Advances in Thermo-Electrochemical (TEC) Cell Performances for Harvesting Low-Grade Heat Energy: A Review
Abstract
:1. Introduction
2. Historical Background
- TEC cells with phase transitions during mass transfer have been studied for a relatively long time (the first publications date back to the 1970s), but these have not found practical application due to their complexity of design and rapid degradation of components. Wang et al. [52] have recently developed electrochemical sodium heat engines based on phase-change reactions. Nevertheless, their important advantage is their continuous operation, which does not require changing hot and cold sources.
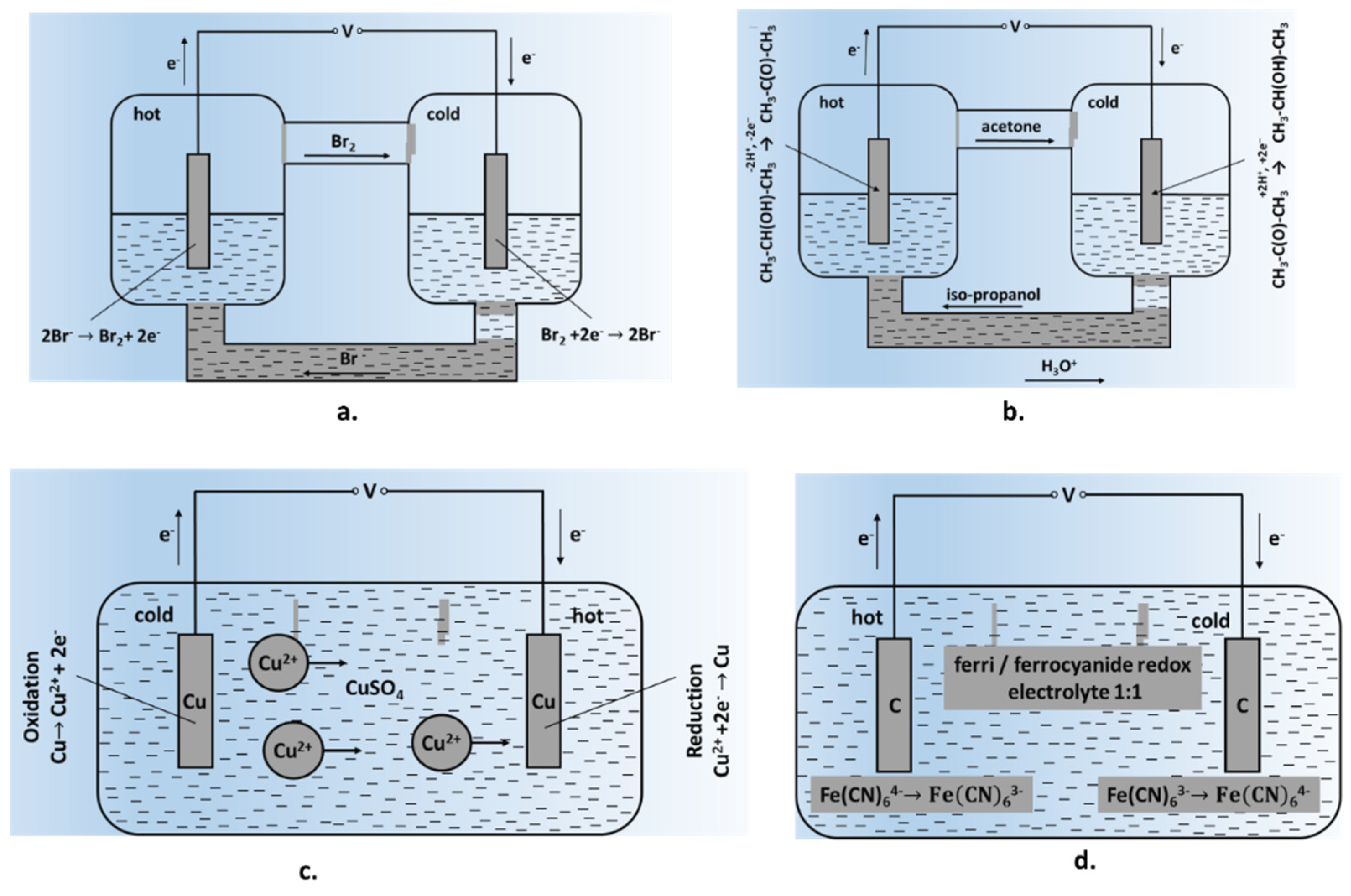
- Typical TECs are non-isothermal electrochemical cell systems consisting of two electrodes, an electrolyte and a separator. The conversion of thermal energy into electrical energy involves electrode kinetics, thermodynamics, and heat and mass transfer. TEC cells involve changes in the aggregate state of oxidized and reduced forms (nitric acid and nitrogen oxides or KBr and Br2) during continuous operation [13,35].
- Thermally regenerative electrochemical cycles (TREC) consist of two electrodes with opposite thermopowers; anodes and cathodes generally have positive and negative thermopowers, respectively. For negative thermopower, the cycle involves cooling, discharging, heating and charging; for positive thermopower, the cycle involves heating, charging, cooling and discharging [53].
- Other cell configurations include thermogalvanic cells (TGC) based on soluble, reversible metal electrodes in solutions of their own salts at different temperatures—TECC cells, using inert electrodes placed in a redox electrolyte or ionic liquid; TGC-Li cells, based on a Li+/Li redox system; TRABs (thermally regenerative ammonia-based batteries); DTCCs (direct thermal charging cells); etc. [54,55,56,57].
3. Redox Couples and Electrolytes
3.1. Redox Couples
3.2. Electrolytes
4. Electrode Materials and Designs
5. Emerging Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forman, C.; Muritala, I.K.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renew. Sustain. Energy Rev. 2016, 57, 1568–1579. [Google Scholar] [CrossRef]
- Straub, A.P.; Yip, N.Y.; Lin, S.; Lee, J.; Elimelech, M. Harvesting low-grade heat energy using thermo-osmotic vapour transport through nanoporous membranes. Nat. Energy 2016, 1, 16090. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, S.W.; Ghasemi, H.; Loomis, J.; Li, X.; Kraemer, D.; Zheng, G.; Cui, Y.; Chen, G. Charging-free electrochemical system for harvesting low-grade thermal energy. Proc. Natl. Acad. Sci. USA 2014, 111, 17011–17016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, S. CarbonBrief/Energy. Solar, Wind and Nuclear Have ‘Amazingly Low’ Carbon Footprints. 2017. Available online: https://www.carbonbrief.org/solar-wind-nuclear-amazingly-low-carbon-footprints/#:~:text=Simon%20Evans,-08.12.2017%20%7C%205&text=Building%20solar%2C%20wind%20or%20nuclear,of%20electricity%20out%20to%202050 (accessed on 10 January 2022).
- COP26 Outcomes. 2021. Available online: https://ukcop26.org/the-conference/cop26-outcomes (accessed on 10 January 2022).
- Bouty, E. Phénomènes thermo-électriques et électro-thermiques au contact d’un métal et d’un liquide. J. Phys. Théorique Appliquée 1880, 9, 306–320. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; Macmillan Publishers, Ltd.: London, UK; World Scientific Publishing Co., Ltd.: Singapore, 2010; pp. 101–110. [Google Scholar]
- Jangonda, C.; Patil, K.; Kinikar, A.; Bhokare, R.; Gavali, M.D. Review of various application of thermoelectric module. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 3393. [Google Scholar]
- Yang, X.; Wang, C.; Lu, R.; Shen, Y.; Zhao, H.; Li, J.; Zheng, X. Progress in Measurement of Thermoelectric Properties of Micro/Nano Thermoelectric Materials: A Critical Review. Nano Energy 2022, 101, 107553. [Google Scholar] [CrossRef]
- Chen, X.Q.; Fan, S.J.; Han, C.; Wu, T.; Wang, L.J.; Jiang, W.; Yang, J.P. Multiscale architectures boosting thermoelectric performance of copper sulfide compound. Rare Met. 2021, 40, 2017–2025. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhao, Y.; Liu, W.D.; Dai, W.; Wu, T.; Yang, J. Carbon-encapsulated copper sulfide leading to enhanced thermoelectric properties. ACS Appl. Mater. Interfaces 2019, 11, 22457–22463. [Google Scholar] [CrossRef] [PubMed]
- Vining, C.B. An inconvenient truth about thermoelectrics. Nat. Mater. 2009, 8, 83–85. [Google Scholar] [CrossRef]
- de Bethune, A.J.; Licht, T.S.; Swendeman, N. The Temperature Coefficients of Electrode Potentials. J. Electrochem. Soc. 1959, 106, 616. [Google Scholar] [CrossRef]
- Landry, B.A. Utilization of waste heat. Science 1953, 3, 3. [Google Scholar] [CrossRef]
- Wakao, N.; Nojo, K. Nitric acid cycle process for extracting thermal energy from low-level heat sources. Nature 1978, 273, 25–27. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef]
- Waske, A.; Dzekan, D.; Sellschopp, K.; Berger, D.; Stork, A.; Nielsch, K.; Fähler, S. Energy harvesting near room temperature using a thermomagnetic generator with a pretzel-like magnetic flux topology. Nat. Energy 2018, 4, 68–74. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Lacey, S.D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat. Mater. 2019, 18, 608–613. [Google Scholar] [CrossRef]
- Yu, B.; Duan, J.; Cong, H.; Xie, W.; Liu, R.; Zhuang, X.; Wang, H.; Qi, B.; Xu, M.; Wang, Z.L.; et al. Thermosensitive crystallization-boosted liquid thermocells for low-grade heat harvesting. Science 2020, 370, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Pandya, S.; Wilbur, J.; Kim, J.; Gao, R.; Dasgupta, A.; Dames, C.; Martin, L.W. Pyroelectric energy conversion with large energy and power density in relaxor ferroelectric thin films. Nat. Mater. 2018, 17, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.; Kumar, A.; Song, H.C.; Jeong, D.Y.; Ryu, J. Pyroelectric Energy Conversion and Its Applications—Flexible Energy Harvesters and Sensors. Sensors 2019, 19, 2170. [Google Scholar] [CrossRef] [Green Version]
- Quoilin, S.; van den Broek, M.; Declaye, S.; Dewallef, P.; Lemort, V. Techno-economic survey of Organic Rankine Cycle (ORC) systems. Renew. Sustain. Energy Rev. 2013, 22, 168–186. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chau, K.T. An automotive thermoelectric photovoltaic hybrid energy system using maximum power point tracking. Energy Convers. Manag. 2011, 52, 641e7. [Google Scholar] [CrossRef]
- Zhang, H.; W Kong, W.; Dong, F.; Xu, H.; Chen, B.; Ni, M. Application of cascading thermoelectric generator and cooler for waste heat recovery from solid oxide fuel cells. Energy Convers. Manag. 2017, 148, 1382–1390. [Google Scholar] [CrossRef]
- Li, P.; Cai, L.; Zhai, P.; Tang, X.; Zhang, Q.; Nino, M. Design of a concentration solar thermoelectric generator. J. Electron. Mater. 2010, 39, 1522–1530. [Google Scholar] [CrossRef]
- Dupont, M.F.; MacFarlane, D.R.; Pringle, J.M. Thermo-electrochemical cells for waste heat harvesting—Progress and perspectives. Chem. Commun. 2017, 53, 6288–6302. [Google Scholar] [CrossRef] [PubMed]
- Chum, H.L.; Osteryoung, R.A. Review of Thermally Regenerative Electrochemical Cells; Solar Energy Research Institute: Selangor, Malaysia, 1981. [Google Scholar]
- Lalancette, J.-M.; Roussel, R. Metals intercalated in graphite. V. A concentration cell with intercalated bromine. Can. J. Chem. 1976, 54, 3541–3544. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Yamagishi, Y.; Inagaki, M. Thermocell with graphite fiber-bromine intercalation compounds. Synth. Met. 1983, 7, 203–209. [Google Scholar] [CrossRef]
- Inagaki, M.; Matsumoto, A.; Sakai, M.; Maeda, Y. A cell of carbon-fibers and nitric acid with temperature difference. Nippon Kagaku Kaishi 1983, 2, 309–311. [Google Scholar] [CrossRef]
- Vigolo, D.; Buzzaccaro, S.; Piazza, R. Thermophoresis and thermoelectricity in surfactant solutions. Langmuir, 2010; 26, 7792. [Google Scholar]
- Lee, J.H.; Shin, G.; Baek, J.Y.; Kang, T.J. An electricity-generating window made of a transparent energy harvester of thermocells. ACS Appl. Mater. Interfaces 2021, 13, 21157–21165. [Google Scholar] [CrossRef]
- Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J.S.; Ovalle-Robles, R.; Yang, H.D.; Kihm, K.D.; Baughman, R.H.; Lee, H.H.; et al. High-efficiency electrochemical thermal energy harvester using carbon nanotube aerogel sheet electrodes. Nat. Commun. 2016, 7, 10600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artyukhov, D.; Kiselev, N.; Gorshkov, N.; Kovyneva, N.; Ganzha, O.; Vikulova, M.; Gorokhovsky, A.; Offor, P.; Boychenko, E.; Burmistrov, I. Harvesting Waste Thermal Energy Using a Surface-Modified Carbon Fiber-Based Thermo-Electrochemical Cell. Sustainability 2021, 13, 1377. [Google Scholar] [CrossRef]
- Inagaki, M.; Itoh, E.; Maeda, Y. Durable Performance of Thermocell with Carbon Cloth and Nitric Acid. TANSO 1985, 1985, 134–136. [Google Scholar] [CrossRef] [Green Version]
- Battistel, A.; Peljo, P. Recent trends in thermoelectrochemical cells and thermally regenerative batteries. Curr. Opin. Electrochem. 2021, 30, 100853. [Google Scholar] [CrossRef]
- Duan, J.; Yu, B.; Huang, L.; Hu, B.; Xu, M.; Feng, G.; Zhou, J. Liquid-state thermocells: Opportunities and challenges for low-grade heat harvesting. Joule 2021, 5, 768–779. [Google Scholar] [CrossRef]
- Disalvo, F.J. Thermoelectric cooling and power generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.J.; Macfarlane, D.R.; Baughman, R.H.; Jin, L.; Li, N.; Pringle, J.M. Towards ionic liquid-based thermoelectrochemical cells for the harvesting of thermal energy. Electrochim. Acta 2013, 113, 87–93. [Google Scholar] [CrossRef]
- Romano, M.S.; Razal, J.M.; Antiohos, D.; Wallace, G.; Chen, J. Nano-carbon electrodes for thermal energy harvesting. J. Nanosci. Nanotechnol. 2015, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Al-Masri, D.; MacFarlane, D.R.; Pringle, J.M. Temperature dependence of the electrode potential of a cobalt-based redox couple in ionic liquid electrolytes for thermal energy harvesting. Faraday Discuss. 2016, 190, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.J.; Fang, S.; Kozlov, M.E.; Haines, C.S.; Li, N.; Kim, Y.H.; Chen, Y.; Baughman, R.H. Electrical Power from Nanotube and Graphene Electrochemical Thermal Energy Harvesters. Adv. Funct. Mater. 2012, 22, 477–489. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, T.; Li, N.; Kang, T.J.; Chen, J.; Pringle, J.M.; Zhang, M.; Kazim, A.H.; Fang, S.; Haines, C.; et al. High Power Density Electrochemical Thermocells for Inexpensively Harvesting Low-Grade Thermal Energy. Adv. Mater. 2017, 29, 1605652. [Google Scholar] [CrossRef]
- Maeda, Y.; Kitamura, H.; Itoh, E.; Inagaki, M. A new carbon fiber and nitric acid cell with a temperature difference between electrodes. Synth. Met. 1983, 7, 211–217. [Google Scholar] [CrossRef]
- Mua, Y.; Quickenden, T.I. Power Conversion Efficiency, Electrode Separation, and Overpotential in the Ferricyanide/Ferrocyanide Thermogalvanic Cell. J. Electrochem. Soc. 1996, 143, 2558–2564. [Google Scholar] [CrossRef]
- Hinterleitner, B.; Knapp, I.; Poneder, M.; Shi, Y.; Müller, H.; Eguchi, G.; Eisenmenger-Sittner, C.; Stöger-Pollach, M.; Kakefuda, Y.; Kawamoto, N.; et al. Thermoelectric performance of a metastable thin-film Heusler alloy. Nature 2019, 576, 85–90. [Google Scholar] [CrossRef]
- Li, D.; Sun, R.R.; Qin, X.Y. Improving thermoelectric properties of p-type Bi2Te3-based alloys by spark plasma sintering. Prog. Nat. Sci. Mater. Int. 2011, 21, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Li, M.; Hong, M.; Dargusch, M.; Zou, J.; Chen, Z.G. High-efficiency thermocells driven by thermo-electrochemical processes. Trends Chem. 2021, 3, 561–574. [Google Scholar] [CrossRef]
- Cho, C.; Stevens, B.; Hsu, J.-H.; Bureau, R.; Hagen, D.A.; Regev, O.; Yu, C.; Grunlan, J.C.; Cho, C.; Stevens, B.; et al. Completely Organic Multilayer Thin Film with Thermoelectric Power Factor Rivaling Inorganic Tellurides. Adv. Mater. 2015, 27, 2996–3001. [Google Scholar] [CrossRef]
- Wang, H.; Hsu, J.-H.; Yi, S.-I.; Kim, S.L.; Choi, K.; Yang, G.; Yu, C.; Wang, H.; Yi, S.; Kim, S.L.; et al. Thermally Driven Large N-Type Voltage Responses from Hybrids of Carbon Nanotubes and Poly(3,4-ethylenedioxythiophene) with Tetrakis(dimethylamino)ethylene. Adv. Mater. 2015, 27, 6855–6861. [Google Scholar] [CrossRef]
- Wang, W.; Shu, G.; Tian, H.; Huo, D.; Zhu, X. A bimetallic thermally-regenerative ammonia-based flow battery for low-grade waste heat recovery. J. Power Sources 2019, 424, 184–192. [Google Scholar] [CrossRef]
- Cheng, C.; Dai, Y.; Yu, J.; Liu, C.; Wang, S.; Feng, S.P.; Ni, M. Review of Liquid-Based Systems to Recover Low-Grade Waste Heat for Electrical Energy Generation. Energy Fuels 2021, 35, 161–175. [Google Scholar] [CrossRef]
- Hu, R.; Xu, D.; Luo, X. Liquid Thermocells Enable Low-Grade Heat Harvesting. Matter 2020, 3, 1400–1402. [Google Scholar] [CrossRef]
- Black, J.J.; Murphy, T.; Atkin, R.; Dolan, A.; Aldous, L. The thermoelectrochemistry of lithium–glyme solvate ionic liquids: Towards waste heat harvesting. Phys. Chem. Chem. Phys. 2016, 18, 20768–20777. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, P. High Seebeck Coefficient Electrochemical Thermocells for Efficient Waste Heat Recovery. ACS Appl. Energy Mater. 2018, 1, 1424–1428. [Google Scholar] [CrossRef]
- Burmistrov, I.; Artyukhov, D.; Shindrov, A.; Gorshkov, N.; Gorokhovsky, A. Thermo-Electrochemical Cells for Low-Grade Waste Heat Conversion. In Proceedings of the Nanotech Middle East 2017 Conference and Exhibition, Dubai, United Arab Emirates, 4–6 December 2017; pp. 4–6. [Google Scholar]
- Sahami, S.; Weaver, M.J. Entropic and enthalpic contributions to the solvent dependence of the thermodynamics of transition-metal redox couples: Part II. Couples containing ammine and ethylenediamine ligands. J. Electroanal. Chem. Interfacial Electrochem. 1981, 122, 171–181. [Google Scholar] [CrossRef]
- Migita, T.; Tachikawa, N.; Katayama, Y.; Miura, T. Thermoelectromotive force of some redox couples in an amide-type room-temperature ionic liquid. Electrochemistry 2009, 77, 639–641. [Google Scholar] [CrossRef] [Green Version]
- Laux, E.; Uhl, S.; Journot, T.; Brossard, J.; Jeandupeux, L.; Keppner, H. Aspects of Protonic Ionic Liquid as Electrolyte in Thermoelectric Generators. J. Electron. Mater. 2016, 45, 3383–3389. [Google Scholar] [CrossRef]
- Anari, E.H.B.; Romano, M.; Teh, W.X.; Black, J.J.; Jiang, E.; Chen, J.; To, T.Q.; Panchompoo, J.; Aldous, L. Substituted ferrocenes and iodine as synergistic thermoelectrochemical heat harvesting redox couples in ionic liquids. Chem. Commun. 2016, 52, 745–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinovyeva, V.; Nakamae, S.; Bonetti, M.; Roger, M. Enhanced Thermoelectric Power in Ionic Liquids. ChemElectroChem 2014, 1, 426–430. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.S.; Lee, G.; Yoon, H.; Yoon, J.; Kang, T.J.; Kim, Y.H. High thermopower of ferri/ferrocyanide redox couple in organic-water solutions. Nano Energy 2017, 31, 160–167. [Google Scholar] [CrossRef]
- Bonetti, M.; Nakamae, S.; Roger, M.; Guenoun, P. Huge Seebeck coefficients in nonaqueous electrolytes. J. Chem. Phys. 2011, 134, 114513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhao, D.; Khan, Z.U.; Puzinas, S.; Jonsson, M.P.; Berggren, M.; Crispin, X.; Wang, H.; Zhao, D.; Khan, Z.U.; et al. Ionic Thermoelectric Figure of Merit for Charging of Supercapacitors. Adv. Electron. Mater. 2017, 3, 1700013. [Google Scholar] [CrossRef]
- Krebs, S. Performance Analysis of a Copper II Sulfate Pentahydrate Based Thermogalvanic Cell. Master’s Thesis, University of Louisville, Louisville, KY, USA, 2015. [Google Scholar]
- Cabral, D.M.; Howlett, P.C.; MacFarlane, D.R. Electrochemistry of the tris(2,2′-bipyridine) complex of iron(II) in ionic liquids and aprotic molecular solvents. Electrochim. Acta 2016, 220, 347–353. [Google Scholar] [CrossRef]
- Yamato, Y.; Katayama, Y.; Miura, T. Effects of the Interaction between Ionic Liquids and Redox Couples on Their Reaction Entropies. J. Electrochem. Soc. 2013, 160, H309–H314. [Google Scholar] [CrossRef]
- Jiao, N.; Abraham, T.J.; MacFarlane, D.R.; Pringle, J.M. Ionic Liquid Electrolytes for Thermal Energy Harvesting Using a Cobalt Redox Couple. J. Electrochem. Soc. 2014, 161, D3061–D3065. [Google Scholar] [CrossRef] [Green Version]
- Salazar, P.F.; Stephens, S.T.; Kazim, A.H.; Pringle, J.M.; Cola, B.A. Enhanced thermo-electrochemical power using carbon nanotube additives in ionic liquid redox electrolytes. J. Mater. Chem. A 2014, 2, 20676–20682. [Google Scholar] [CrossRef] [Green Version]
- Kazim, A.H.; Cola, B.A. Electrochemical Characterization of Carbon Nanotube and Poly (3,4-ethylenedioxythiophene)−Poly(styrenesulfonate) Composite Aqueous Electrolyte for Thermo-Electrochemical Cells. J. Electrochem. Soc. 2016, 163, F867–F871. [Google Scholar] [CrossRef]
- Wu, J.; Black, J.J.; Aldous, L. Thermoelectrochemistry using conventional and novel gelled electrolytes in heat-to-current thermocells. Electrochim. Acta 2017, 225, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Greene, G.W.; MacFarlane, D.R.; Pringle, J.M. Redox-Active Quasi-Solid-State Electrolytes for Thermal Energy Harvesting. ACS Energy Lett. 2016, 1, 654–658. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, X.; Guo, J.; Zhou, C.; Zuo, H.; Liu, Q.; Huang, Q.; Zhang, Q.; Diao, X. The role of interface between LiPON solid electrolyte and electrode in inorganic monolithic electrochromic devices. Electrochim. Acta 2018, 260, 254–263. [Google Scholar] [CrossRef]
- Yang, P.; Liu, K.; Chen, Q.; Mo, X.; Zhou, Y.; Li, S.; Feng, G.; Zhou, J. Wearable thermocells based on gel electrolytes for the utilization of body heat. Angew. Chem. 2016, 128, 12229–12232. [Google Scholar] [CrossRef]
- Quickenden, T.I.; Mua, Y. A Review of Power Generation in Aqueous Thermogalvanic Cells. J. Electrochem. Soc. 1995, 142, 3985–3994. [Google Scholar] [CrossRef]
- Gunawan, A.; Li, H.; Lin, C.H.; Buttry, D.A.; Mujica, V.; Taylor, R.A.; Prasher, R.S.; Phelan, P.E. The amplifying effect of natural convection on power generation of thermogalvanic cells. Int. J. Heat Mass Transf. 2014, 78, 423–434. [Google Scholar] [CrossRef]
- Burmistrov, I.; Kovyneva, N.; Gorshkov, N.; Gorokhovsky, A.; Durakov, A.; Artyukhov, D.; Kiselev, N. Development of new electrode materials for thermo-electrochemical cells for waste heat harvesting. Renew. Energy Focus 2019, 29, 42–48. [Google Scholar] [CrossRef]
- Koo, M.H.; Yoon, H.H. Fabrication of carbon nanotubes and charge transfer complex-based electrodes for a glucose/oxygen biofuel cell. J. Nanosci. Nanotechnol. 2013, 13, 7434–7438. [Google Scholar] [CrossRef] [PubMed]
- Nugent, J.M.; Santhanam, K.S.V.; Rubio, A.; Ajayan, P.M. Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube Microbundle Electrodes. Nano Lett. 2001, 1, 87–91. [Google Scholar] [CrossRef]
- Hu, R.; Cola, B.A.; Haram, N.; Barisci, J.N.; Lee, S.; Stoughton, S.; Wallace, G.; Too, C.; Thomas, M.; Gestos, A.; et al. Harvesting Waste Thermal Energy Using a Carbon-Nanotube-Based Thermo-Electrochemical Cell. Nano Lett. 2010, 10, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, I.; Gorshkov, N.; Kovyneva, N.; Kolesnikov, E.; Khaidarov, B.; Karunakaran, G.; Cho, E.B.; Kiselev, N.; Artyukhov, D.; Kuznetsov, D.; et al. High seebeck coefficient thermo-electrochemical cell using nickel hollow microspheres electrodes. Renew. Energy 2020, 157, 1–8. [Google Scholar] [CrossRef]
- Kiselev, N.; Artyukhov, D.; Boychenko, E.; Gorshkov, N.; Glubokaya, A.; Burmistrov, I. Electrolyte concentration dependences of NiO based thermoelectrochemical cells performance. AIP Conf. Proc. 2022, 2456, 020005. [Google Scholar]
- Taganova, A.A.; Boychenko, E.A.; Kiselev, N.v.; Khaidarov, B.B.; Kolesnikov, E.A.; Yudin, A.G.; Vikulova, M.A.; Gorshkov, N.v.; Kuznetsov, D.v.; Burmistrov, I.N. Synthesis and Study of the Composition of Hollow Microspheres of NiO and NiO/Ni Composition for Thermoelectrochemical Energy Converters of Low-Potential Temperature Gradients of Thermal Units into Electricity. Refract. Ind. Ceram. 2021, 61, 715–719. [Google Scholar] [CrossRef]
- Marquardt, T.; Kube, J.; Radici, P.; Kabelac, S. Experimental investigation of a thermocell with proton exchange membrane and hydrogen electrodes. Int. J. Hydrog. Energy 2020, 45, 12680–12690. [Google Scholar] [CrossRef]
- Misra, V.; Bozkurt, A.; Calhoun, B.; Jackson, T.; Jur, J.; Lach, J.; Lee, B.; Muth, J.; Oralkan, O.; Ozturk, M.; et al. Flexible technologies for self-powered wearable health and environmental sensing. Proc. IEEE 2015, 103, 665–681. [Google Scholar] [CrossRef]
- Ando Junior, O.H.; Maran, A.L.O.; Henao, N.C. A review of the development and applications of thermoelectric microgenerators for energy harvesting. Renew. Sustain. Energy Rev. 2018, 91, 376–393. [Google Scholar] [CrossRef]
- Riemer, R.; Shapiro, A. Biomechanical energy harvesting from human motion: Theory, state of the art, design guidelines, and future directions. J. Neuroeng. Rehabil. 2011, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, F.; Dulio, S.; Patrini, M.; Guizzetti, G.; Mustarelli, P. Energy harvesting from human motion: Materials and techniques. Chem. Soc. Rev. 2016, 45, 5455–5473. [Google Scholar] [CrossRef]
- Llamas, R.; IDC Media Center. Worldwide Wearables Market to Nearly Double by 2021, According to IDC Internet. Available online: https://www.idc.com/getdoc.jsp?containerId=prUS42818517 (accessed on 22 November 2021).
- Xu, Z.; Wu, H.; Zhu, T.; Fu, C.; Liu, X.; Hu, L.; He, J.; He, J.; Zhao, X. Attaining high mid-temperature performance in (Bi,Sb)2Te3 thermoelectric materials via synergistic optimization. NPG Asia Mater. 2016, 8, e302. [Google Scholar] [CrossRef]
- Wang, S.; Tan, G.; Xie, W.; Zheng, G.; Li, H.; Yang, J.; Tang, X. Enhanced thermoelectric properties of Bi2(Te1−xSex)3-based compounds as n-type legs for low-temperature power generation. J. Mater. Chem. 2012, 22, 20943–20951. [Google Scholar] [CrossRef]
- Leonov, V. Thermoelectric energy harvester on the heated human machine. J. Micromech. Microeng. 2011, 21, 125013. [Google Scholar] [CrossRef]
- Suarez, F.; Parekh, D.P.; Ladd, C.; Vashaee, D.; Dickey, M.D.; Öztürk, M.C. Flexible thermoelectric generator using bulk legs and liquid metal interconnects for wearable electronics. Appl. Energy 2017, 202, 736–745. [Google Scholar] [CrossRef]
- Park, H.; Lee, D.; Kim, D.; Cho, H.; Eom, Y.; Hwang, J.; Kim, H.; Kim, J.; Han, S.; Kim, W. High power output from body heat harvesting based on flexible thermoelectric system with low thermal contact resistance. J. Phys. D Appl. Phys. 2018, 51, 365501. [Google Scholar] [CrossRef]
- Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT:PSS. Adv. Electron. Mater. 2019, 5, 1800769. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Sherrell, P.C.; Liu, L.; Wang, Y.; Chen, J. Potentially Wearable Thermo-Electrochemical Cells for Body Heat Harvesting: From Mechanism, Materials, Strategies to Applications. Adv. Sci. 2021, 8, 2100669. [Google Scholar] [CrossRef]
- Masoumi, S.; O’Shaughnessy, S.; Pakdel, A. Organic-based flexible thermoelectric generators: From materials to devices. Nano Energy 2022, 92, 106774. [Google Scholar] [CrossRef]
- Peng, S.; Wang, D.; Lu, J.; He, M.; Xu, C.; Li, Y.; Zhu, S. A Review on Organic Polymer-Based Thermoelectric Materials. J. Polym. Environ. 2017, 25, 1208–1218. [Google Scholar] [CrossRef]
- Guan, X.; Cheng, H.; Ouyang, J. Significant enhancement in the Seebeck coefficient and power factor of thermoelectric polymers by the Soret effect of polyelectrolytes. J. Mater. Chem. A 2018, 6, 19347–19352. [Google Scholar] [CrossRef]
- Yang, S.; Cho, K.; Park, Y.; Kim, S. Bendable thermoelectric generators composed of p- and n-type silver chalcogenide nanoparticle thin films. Nano Energy 2018, 49, 333–337. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, H.E.; Choi, H.; Kim, Y.; We, J.H.; Shin, J.S.; Lee, K.J.; Cho, B.J. High-Performance Flexible Thermoelectric Power Generator Using Laser Multiscanning Lift-Off Process. ACS Nano 2016, 10, 10851–10857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, H.; Khan, Z.U.; Chen, J.C.; Gabrielsson, R.; Jonsson, M.P.; Berggren, M.; Crispin, X. Ionic thermoelectric supercapacitors. Energy Environ. Sci. 2016, 9, 1450–1457. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Cho, K.; Yang, S.; Park, Y.; Kim, S. A laterally designed all-in-one energy device using a thermoelectric generator-coupled micro supercapacitor. Nano Energy 2019, 60, 667–672. [Google Scholar] [CrossRef]
- Wu, X.; Huang, B.; Wang, Q.; Wang, Y. Thermally chargeable supercapacitor using a conjugated conducting polymer: Insight into the mechanism of charge-discharge cycle. Chem. Eng. J. 2019, 373, 493–500. [Google Scholar] [CrossRef]
- Park, Y.; Cho, K.; Kim, S. Vertical all-in-one energy systems constructed with thermoelectric generators and microsupercapacitors. J. Power Sources 2021, 510, 230402. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, X.; Wang, B.; Xia, M.; Lin, S.; Guo, Z.; Zhang, X.; Gao, S. Coupling thermoelectricity and electrocatalysis for hydrogen production via PbTePbS/TiO2 heterojunction. J. Power Sources 2017, 342, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Li, K.; Yang, Z.; Zhang, C.; Deng, J. An advanced Lithium-ion battery optimal charging strategy based on a coupled thermoelectric model. Electrochim. Acta 2017, 225, 330–344. [Google Scholar] [CrossRef]
- Richards, G.; Gemmen, R.S.; Williams, M.C. Solid–state electrochemical heat engines. Int. J. Hydrog. Energy 2015, 40, 3719–3725. [Google Scholar] [CrossRef] [Green Version]
- Liao, G.; Jiang, K.; Zhang, F.; Jiaqiang, E.; Liu, L.; Chen, J.; Leng, E. Thermal performance of battery thermal management system coupled with phase change material and thermoelectric elements. J. Energy Storage 2021, 43, 103217. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Proton exchange membrane fuel cells heat recovery opportunities for combined heating/cooling and power applications. Energy Convers. Manag. 2020, 204, 112328. [Google Scholar] [CrossRef]
- Geffroy, C.; Lilley, D.; Parez, P.S.; Prasher, R. Techno-economic analysis of waste-heat conversion. Joule 2021, 5, 3080–3096. [Google Scholar] [CrossRef]


| TEC Devices | Seebeck Coefficient (mV/K) | Electrical Conductivity (S/cm) | Thermal Conductivity (W/(cm·K) | ZT |
|---|---|---|---|---|
| Thermoelectric Semiconductor Materials | ||||
| Heusler alloy [46] | −0.1–0.6 | 1000–2000 | 2.7–3.0 | 1–6 |
| Copper chalcogenides Cu2−xE (E = S, Se, Te) [11,47] | 0.15–0.2 | 1500–4000 | >0.009 | 1 |
| Bi2Te3 alloys [48] | 0.18–0.25 | ~1000 | ~0.02 | 0.5–1.5 |
| Thermogalvanic Cells Based on Ferri/Ferrocyanide | ||||
| Organic solvent [49] | 1.5–10 | <0.01 | 0.002 | 0.002 |
| Aqueous solvent [50] | 1–13 | 0.6 | 0.006 | 0.03 |
| Organic and organometallic polymers | ||||
| polyaniline/graphene/polyaniline/double-walled carbon nanotube [50] | −1.14 | 8 | ~0.006 | 1.05 |
| poly (3,4-ethylenedioxy thiophene)/carbon nanotube [51] | 0.13 | 10.8 | - | 1.825 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burmistrov, I.; Khanna, R.; Gorshkov, N.; Kiselev, N.; Artyukhov, D.; Boychenko, E.; Yudin, A.; Konyukhov, Y.; Kravchenko, M.; Gorokhovsky, A.; et al. Advances in Thermo-Electrochemical (TEC) Cell Performances for Harvesting Low-Grade Heat Energy: A Review. Sustainability 2022, 14, 9483. https://doi.org/10.3390/su14159483
Burmistrov I, Khanna R, Gorshkov N, Kiselev N, Artyukhov D, Boychenko E, Yudin A, Konyukhov Y, Kravchenko M, Gorokhovsky A, et al. Advances in Thermo-Electrochemical (TEC) Cell Performances for Harvesting Low-Grade Heat Energy: A Review. Sustainability. 2022; 14(15):9483. https://doi.org/10.3390/su14159483
Chicago/Turabian StyleBurmistrov, Igor, Rita Khanna, Nikolay Gorshkov, Nikolay Kiselev, Denis Artyukhov, Elena Boychenko, Andrey Yudin, Yuri Konyukhov, Maksim Kravchenko, Alexander Gorokhovsky, and et al. 2022. "Advances in Thermo-Electrochemical (TEC) Cell Performances for Harvesting Low-Grade Heat Energy: A Review" Sustainability 14, no. 15: 9483. https://doi.org/10.3390/su14159483










