Parameters Affecting the Water Vapour Permeability of Gelatin Films as Evaluated by the Infrared Detecting Method ASTM F1249
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Method of Selection and Preparation of Gelatin Films
2.3. Film Thickness
2.4. Film Uniformity
2.5. WVP Studies
2.6. The Effect of RH and Flow Rate on WVP Measurements Using Gelatin Films
3. Results
3.1. The Effect of Film Thickness on WVP Measurements in Gelatin Films
3.2. ASTM F1249 Method Results Compatibility for Gelatin Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bourtoom, T. Review Article Edible protein films: Properties enhancement. Int. Food Res. J. 2009, 16, 1–9. [Google Scholar]
- Wang, L.Z.; Liu, L.; Holmes, J.; Kerry, J.F.; Kerry, J.P. Assessment of film-forming potential and properties of protein and polysaccharide-based biopolymer films. Int. J. Food Sci. Technol. 2007, 42, 1128–1138. [Google Scholar] [CrossRef]
- Wang, L.; Auty, M.A.; Rau, A.; Kerry, J.F.; Kerry, J.P. Effect of pH and addition of corn oil on the properties of gelatin-based biopolymer films. J. Food Eng. 2009, 90, 11–19. [Google Scholar] [CrossRef]
- Wang, L.; Auty, M.A.; Kerry, J.P. Physical assessment of composite biodegradable films manufactured using whey protein isolate, gelatin and sodium alginate. J. Food Eng. 2010, 96, 199–207. [Google Scholar] [CrossRef]
- Kerry, J.P.; Tyuftin, A.A. Chapter 10–Storage and Preservation of Raw Meat and Muscle-Based Food Products: IV Storage and Packaging. In Lawrie’s Meat Science, 8th ed.; Fidel, T., Ed.; Woodhead Publishing: Sawston, UK, 2017; p. 718. [Google Scholar]
- Clarke, D.; Tyuftin, A.A.; Cruz-Romero, M.C.; Bolton, D.; Fanning, S.; Pankaj, S.K.; Bueno-Ferrer, C.; Cullen, P.J.; Kerry, J.P. Surface attachment of active antimicrobial coatings onto conventional plastic-based laminates and performance assessment of these materials on the storage life of vacuum packaged beef sub-primals. Food Microbiol. 2017, 62, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Hanani, Z.A.N.; Beatty, E.; Roos, Y.; Morris, M.; Kerry, J. Manufacture and characterization of gelatin films derived from beef, pork and fish sources using twin screw extrusion. J. Food Eng. 2012, 113, 606–614. [Google Scholar] [CrossRef]
- Park, H.J.; Chinnan, M.S. Gas and water vapor barrier properties of edible films from protein and cellulosic materials. J. Food Eng. 1995, 25, 497–507. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.D.C. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Hanani, Z.A.N.; McNamara, J.; Roos, Y.; Kerry, J. Effect of plasticizer content on the functional properties of extruded gelatin-based composite films. Food Hydrocoll. 2013, 31, 264–269. [Google Scholar] [CrossRef]
- Clarke, D.; Molinaro, S.; Tyuftin, A.; Bolton, D.; Fanning, S.; Kerry, J.P. Incorporation of commercially-derived antimicrobials into gelatin-based films and assessment of their antimicrobial activity and impact on physical film properties. Food Control 2016, 64, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Tyuftin, A.A.; Kerry, J.P. Gelatin films: Study review of barrier properties and implications for future studies employing biopolymer films. Food Packag. Shelf Life 2021, 29, 100688. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Grosso, C.R.F.; Sobral, P.J.A. Effect of chemical treatment on the mechanical properties, water vapour permeability and sorption isotherms of gelatin-based films. Packag. Technol. Sci. 2008, 21, 165–169. [Google Scholar] [CrossRef]
- Cao, N.; Yang, X.; Fu, Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll. 2009, 23, 729–735. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Castillo, L.A.; Barbosa, S.E.; De Angelis, M.G. Barrier properties and mechanical strength of bio-renewable, heat-sealable films based on gelatin, glycerol and soybean oil for sustainable food packaging. React. Funct. Polym. 2018, 125, 29–36. [Google Scholar] [CrossRef]
- Martucci, J.; Ruseckaite, R. Biodegradable three-layer film derived from bovine gelatin. J. Food Eng. 2010, 99, 377–383. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM E96 vs. F1249. MOCON White Paper. Which Provides More Accurate Test Results? 2017. Available online: https://www.mocon.com/assets/documents/mocon-wp-astm-e96-vs-f1249-method-for-wvtr-permeatio.pdf (accessed on 16 May 2017).
- ASTM F1249-13; Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2013.
- Yi, J.B.; Kim, Y.T.; Bae, H.J.; Whiteside, W.S.; Park, H.J. Influence of Transglutaminase-Induced Cross-Linking on Properties of Fish Gelatin Films. J. Food Sci. 2006, 71, E376–E383. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- MOCON Manual PERMATRAN-W® Model 3/33 Operator’s Manual; Revision C; MOCON: Minneapolis, MN, USA, 2014; Available online: http://www.mocon.com. (accessed on 14 November 2021).
- Lim, L.-T.; Mine, Y.; Tung, M. Barrier and Tensile Properties of Transglutaminase Cross-linked Gelatin Films as Affected by Relative Humidity, Temperature, and Glycerol Content. J. Food Sci. 1999, 64, 616–622. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Cerisuelo, J.P.; Gavara, R.; Hernandez-Muñoz, P. Mass transport properties of gliadin films: Effect of cross-linking degree, relative humidity, and temperature. J. Membr. Sci. 2013, 428, 380–392. [Google Scholar] [CrossRef]
- Roy, S.; Gennadios, A.; Weller, C.L.; Testin, R.F. Water vapor transport parameters of a cast wheat gluten film. Ind. Crop. Prod. 2000, 11, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Bourlieu, C.; Guillard, V.; Vallès-Pamiès, B.; Guilbert, S.; Gontard, N. Edible Moisture Barriers: How to Assess of their Potential and Limits in Food Products Shelf-Life Extension? Crit. Rev. Food Sci. Nutr. 2009, 49, 474–499. [Google Scholar] [CrossRef] [PubMed]
- Todd, W.G. Variables that Affect/Control High-Density Polyethylene Film Oxygen–Moisture Barrier. J. Plast. Film Sheeting 2003, 19, 209–220. [Google Scholar] [CrossRef]
- Hülsmann, P.; Wallner, G. Permeation of water vapour through polyethylene terephthalate (PET) films for back-sheets of photovoltaic modules. Polym. Test. 2017, 58, 153–158. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Basics of Barriers–II; Internet Seminar Series; MOCON: Minneapolis, MN, USA, 2007; Available online: www.mocon.com (accessed on 14 November 2021).
- Buonocore, G.G.; Conte, A.; Del Nobile, M.A. Use of a Mathematical Model to Describe the Barrier Properties of Edible Films. J. Food Sci. 2005, 70, E142–E147. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Wang, L.; Auty, M.A.E.; Kerry, J.P. Development and Assessment of Duplex and Triplex Laminated Edible Films Using Whey Protein Isolate, Gelatin and Sodium Alginate. Int. J. Mol. Sci. 2020, 21, 2486. [Google Scholar] [CrossRef] [Green Version]
 ) 50 µm, (
) 50 µm, (  ) 100 µm and (
) 100 µm and (  ) 150 µm film thickness, ASTM F1249, 37.9 °C.
) 150 µm film thickness, ASTM F1249, 37.9 °C.
 ) 50 µm, (
) 50 µm, (  ) 100 µm and (
) 100 µm and (  ) 150 µm film thickness, ASTM F1249, 37.9 °C.
) 150 µm film thickness, ASTM F1249, 37.9 °C.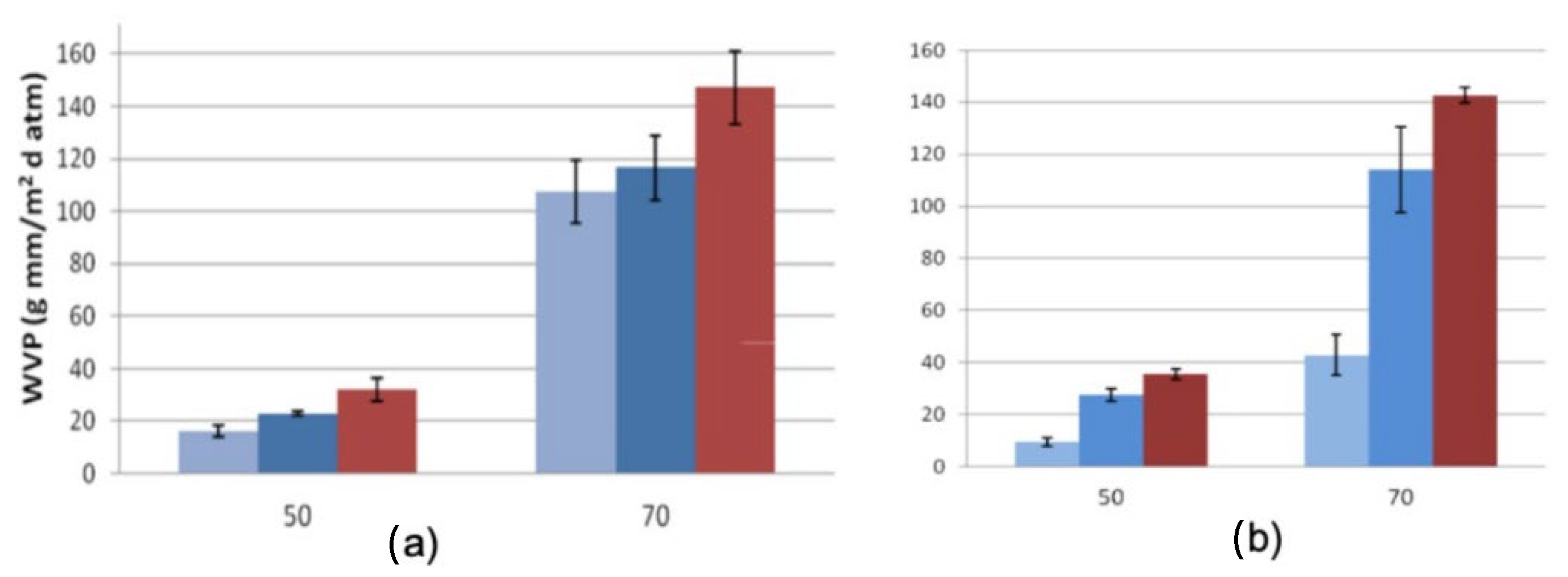
 ) 100 and (
) 100 and (  ) 150 cm3 RH cell gas flow rates, ASTM F1249, 37.9 °C.
) 150 cm3 RH cell gas flow rates, ASTM F1249, 37.9 °C.
 ) 100 and (
) 100 and (  ) 150 cm3 RH cell gas flow rates, ASTM F1249, 37.9 °C.
) 150 cm3 RH cell gas flow rates, ASTM F1249, 37.9 °C.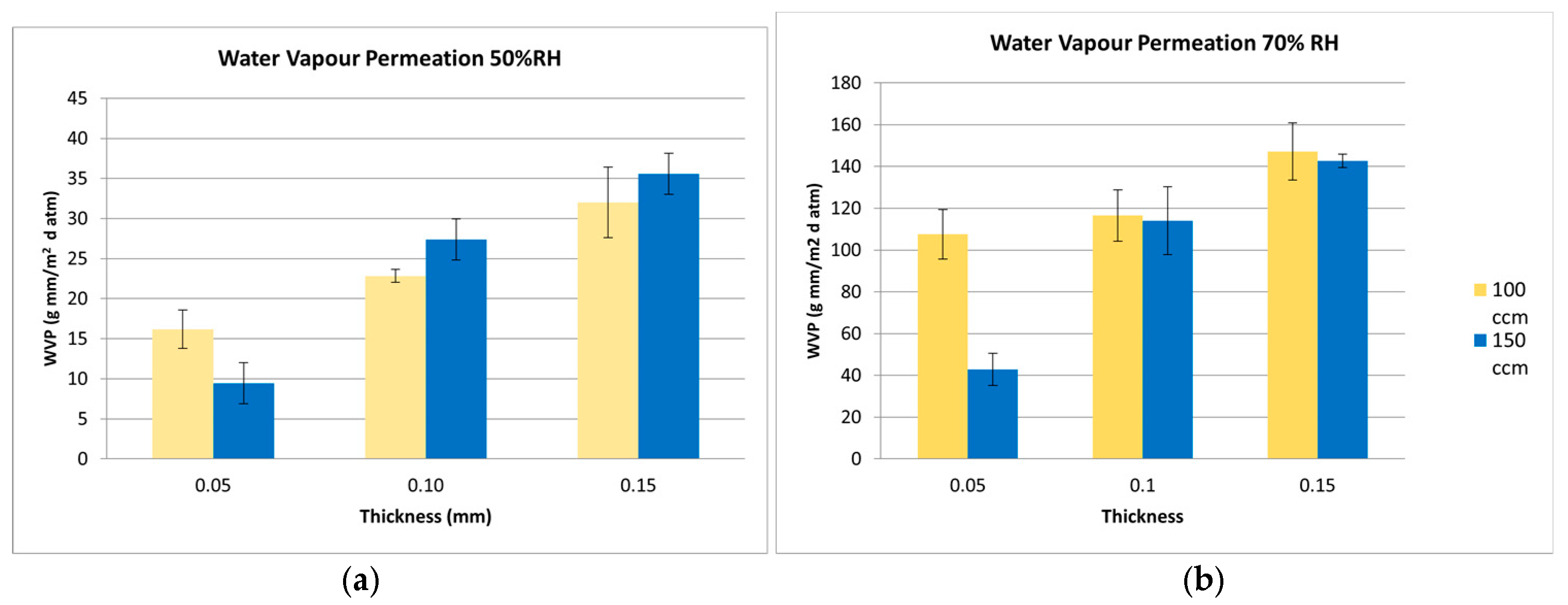
| Bovine Gelatin Content of Film | Plasticizer Type and Concentration | Thickness, (µm) | WVP (g mm/m2 d atm) | Test Relative Humidity RH, (%) | Temperature, (°C) | ASTM |
|---|---|---|---|---|---|---|
| Gelatin 10% | Glycerol 4.5% to gelatin | 80.0 ± 4.0 | 481.5 ± 7.30 | 50 | 25 | E96 |
| Gelatin 1% | Sorbitol 15% to gelatin Sorbitol 45% to gelatin Sorbitol 65% to gelatin | 43 ± 9 | 413.406 705.222 924.084 | 100 | 22 | E96 |
| Gelatin 12% | Malic acid 20% to gelatin Polyethylene glycol 20% to gelatin Sorbitol 20% to gelatin Ethylene glycol 20% to gelatin Diethylene glycol 20% to gelatin Triethylene glycol 20% to gelatin Ethanolamine 20% to gelatin Diethanolamine 20% to gelatin Triethanolamine 20% to gelatin | 21.0 ± 0.6 23.2 ± 0.7 23.7 ± 0.4 22.4 ± 0.7 23.0 ± 0.8 22.5 ± 0.5 23.2 ± 0.7 21.5 ± 0.5 23.4 ± 0.5 | 0.5 ± 0.05 3.3 ± 0.15 0.7 ± 0.05 5.6 ± 0.05 4.7 ± 0.05 4.6 ± 0.07 3.0 ± 0.02 2.4 ± 0.05 3.1 ± 0.05 | 50 | 25 | E96 |
| Gelatin | Glycerol 0.25% water | 56.5 ± 7.43 43.0 ± 9.50 40.8 ± 5.36 50.3 ± 3.79 46.9 ± 4.15 55.0 ± 4.06 | 10,993.8 ± 531.96 7802.0 ± 531.96 6383.5 ± 106.39 9023.0 ± 106.39 9976.5 ± 623.15 7014.7 ± 373.89 | 50 | 23 ± 2 | E96 |
| Gelatin | Glycerol 0.2% to gelatin Glycerol0.5% Glycerol 0.8% Glycerol 1.1% | 20.7 ± 3.2 23.3 ± 2.2 21.1 ± 6.6 23.4 ± 2.5 | 4063.1 ± 137.80 4352.9 ± 15.20 3959.7 ± 45.60 4869.7 ± 122.60 | 50 | 23 ± 2 | E96 |
| Gelatin 5% | Glycerol 33% to gelatin | 57 ± 6 | 37.6 ± 3.42 | 50 | 23 ± 2 | E96 |
| Gelatin 3.34% | Sorbitol 2% to solution | N/A | 26438.5 ± 1225.63 | 50 | 25 | E96 |
| Gelatin | Glycerol 40% to gelatin | 125 ± 25 | 2.8 ± 0.33 14.7 ± 1.18 77.8 ± 0.48 196.1 ± 0.89 | 35 50 70 90 | 23 | F1249 |
| Gelatin | Glycerol 30% to gelatin | 58 ± 4 | 2188.62 | 65 | 25 | E96 |
| Gelatin (only fish gelatin) 20% | - | None | 1630 ± 300 | 50 | 25 | F1249 |
| V, (mL) | Average Film Thickness Obtained (µm) |
|---|---|
| 4 | 20 ± 2 |
| 7 | 50 ± 1 |
| 13 | 100 ± 6 |
| 18 | 150 ± 10 |
| RH Cell Gas Flow Rate, (sccm) | Cells A, B, Z, Ref Gas Flow Rate, (sccm) | Test Area, (cm2) | RH, (%) | Temperature, (°C) |
|---|---|---|---|---|
| 20 | 10 | 1.131 | 50 | 37.8 |
| 100 | 100 | 1.131 | 50 or 70 | 37.8 |
| 150 | 100 | 5 | 50 or 70 | 37.8 |
| 100 | 100 | 5 | 50 | 25 |
| Gelatin Film Thickness, (mm) | WVP, (g mm/m2 d atm) |
|---|---|
| 0.020 ± 0.0008 | 84.4 ± 10.99 |
| 0.030 ± 0.0004 | 91.8 ± 18.36 |
| 0.041 ± 0.0001 | 104.5 ± 24.15 |
| Thickness, (µm) | Temperature, (°C) | WVP, (g mm/m2 day atm) | Reference (@ RH = 50%) |
|---|---|---|---|
| 125 ± 25 | 25 | 12.9 ± 2.36 | Current study: RH cell flow = 150 cm3, gas flow for other cells = 100 cm3 |
| 125 ± 25 | 23 | 14.7 ± 1.18 | [16]: RH cell flow = 150 cm3, gas flow for other cells = 100 cm3 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyuftin, A.A.; Pecorini, F.; Zanardi, E.; Kerry, J.P. Parameters Affecting the Water Vapour Permeability of Gelatin Films as Evaluated by the Infrared Detecting Method ASTM F1249. Sustainability 2022, 14, 9018. https://doi.org/10.3390/su14159018
Tyuftin AA, Pecorini F, Zanardi E, Kerry JP. Parameters Affecting the Water Vapour Permeability of Gelatin Films as Evaluated by the Infrared Detecting Method ASTM F1249. Sustainability. 2022; 14(15):9018. https://doi.org/10.3390/su14159018
Chicago/Turabian StyleTyuftin, Andrey A., Francesca Pecorini, Emanuela Zanardi, and Joe P. Kerry. 2022. "Parameters Affecting the Water Vapour Permeability of Gelatin Films as Evaluated by the Infrared Detecting Method ASTM F1249" Sustainability 14, no. 15: 9018. https://doi.org/10.3390/su14159018
APA StyleTyuftin, A. A., Pecorini, F., Zanardi, E., & Kerry, J. P. (2022). Parameters Affecting the Water Vapour Permeability of Gelatin Films as Evaluated by the Infrared Detecting Method ASTM F1249. Sustainability, 14(15), 9018. https://doi.org/10.3390/su14159018