Development of Sustainable Inhibitors for Corrosion Control
Abstract
:1. Introduction
2. Frequently Utilized Corrosion Control Methods
- (a)
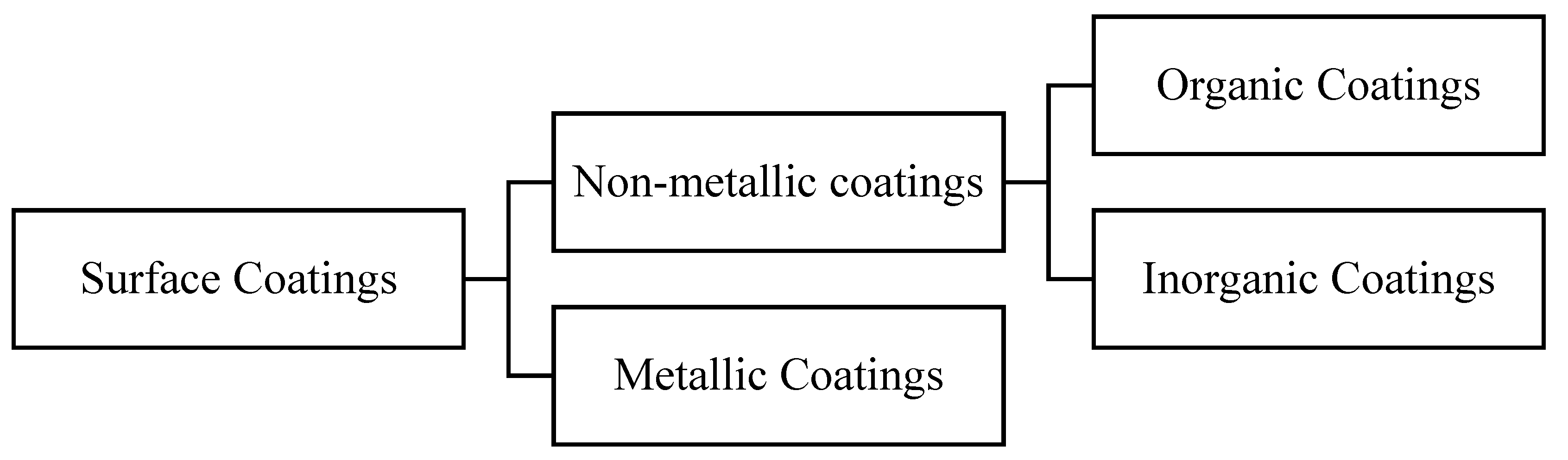
- Surface coatings
- (b)
- Chemical inhibitors
- (c)
- Modified material of construction
- (d)
- Alteration in equipment design
3. Corrosion of Modern Materials
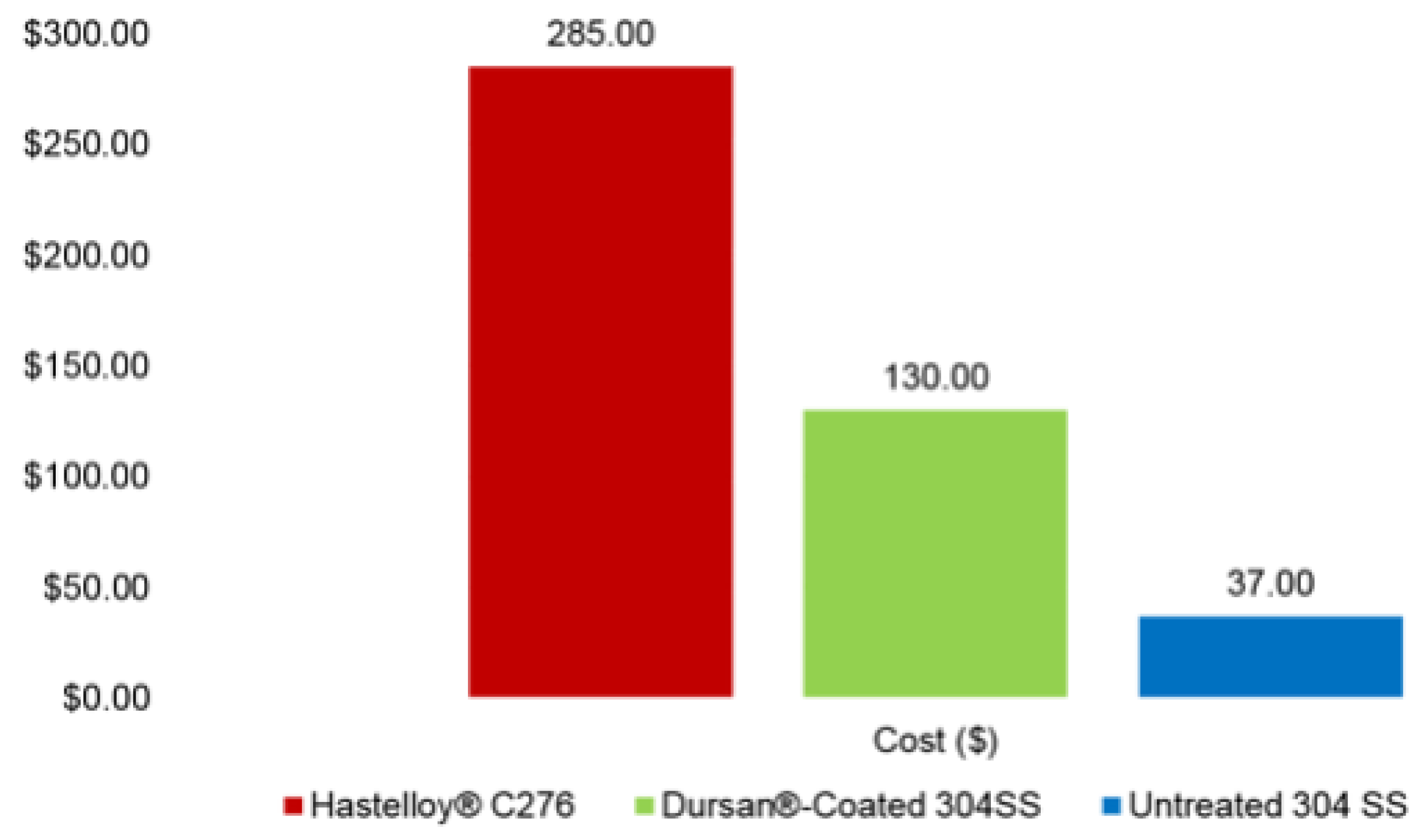
4. Corrosion Prevention through Coatings and Encapsulation
5. Contemporary Materials Development for Sustainable Corrosion Inhibition
6. Comparative Analysis of Corrosion Prevention Methods
7. Conclusions
8. Recommendations and Future Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, C.D.; Lu, P.; Saal, J.; Frankel, G.S.; Scully, J.R. Integrated computational materials engineering of corrosion resistant alloys. NPJ Mater. Degrad. 2018, 2, 6. [Google Scholar] [CrossRef]
- NACE International. Available online: http://impact.nace.org/economic-impact.aspx (accessed on 13 July 2022).
- Wang, Z.; Zhu, Z.; Sun, X.; Wang, X. Deterioration of fracture toughness of concrete under acid rain environment. Eng. Fail. Anal. 2017, 77, 76–84. [Google Scholar] [CrossRef]
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion, 2nd ed.; Wiley: Hoboken, NJ, USA, 2016; Available online: https://www.wiley.com/en-us/Atmospheric+Corrosion%2C+2nd+Edition-p-9781118762271 (accessed on 13 July 2022).
- Mansoori, H.; Mirzaee, R.; Esmaeilzadeh, F.; Vojood, A.; Dowrani, A.S. Pitting corrosion failure analysis of a wet gas pipeline. Eng. Fail. Anal. 2017, 82, 16–25. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Ho, S.C.M.; Wang, B.; Song, G. Monitoring Concrete Deterioration Due to Reinforcement Corrosion by Integrating Acoustic Emission and FBG Strain Measurements. Sensors 2017, 17, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habeeb, H.J.; Luaibi, H.M.; Dakhil, R.M.; Kadhum, A.A.H.; Al-Amiery, A.A.; Gaaz, T.S. Development of new corrosion inhibitor tested on mild steel supported by electrochemical study. Results Phys. 2018, 8, 1260–1267. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- Richardson, J.; Kalakodimi, R.; Wills-Guy, D. Corrosion Control Methods. U.S. Patent 9,290,849 B2, 22 March 2016. [Google Scholar]
- Gillanders, L.; Williams, S.; Ryan, G.; Trout, J.F. Barrier Coating Corrosion Control Methods and Systems for Interior Walls of Pipe in Underground Piping. U.S. Patent 8,887,660, 18 November 2014. [Google Scholar]
- Kain, V. Corrosion-Resistant Materials. Funct. Mater. 2012, 507–547. [Google Scholar] [CrossRef]
- Arthur, D.E.; Jonathan, A.; Ameh, P.O.; Anya, C. A review on the assessment of polymeric materials used as corrosion inhibitor of metals and alloys. Int. J. Ind. Chem. 2013, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Lan, W.; Zhang, S.; Deng, H.; Qiang, Y.; Fu, A.; Ran, Y.; Xiong, J.; Marzouki, R.; Li, W. Passiflora edulia Sims leaves Extract as renewable and degradable inhibitor for copper in sulfuric acid solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128892. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; Cao, X.; Fu, A.; Guo, L.; Marzouki, R.; Li, W. Insight into the anti-corrosion performance of two food flavors as eco-friendly and ultra-high performance inhibitors for copper in sulfuric acid medium. J. Colloid Interface Sci. 2022, 609, 838–851. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion Inhibitors—Principles, Mechanisms and Applications. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech: London, UK, 2014; ISBN 978-953-51-1223-5. [Google Scholar]
- Rashid, K.H.; Khadom, A.A. Sodium sulfite as an oxygen scavenger for the corrosion control of mild steel in petroleum refinery wastewater: Optimization, mathematical modeling, surface morphology and reaction kinetics studies. React. Kinet. Mech. Catal. 2020, 129, 1027–1046. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Devarayan, K.; Mayakrishnan, G.; Sulochana, N. Green Inhibitors for Corrosion of Metals: A Review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. [Google Scholar]
- Al-Sherrawi, M.; Lyashenko, V.; Edaan, E.; Sotnik, L. Corrosion as a Source of Destruction in Construction. Int. J. Civ. Eng. Technol. 2018, 9, 306–314. [Google Scholar]
- Koch, G. 1—Cost of corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Boston, MA, USA, 2017; pp. 3–30. ISBN 978-0-08-101105-8. [Google Scholar]
- Eckert, R.B.; Koch, G.H. Improving Corrosion Control Efficiencies Using Corrosion Management Principles. In Proceedings of the SPE International Oilfield Corrosion Conference and Exhibition, Aberdeen, UK, 11–12 May 2016. [Google Scholar]
- Shores, D.A.; Deluga, G.A. Basic materials corrosion issues. In Handbook of Fuel Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-97400-1. [Google Scholar]
- Kim, B.S.; Kang, B.G.; Choi, S.H.; Kim, T.G. Data modeling versus simulation modeling in the big data era: Case study of a greenhouse control system. Simulation 2017, 93, 579–594. [Google Scholar] [CrossRef]
- Davis, E.; Marcus, G. The scope and limits of simulation in automated reasoning. Artif. Intell. 2016, 233, 60–72. [Google Scholar] [CrossRef]
- Srinivasan, S.; Kane, R.D. Critical Issues in the Application and Evaluation of a Corrosion Prediction Model for Oil and Gas Systems. In Proceedings of the CORROSION 2003, San Diego, CA, USA, 16–20 March 2003. [Google Scholar]
- Faes, W.; Lecompte, S.; Ahmed, Z.Y.; Bael, J.V.; Salenbien, R.; Verbeken, K.; Paepe, M.D. Corrosion and corrosion prevention in heat exchangers. Corros. Rev. 2019, 37, 131–155. [Google Scholar] [CrossRef]
- Moskvicheva, E.V.; Sidyakin, P.A.; Shitov, D.V. Method of Corrosion Prevention in Steel Pressure Pipelines in Sewerage Systems. Procedia Eng. 2016, 150, 2381–2386. [Google Scholar] [CrossRef] [Green Version]
- Snihirova, D.; Lamaka, S.V.; Cardoso, M.M.; Condeço, J.A.D.; Ferreira, H.E.C.S.; de Fatima Montemor, M. pH-sensitive polymeric particles with increased inhibitor-loading capacity as smart additives for corrosion protective coatings for AA2024. Electrochim. Acta 2014, 145, 123–131. [Google Scholar] [CrossRef]
- Thellaputta, G.R.; Chandra, P.S.; Rao, C.S.P. Machinability of Nickel Based Superalloys: A Review. Mater. Today Proc. 2017, 4, 3712–3721. [Google Scholar] [CrossRef]
- Higgins, M. Silicon Coatings Can Help Ease the Pain of High Nickel Alloy Prices. Available online: https://www.silcotek.com/semi-coating-blog/silicon-coatings-can-help-ease-the-pain-of-high-nickel-alloy-prices (accessed on 14 July 2022).
- Dararatana, N.; Seidi, F.; Crespy, D. pH-Sensitive Polymer Conjugates for Anticorrosion and Corrosion Sensing. ACS Appl. Mater. Interfaces 2018, 10, 20876–20883. [Google Scholar] [CrossRef] [PubMed]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Kong, P.; Chen, N.; Lu, Y.; Feng, H.; Qiu, J. Corrosion by Polyaniline/Salicylaldehyde Modified Chitosan in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2019, 14, 9774–9784. [Google Scholar] [CrossRef]
- Hamadi, L.; Mansouri, S.; Oulmi, K.; Kareche, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Palraj, S.; Selvaraj, M.; Vidhya, M.; Rajagopal, G. Synthesis and characterization of epoxy–silicone–polythiophene interpenetrating polymer network for corrosion protection of steel. Prog. Org. Coat. 2012, 75, 356–363. [Google Scholar] [CrossRef]
- Ates, M. A review on conducting polymer coatings for corrosion protection. J. Adhes. Sci. Technol. 2016, 30, 1510–1536. [Google Scholar] [CrossRef]
- Riaz, U.; Nwaoha, C.; Ashraf, S.M. Recent advances in corrosion protective composite coatings based on conducting polymers and natural resource derived polymers. Prog. Org. Coat. 2014, 77, 743–756. [Google Scholar] [CrossRef]
- Augustyniak, A.; Tsavalas, J.; Ming, W. Early Detection of Steel Corrosion via “Turn-On” Fluorescence in Smart Epoxy Coatings. ACS Appl. Mater. Interfaces 2009, 1, 2618–2623. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, X.; Schenderlein, M.; Borisova, D.; Cao, R.; Möhwald, H.; Shchukin, D. Self-Healing and Antifouling Multifunctional Coatings Based on pH and Sulfide Ion Sensitive Nanocontainers. Adv. Funct. Mater. 2013, 23, 3307–3314. [Google Scholar] [CrossRef]
- Shchukina, E.; Shchukin, D.G. Nanocontainer-Based Active Systems: From Self-Healing Coatings to Thermal Energy Storage. Langmuir 2019, 35, 8603–8611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Suo, X.; Wang, Z.; Gong, Y.; Wang, X.; Li, H. Developing polyimide-copper antifouling coatings with capsule structures for sustainable release of copper. Mater. Des. 2017, 130, 285–293. [Google Scholar] [CrossRef]
- Kamtsikakis, A.; Kavetsou, E.; Chronaki, K.; Kiosidou, E.; Pavlatou, E.; Karana, A.; Papaspyrides, C.; Detsi, A.; Karantonis, A.; Vouyiouka, S. Encapsulation of Antifouling Organic Biocides in Poly(lactic acid) Nanoparticles. Bioengineering 2017, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Suo, X.; Gong, Y.; Li, H. Suspension Flame Spray Construction of Polyimide-Copper Layers for Marine Antifouling Applications. J. Therm. Spray Technol. 2018, 27, 98–105. [Google Scholar] [CrossRef]
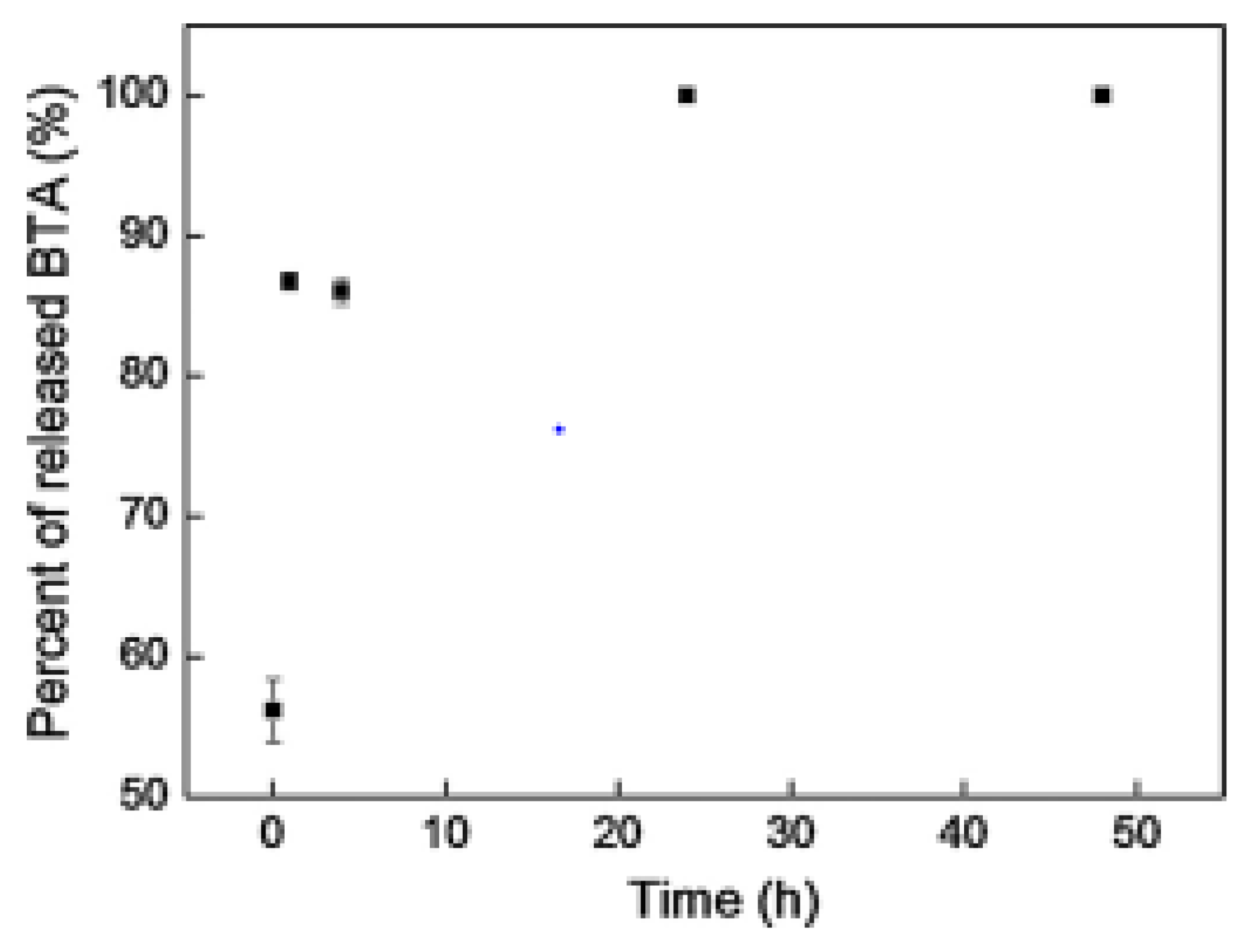
- Calegari, F.; da Silva, B.C.; Tedim, J.; Ferreira, M.G.S.; Berton, M.A.C.; Marino, C.E.B. Benzotriazole encapsulation in spray-dried carboxymethylcellulose microspheres for active corrosion protection of carbon steel. Prog. Org. Coat. 2020, 138, 105329. [Google Scholar] [CrossRef]
- Du, P.; Wang, J.; Zhao, H.; Liu, G.; Wang, L. Graphene oxide encapsulated by mesoporous silica for intelligent anticorrosive coating: Studies on release models and self-healing ability. Dalton Trans. 2019, 48, 13064–13073. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Zhou, D.; Tang, E.; Liu, S.; Chu, X.; Xu, X.; Xu, Y. A novel method to control the release rate of halloysite encapsulated Na2MoO4 with Ca2+ and corrosion resistance for Q235 steel. Appl. Clay Sci. 2020, 188, 105492. [Google Scholar] [CrossRef]
- Alrashed, M.M.; Jana, S.; Soucek, M.D. Corrosion performance of polyurethane hybrid coatings with encapsulated inhibitor. Prog. Org. Coat. 2019, 130, 235–243. [Google Scholar] [CrossRef]
- de Miranda, L.S.; Nele, M.; Pinto, J.C. Production of Polymer Particles Loaded with Corrosion Inhibitor for Applications in Oil Wells. Macromol. React. Eng. 2019, 13, 1900027. [Google Scholar] [CrossRef]
- Ubaid, F.; Radwan, A.B.; Naeem, N.; Shakoor, R.A.; Ahmad, Z.; Montemor, M.F.; Kahraman, R.; Abdullah, A.M.; Soliman, A. Multifunctional self-healing polymeric nanocomposite coatings for corrosion inhibition of steel. Surf. Coat. Technol. 2019, 372, 121–133. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Kardar, P.; Arabi, A.M.; Amini, R.; Pasbakhsh, P. Acid-modification and praseodymium loading of halloysite nanotubes as a corrosion inhibitor. Appl. Clay Sci. 2020, 184, 105355. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, S.; Hu, Z.; Chen, W.; Qu, Z.; Xu, C.; Zhang, Q.; Wu, K.; Shi, J.; Lu, M. pH-Responsive Self-Healing Anticorrosion Coating Based on a Lignin Microsphere Encapsulating Inhibitor. Ind. Eng. Chem. Res. 2020, 59, 2657–2666. [Google Scholar] [CrossRef]
- Dong, Y.; Geng, C.; Wang, X.; Zhou, Q. Porous polystyrene nanoparticles as nanocontainers of inhibitors for corrosion protection of low-alloy steel. Pigment. Resin Technol. 2020, 49, 305–313. [Google Scholar] [CrossRef]
- Davoodi, M.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M. Designing a zinc-encapsulated Feldspar as a unique rock-forming tectosilicate nanocontainer in the epoxy coating; improving the robust barrier and self-healing anti-corrosion properties. Constr. Build. Mater. 2020. [Google Scholar] [CrossRef]
- Izadi, M.; Shahrabi, T.; Ramezanzadeh, B. Electrochemical investigations of the corrosion resistance of a hybrid sol–gel film containing green corrosion inhibitor-encapsulated nanocontainers. J. Taiwan Inst. Chem. Eng. 2017, 81, 356–372. [Google Scholar] [CrossRef]
- Karekar, S.E.; Bagale, U.D.; Sonawane, S.H.; Bhanvase, B.A.; Pinjari, D.V. A smart coating established with encapsulation of Zinc Molybdate centred nanocontainer for active corrosion protection of mild steel: Release kinetics of corrosion inhibitor. Compos. Interfaces 2018, 25, 785–808. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.S.; Alves, F.J.; Silva, E.C.S.; Andrade, M.A.S. A novel and efficient epoxy/chitosan cement slurry for use in severe acidic environments of oil wells—Structural characterization and kinetic modeling. J. Hazard. Mater. 2012, 213–214, 109–116. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan as a Smart Coating for Controlled Release of Corrosion Inhibitor 2-Mercaptobenzothiazole. ECS Electrochem. Lett. 2013, 2, C19. [Google Scholar] [CrossRef]
- Santos, L.R.L.; Marino, C.E.B.; Riegel-Vidotti, I.C. Silica/chitosan hybrid particles for smart release of the corrosion inhibitor benzotriazole. Eur. Polym. J. 2019, 115, 86–98. [Google Scholar] [CrossRef]
- Srivastava, V.; Haque, J.; Verma, C.; Singh, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Amino acid based imidazolium zwitterions as novel and green corrosion inhibitors for mild steel: Experimental, DFT and MD studies. J. Mol. Liq. 2017, 244, 340–352. [Google Scholar] [CrossRef]
- Karthikaiselvi, R.; Subhashini, S. Study of adsorption properties and inhibition of mild steel corrosion in hydrochloric acid media by water soluble composite poly (vinyl alcohol-o-methoxy aniline). J. Assoc. Arab. Univ. Basic Appl. Sci. 2014, 16, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Goni, L.K.M.O.; Mazumder, M.A.J.; Ali, S.A.; Nazal, M.K.; Al-Muallem, H.A. Biogenic amino acid methionine-based corrosion inhibitors of mild steel in acidic media. Int. J. Miner. Metall. Mater. 2019, 26, 467–482. [Google Scholar] [CrossRef]
- Prathipa, V.; Raja, A.S. Zinc as a Synergist to L-Glutamic Acid for Corrosion Behaviour of Carbon Steel in Nearly Neutral Aqueous Environment. J. Adv. Chem. Sci. 2016, 3. [Google Scholar]
- Aouniti, A.; Khaled, K.; Hammouti, B. Correlation Between Inhibition Efficiency and Chemical Structure of Some Amino Acids on the Corrosion of Armco Iron in Molar HCl. Int. J. Electrochem. Sci. 2013, 8, 5925–5943. [Google Scholar]
- Gowri, S.; Sathiyabama, J.; Rajendran, S. The Inhibitive Effect Of Glutamic Acid On The Corrosion Of Carbon Steel In Sea Water. Int. J. ChemTech Res. 2013, 5, 347–352. [Google Scholar]
- Dai, W.; Zhang, Y.Y. Molecular Dynamics Simulation of the Adsorption Behavior of Amino Acid Corrosion Inhibitor on Cu (001) Surface. Appl. Mech. Mater. 2012, 121–126, 226–230. [Google Scholar] [CrossRef]
- Barouni, K.; Kassale, A.; Albourine, A.; Jbara, O.; Hammouti, B.; Bazzi, L. Amino acids as corrosion inhibitors for copper in nitric acid medium: Experimental and theoretical study. J. Mater. Environ. Sci. 2014, 5, 456–463. [Google Scholar]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; El Mouaden, K.; Jmiai, A.; Baddouh, A.; El Issami, S.; Bazzi, L.; Hilali, M. Understanding the influence of solution’s pH on the corrosion of tin in saline solution containing functional amino acids using electrochemical techniques and molecular modeling. Surf. Interfaces 2019, 17, 100343. [Google Scholar] [CrossRef]
- Yeganeh, M.; Khosravi-Bigdeli, I.; Eskandari, M.; Alavi Zaree, S.R. Corrosion Inhibition of l-Methionine Amino Acid as a Green Corrosion Inhibitor for Stainless Steel in the H2SO4 Solution. J. Mater. Eng. Perform. 2020, 29, 3983–3994. [Google Scholar] [CrossRef]
- Saifi, H.; Ouchenane, S.; Bourenane, R.; Boukerche, S.; Joiret, S.; Takenouti, H. Electrochemical Behavior Investigation of Cysteine on Nickel Corrosion in Acidic Medium. J. Fail. Anal. Preven. 2019, 19, 1597–1606. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Jafari, R.; Kazempour, A.; Asghari, E. Effect of amino acids and montmorillonite nanoparticles on improving the corrosion protection characteristics of hybrid sol-gel coating applied on AZ91 Mg alloy. Mater. Chem. Phys. 2019, 225, 298–308. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J. (Eds.) Materials Corrosion and Protection; De Gruyter: Berlin, Germany, 2018; ISBN 978-3-11-031005-4. [Google Scholar]
- Shehata, O.S.; Korshed, L.A.; Attia, A. Green Corrosion Inhibitors, Past, Present, and Future; IntechOpen: London, UK, 2017; ISBN 978-953-51-3918-8. [Google Scholar]
- Stankiewicz, A.; Barker, M.B. Development of self-healing coatings for corrosion protection on metallic structures. Smart Mater. Struct. 2016, 25, 084013. [Google Scholar] [CrossRef]
- Shchukina, E.; Wang, H.; Shchukin, D.G. Nanocontainer-based self-healing coatings: Current progress and future perspectives. Chem. Commun. 2019, 55, 3859–3867. [Google Scholar] [CrossRef] [PubMed]
- Meng-Lund, H.; Friis, N.; van de Weert, M.; Rantanen, J.; Poso, A.; Grohganz, H.; Jorgensen, L. Correlation between calculated molecular descriptors of excipient amino acids and experimentally observed thermal stability of lysozyme. Int. J. Pharm. 2017, 523, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Atta, A. Corrosion Inhibition Efficiency of Modified Silver Nanoparticles For Carbon Steel in 1 M HCl. Int. J. Electrochem. Sci. 2013, 8, 4873–4885. [Google Scholar]
- Bao, J.; Zhang, H.; Zhao, X.; Deng, J. Biomass polymeric microspheres containing aldehyde groups: Immobilizing and controlled-releasing amino acids as green metal corrosion inhibitor. Chem. Eng. J. 2018, 341, 146–156. [Google Scholar] [CrossRef]
- Jiang, S.; Lv, L.; Li, Q.; Wang, J.; Landfester, K.; Crespy, D. Tailoring nanoarchitectonics to control the release profile of payloads. Nanoscale 2016, 8, 11511–11517. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Qin, B.; Zhang, X.; Wang, K.; Wei, Y.; Ji, Y. Self-healing anti-corrosion coatings based on polymers of intrinsic microporosity for the protection of aluminum alloy. RSC Adv. 2015, 5, 104451–104457. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Tiu, B.D.B.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Hooshmand Zaferani, S.; Sharifi, M.; Zaarei, D.; Shishesaz, M.R. Application of eco-friendly products as corrosion inhibitors for metals in acid pickling processes—A review. J. Environ. Chem. Eng. 2013, 1, 652–657. [Google Scholar] [CrossRef]
- Chigondo, M.; Chigondo, F. Recent Natural Corrosion Inhibitors for Mild Steel: An Overview. J. Chem. 2016, 2016, e6208937. [Google Scholar] [CrossRef] [Green Version]
- Brzeszcz, J.; Turkiewicz, A. Corrosion inhibitors—Application in oil industry. Nafta-Gaz 2015, 71, 67–75. [Google Scholar]
- Chen, X.; Ebert, W.L.; Indacochea, J.E. Electrochemical corrosion of a noble metal-bearing alloy-oxide composite. Corros. Sci. 2017, 124, 10–24. [Google Scholar] [CrossRef]
- Kozhukharov, S.; Girginov, C. Recent Trends of the Use of Rare Earth Elements for Efficient Environmentally Compliant Corrosion Protection of Aluminum and Its Alloys. In Proceedings of the Nanoscience and Nanotechnology in Security and Protection against CBRN Threats; Petkov, P., Achour, M.E., Popov, C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2020; pp. 437–445. [Google Scholar]
- Obot, I.B.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Umoren, S.A.; Quraishi, M.A.; Sorour, A.A.; Chen, T.; Aljeaban, N.; Wang, Q. High temperature sweet corrosion and inhibition in the oil and gas industry: Progress, challenges and future perspectives. J. Pet. Sci. Eng. 2020, 185, 106469. [Google Scholar] [CrossRef]








| Sample | H2O (15 Days) | 5% NaOH (15 Days) | 5% HCl (15 Days) | 5% NaCl (15 Days) | ||||
|---|---|---|---|---|---|---|---|---|
| Before Aging | After Aging | Before Aging | After Aging | Before Aging | After Aging | Before Aging | After Aging | |
| CPEAU | a | - | f | - | e | - | f | - |
| 2-PANI/CPEAU | a | b | c | e | b | c | d | e |
| 4-PANI/CPEAU | a | a | c | e | a | c | c | d |
| 8-PANI/CPEAU | a | a | b | e | a | b | b | c |
| Material | Encapsulation Material | Corrosion Inhibitor | Performance | Reference |
|---|---|---|---|---|
| Carbon Steel | Biopolymeric microspheres, carboxymethylcellulose (CMC-Na) | Benzotriazole | BTA enhanced the coating performance on surface metal | [44] |
| Q235 steel | Halloysite nanotubes with CaMoO4 | Na2MoO4 | Highest performance could be attained by 10 wt% Ca2+ treated Na2MoO4 loaded halloysite | [46] |
| N/A; General study | Mesoporous silica; benzotriazole-loaded nanoreservoir | Graphene oxide | Increased IE with self-healing properties | [45] |
| 2024–T3 aluminum alloy | polylactic acid (PLA) nanoparticles combined in polyurethane/polysiloxane hybrid coating | 2-mercaptobenzothiazole | 200 times improvement in corrosion induction time | [47] |
| Coatings of oil wells | Polymer particles (Styrene) | Various inhibitors | Improvement in release timing | [48] |
| Steel | synthesized titania nanotubes | Epoxy monomer and dodecylamine | Improvement in release rates and self-healing due to polymer | [49] |
| AZ31 magnesium alloy | Acid-modified halloysite nanotubes | Praseodymium ions | Substantially lowered corrosion rates | [50] |
| N/A; General study | Lignin microspheres | Benzotriazole | pH responsive behavior with improved self-healing | [51] |
| Low alloy steel | Porous polystyrene | Benzotriazole | Long term improvement of protection | [52] |
| Steel | Feldspar nano-container | Zinc cations | Improvement of 83% in charge transfer resistance and improved self-healing | [53] |
| Mild steel | Nettle-loaded nanocontainers with Zinc Acetate | Silane | Enhancement of synergetic inhibition effects | [54] |
| Mild steel | Zinc Molybdate | Myristic acid, Polyaniline layer, benzotriazole layer and polyacrylic acid | Successful use in multifunctional coatings | [55] |
| Material | Inhibitor | Technique | Medium | Results Obtained | References |
|---|---|---|---|---|---|
| Carbon steel | Leucine, Alanine, glutamic acid, Methionine | Optical microscopic method | Hydrocyanic acid | Good inhibition efficiency but glutamic acid cannot be absorbed on surface | [34] |
| Carbon steel | L-glutamic acid-Zn2+ | Weight loss and electrochemical | Well-water of varying pH | IE = 90% at pH = 6.7, and IE = 33% at pH = 3.1 | [62] |
| Iron | Serine, Glutamic acid, Ornithine Lysine Aspartic acid, Alanine Valine Asparagine, Glutamine, Threonine, Methionine, Cysteine, Cystine Glycine Leucine Arginine, Asparagine, Glutamine, Threonine | Chemical measurements | HCl; 1 M | Highest efficiencies shown by Methionine, cysteine and cystine | [63] |
| Carbon Steel | Glutamic acid-Zn2+ | AFM analysis, electrochemical methods | Sea water | IE = 87% | [64] |
| Copper | Aspartic and glutamic acid | Molecular dynamics simulations | Cu surface | Glutamic acid shows better performance than aspartic acid | [65] |
| Copper surface | Glutamine, Leucine Methionine Threonine, Acid aspartic Acid Glutamic Alanine Asparagine | Electrochemical methods and quantum chemical computations | HNO3; 1 M | Highest IE shown by Methionine (80.38%) | [66] |
| Copper | Cysteine glutamic acid, glycine and their derivative glutathione | Density functional theory | N/A | Glutathione exhibited highest IE of above 90% in all simulations | [67] |
| Tin | Threonine, Asparagine and Glutamine | Electrochemical impedance spectroscopy and potentiodynamic polarization | NaCl Solution; 2 wt.% | Highest efficiency was shown by Asparagine and Glutamine at pH 2 and 5 respectively | [68] |
| Stainless Steel AISI309S | L-methionine | Tafel polarization, electrochemical noise methods, and electrochemical impedance spectroscopy | H2SO4; 1 M | 97% and 95% | [69] |
| Nickel | Cysteine | Polarization and electrochemical impedance spectroscopy | H2SO4; 0.5 M | Poor inhibitive efficiency | [70] |
| Mg alloy | Montmorillonite nanoparticles with amino acids | Electrochemical impedance spectroscopy | Varying proportions of amino acids with MMT | Highest efficiency shown by 0.5 wt.% methionine | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.A.; Irfan, O.M.; Djavanroodi, F.; Asad, M. Development of Sustainable Inhibitors for Corrosion Control. Sustainability 2022, 14, 9502. https://doi.org/10.3390/su14159502
Khan MAA, Irfan OM, Djavanroodi F, Asad M. Development of Sustainable Inhibitors for Corrosion Control. Sustainability. 2022; 14(15):9502. https://doi.org/10.3390/su14159502
Chicago/Turabian StyleKhan, Muhammad Azhar Ali, Osama Mohamed Irfan, Faramarz Djavanroodi, and Muhammad Asad. 2022. "Development of Sustainable Inhibitors for Corrosion Control" Sustainability 14, no. 15: 9502. https://doi.org/10.3390/su14159502
APA StyleKhan, M. A. A., Irfan, O. M., Djavanroodi, F., & Asad, M. (2022). Development of Sustainable Inhibitors for Corrosion Control. Sustainability, 14(15), 9502. https://doi.org/10.3390/su14159502






