoring for Agricultural Sustainability in Sri Lanka. Sustainability 2021, 13, 3427. [Google Scholar] [CrossRef]
- Alongi, D.M. Impact of Global Change on Nutrient Dynamics in Mangrove Forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef] [Green Version]
- Lovelock, C.E.; Ball, M.C.; Martin, K.C.; Feller, I.C. Nutrient Enrichment Increases Mortality of Mangroves. PLoS ONE 2009, 4, e5600. [Google Scholar] [CrossRef] [PubMed] [Green Version]



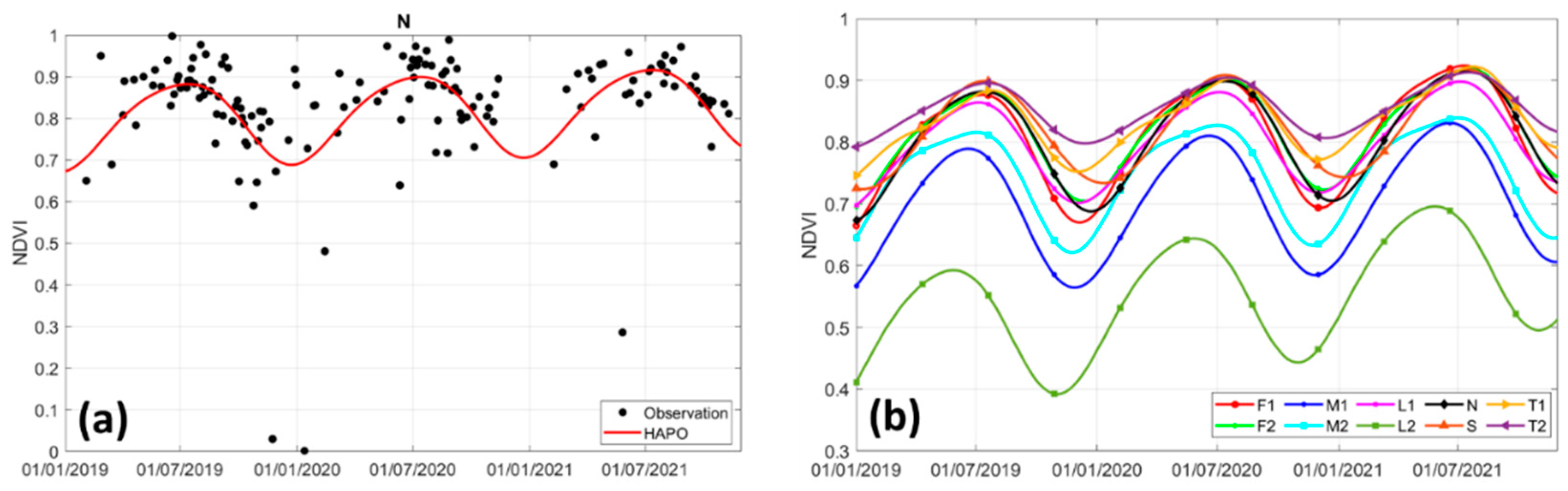



| F1 | F2 | M1 | M2 | L1 | L2 | N | S | T1 | T2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| RMSE | 0.091 | 0.075 | 0.056 | 0.053 | 0.052 | 0.048 | 0.052 | 0.066 | 0.049 | 0.047 |
| Correlation coefficient | 0.621 | 0.598 | 0.803 | 0.792 | 0.706 | 0.892 | 0.700 | 0.545 | 0.666 | 0.603 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prihantono, J.; Nakamura, T.; Nadaoka, K.; Wirasatriya, A.; Adi, N.S. Rainfall Variability and Tidal Inundation Influences on Mangrove Greenness in Karimunjawa National Park, Indonesia. Sustainability 2022, 14, 8948. https://doi.org/10.3390/su14148948
Prihantono J, Nakamura T, Nadaoka K, Wirasatriya A, Adi NS. Rainfall Variability and Tidal Inundation Influences on Mangrove Greenness in Karimunjawa National Park, Indonesia. Sustainability. 2022; 14(14):8948. https://doi.org/10.3390/su14148948
Chicago/Turabian StylePrihantono, Joko, Takashi Nakamura, Kazuo Nadaoka, Anindya Wirasatriya, and Novi Susetyo Adi. 2022. "Rainfall Variability and Tidal Inundation Influences on Mangrove Greenness in Karimunjawa National Park, Indonesia" Sustainability 14, no. 14: 8948. https://doi.org/10.3390/su14148948
APA StylePrihantono, J., Nakamura, T., Nadaoka, K., Wirasatriya, A., & Adi, N. S. (2022). Rainfall Variability and Tidal Inundation Influences on Mangrove Greenness in Karimunjawa National Park, Indonesia. Sustainability, 14(14), 8948. https://doi.org/10.3390/su14148948







