Shark Fishing vs. Conservation: Analysis and Synthesis
Abstract
:1. Introduction
2. The Impact of Industrial Fishing
3. The Ecological Consequences
4. A Barrier to Shark Conservation
5. The Uncertainties
5.1. Illegal, Unreported, and Unregulated Fishing
5.2. Other Markets
5.3. Substituting Disappearing Species
6. Shark Conservation Measures
6.1. Finning Bans and ‘Fins Naturally Attached’ Policies
6.2. Shark Sanctuaries and Fishing Bans
6.3. The Fisheries Certification Standard for Sustainable Seafood
7. The Shark Meat Problem
“may contain amounts of mercury in excess of the recommendation of the USA Food and Drug Administration’s (FDA) recommended limit”.
“pregnant and nursing women, women who may get pregnant, and children under 8 years of age”
8. Sustainability in Shark Fisheries
8.1. The Spiny Dogfish
8.2. Sustainability in the Shark Fin Trade
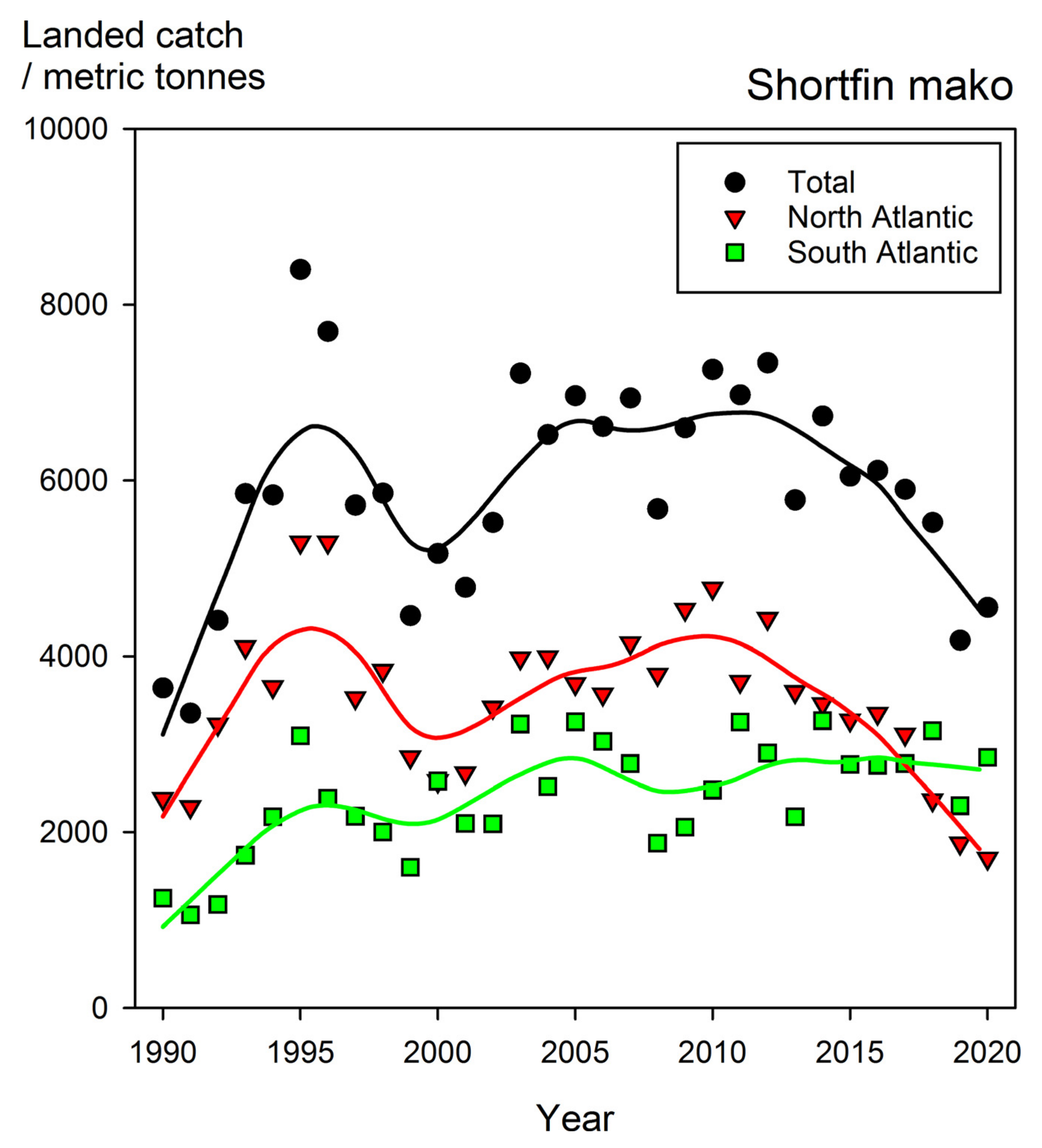
8.3. The Shortfin Mako
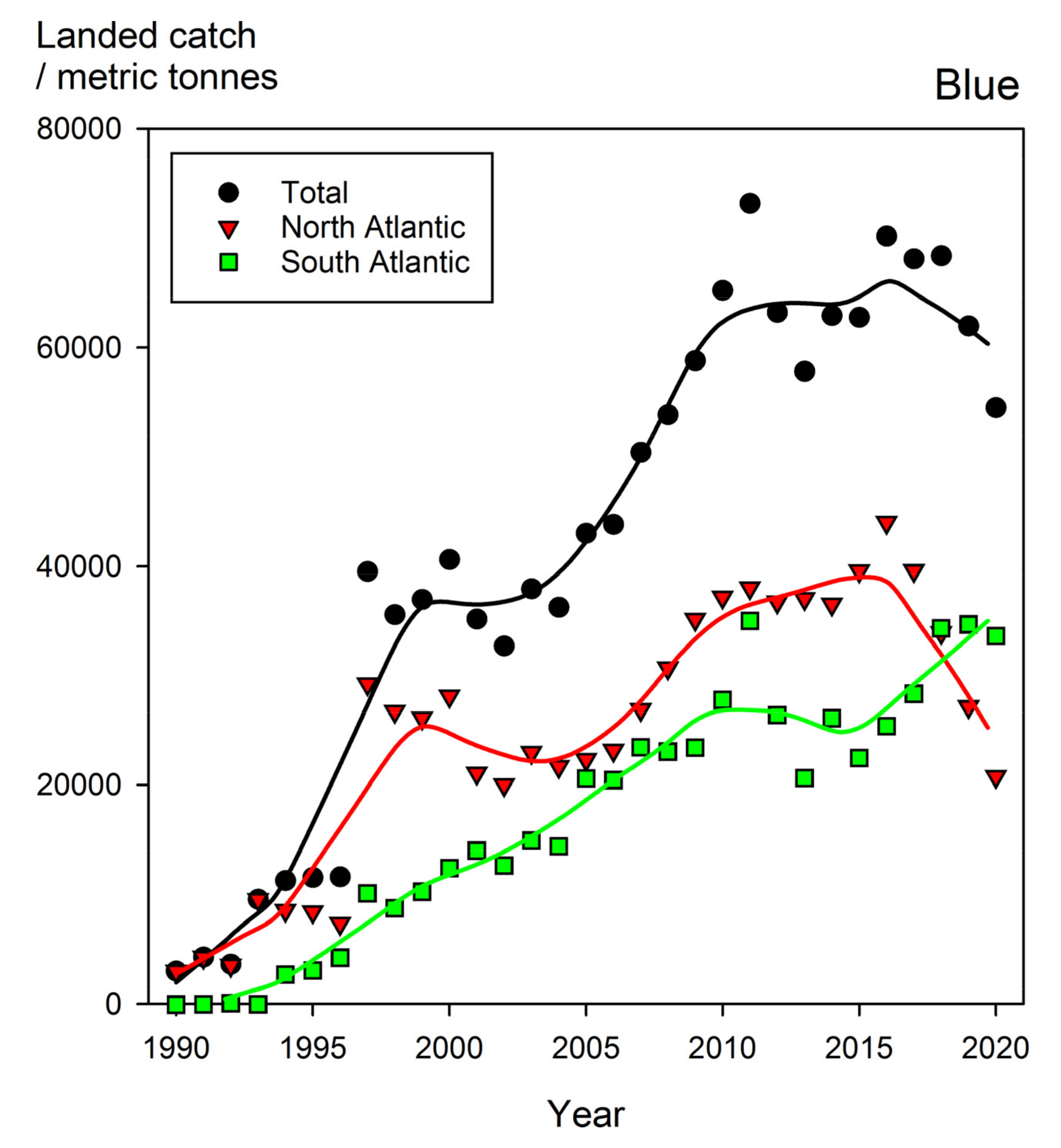
8.4. The Blue Shark
“current biomass being greater than that required to achieve MSY or current fishing mortality being less than that which will yield MSY”.[28]
9. Fishing Economics
10. Fisheries Management
10.1. The Impossibility of Establishing Global Sustainability
“transparent certification program for countries seeking to import shark products into the United States,”
“shark and ray management policies comparable to those under the U.S. Magnuson-Stevens Act”.
- how the determination of what is a sustainable catch rate for every shark fishery in the world will be made
- how the baseline will be determined
- how management plans will be implemented
- how they will be funded
- how they will be enforced
- how RFMOs could be made to agree to base quotas and rules
10.2. The Concept of ‘Sustainable Use’
“use of components of biological diversity in a way and at a rate that does not lead to the long-term decline of biological diversity, thereby maintaining its potential to meet the needs and aspirations of present and future generations (Article 2)”.[156]
“[the phrase] supporting sustainable use can be used in order to justify any specific use, regardless of its sustainability. This will usually be used as a political or rhetorical strategy to defeat opposition to use … it has no biocentric rationale, and … it is not consistent with a conservation strategy. It will be motivated by anthropocentric reasons, to justify gaining the benefits of use to humans”.[157]
- the use of biodiversity and its components at unsustainable levels
- the use of components of biodiversity causing environmental damage
- consumption of anything that impacts unsustainably on biodiversity [157].
10.3. Instinct Versus Science
11. Conclusions
11.1. Protection from International Trade
11.2. Fishing Effort Reduction
11.3. RFMO Policies
11.4. Cultural Change
11.5. True Sustainability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMCS | Australian Marine Conservation Society |
| CAB | Conformity Assessment Body |
| CBD | United Nations Convention on Biological Diversity |
| CCRF FAO | Code of Conduct for Responsible Fisheries |
| CITES | Convention on International Trade in Endangered Species of Wild Fauna and Flora |
| CMS | Convention on the Conservation of Migratory Species of Wild Animals |
| CREMA | Centro de Rescate de Especies Marinas Amenazada |
| DFAD | Drifting fish aggregating device |
| DNA | Deoxyribonucleic Acid |
| EEZ | Exclusive Economic Zone |
| EPBC | Commonwealth Environment Protection and Biodiversity Conservation Act |
| EU | European Union |
| FAO | Food and Agriculture Organization of the United Nations |
| FNA | Fins naturally attached |
| FWC | Florida Fish and Wildlife Conservation Commission |
| GFCM | General Fisheries Commission for the Mediterranean |
| IATTC | Inter-American Tropical Tuna Commission |
| ICCAT | International Commission for the Conservation of Atlantic Tunas |
| IOTC | Indian Ocean Tuna Commission |
| IPBES | Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services |
| ISC | International Scientific Committee for Tuna and Tuna-like Species in the North Pacific Ocean |
| IUCN | International Union for Conservation of Nature |
| IUU | Illegal, unreported, and unregulated |
| MPA | Marine protected area |
| MSC | Marine Stewardship Council |
| MSY | Maximum sustainable yield |
| NAFO | Northwest Atlantic Fisheries Organization |
| NEAFC | North East Atlantic Fisheries Commission |
| NM | Nautical mile |
| NOAA | US Department of Commerce’s National Oceanic and Atmospheric Administration |
| OED | Oxford English Dictionary |
| OMZ | Oxygen minimum zone |
| PADI | Professional Association of Diving Instructors |
| RFMO | Regional Fisheries Management Organization |
| SSA | Sustainable Shark Alliance |
| SSFTA | Sustainable Shark Fisheries and Trade Act |
| t | Metric tonne |
| TAC | Total allowable catch limit |
| UNCLOS | United Nations Convention on the Law of the Sea |
| UNFSA | UN Fish Stocks Agreement |
| US EPA | United States Environmental Protection Agency |
| USA | United States of America |
| USD | United States Dollar |
| WCPFC | Western and Central Pacific Fisheries Commission |
References
- Clarke, S.; Magnussen, J.E.; Abercrombie, D.L.; McAllister, M.; Shivji, M. Identification of shark species composition and proportion in the Hong Kong shark fin market based on molecular genetics and trade records. Conserv. Biol. 2006, 20, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Murdoch, K.; McAllister, M.; Milner-Gulland, E.J.; Kirkwood, G.P.; Michielsens, C.; Agnew, D.J.; Pikitch, E.K.; Nakano, H.; Shivji, M.S. Global estimates of shark catches using trade records from commercial markets. Ecol. Lett. 2006, 9, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Dulvy, N.K.; Baum, J.K.; Clarke, S.; Compagno, L.J.V.; Cortés, E.; Domingo, A.; Fordham, S.; Fowler, S.; Francis, M.P.; Gibson, C.; et al. You can swim but you can’t hide: The global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 459–482. [Google Scholar] [CrossRef]
- Worm, B.; Davis, B.; Kettemer, L.; Ward-Paige, C.A.; Chapman, D.; Heithaus, M.R.; Kessel, S.T.; Gruber, S.H. Global catches, exploitation rates, and rebuilding options for sharks. Mar. Pol. 2013, 40, 194–204. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef] [Green Version]
- Fields, A.T.; Fischer, G.A.; Shea, S.K.H.; Zhang, H.; Abercrombie, D.L.; Feldheim, K.A.; Babcock, E.A.; Chapman, D.D. Species composition of the international shark fin trade assessed through a retail-market survey in Hong Kong. Conserv. Biol. 2017, 32, 376–389. [Google Scholar] [CrossRef] [Green Version]
- Myers, R.A.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 423, 280–283. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- World Bank. The Sunken Billions Revisited: Progress and Challenges in Global Marine Fisheries; Environment and Sustainable Development Series; World Bank: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Clarke, S.; Milner-Gulland, E.J.; Bjørndal, T. Social, economic and regulatory drivers of the shark fin trade. Mar. Resour. Econ. 2007, 22, 305–327. [Google Scholar] [CrossRef]
- Hareide, N.R.; Carlson, J.; Clarke, M.; Clarke, S.; Ellis, J.; Fordham, S.; Fowler, S.; Pinho, M.; Raymakers, C.; Serena, F.; et al. European shark fisheries: A preliminary investigation into fisheries, conversion factors, trade products, markets and management measures. In Proceedings of the European Elasmobranch Association 2008, Lisbon, Portugal, 14–16 November 2008; Available online: www.iotc.org/documents/european-shark-fisheries-preliminary-investigation-fisheries-conversion-factors-trade/IOTC-2008-WPEB-INF04.pdf (accessed on 10 March 2021).
- Da Silva, T.E.F.; Lessa, R.; Santana, F.M. Current knowledge on biology, fishing and conservation of the blue shark (Prionace glauca). Neotrop. Biol. Conserv. 2021, 16, 71–88. [Google Scholar] [CrossRef]
- Rosello, M.; Vilata, J.; Belhabib, D. Atlantic shortfin mako: Chronicle of a death foretold? Laws 2021, 10, 52. [Google Scholar] [CrossRef]
- Oliver, S.; Braccini, M.; Newman, S.J.; Harvey, E.S. Global patterns in the bycatch of sharks and rays. Mar. Pol. 2015, 54, 86–97. [Google Scholar] [CrossRef]
- Van Houtan, K.S.; Gagné, T.O.; Reygondeau, G.; Tanaka, K.R.; Palumbi, S.R.; Jorgensen, S.J. Coastal sharks supply the global shark fin trade. Biol. Lett. 2020, 16, 20200609. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.; Myers, R.A. Shifts in open-ocean fish communities coinciding with the commencement of commercial fishing. Ecology 2005, 86, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Lam, V.Y.Y.; Sadovy de Mitcheson, Y. The sharks of South East Asia—Unknown, unmonitored and unmanaged. Fish Fish. 2011, 12, 51–74. [Google Scholar] [CrossRef]
- Doherty, P.D.; Alfaro-Shigueto, J.; Hodgson, D.J.; Mangel, J.C.; Witt, M.J.; Godley, B.J. Big catch, little sharks: Insight into Peruvian small-scale longline fisheries. Ecol. Evol. 2014, 4, 2375–2383. [Google Scholar] [CrossRef] [Green Version]
- ICCAT. International Commission for the Conservation of Atlantic Tunas Report of the Standing Committee on Research and Statistics (SCRS). In Proceedings of the Standing Committee on Research and Statistics (SCRS), Madrid, Spain, 30 September–4 October 2019; Available online: www.iccat.int/Documents/Meetings/Docs/2019/REPORTS/2019_SCRS_ENG.pdf (accessed on 22 April 2020).
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystem. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- IUCN Red List. Available online: www.iucnredlist.org (accessed on 28 May 2022).
- Davidson, L.N.K.; Krawchuk, M.A.; Dulvy, N.K. Why have global shark and ray landings declined: Improved management or overfishing? Fish Fish. 2016, 17, 438–458. [Google Scholar] [CrossRef]
- Cashion, M.S.; Bailly, N.; Pauly, D. Official catch data under-represent shark and ray taxa caught in Mediterranean and Black Sea fisheries. Mar. Pol. 2019, 105, 1–9. [Google Scholar] [CrossRef]
- Sadovy de Mitcheson, Y.; Andersson, A.A.; Hofford, A.; Law, C.S.W.; Hau, L.C.Y.; Pauly, D. Out of control means off the menu: The case for ceasing consumption of luxury products from highly vulnerable species when international trade cannot be adequately controlled; shark fin as a case study. Mar. Pol. 2018, 98, 115–120. [Google Scholar] [CrossRef]
- Dent, F.; Clarke, S.C. State of the Global Market for Shark Products; FAO Fisheries and Aquaculture technical paper, Technical Paper 590; FAO: Rome, Italy, 2015. [Google Scholar]
- Okes, N.; Sant, G. An Overview of Major Shark Traders, Catchers and Species; TRAFFIC: Cambridge, UK, 2019; Available online: www.traffic.org/publications/reports/an-overview-of-major-shark-and-ray-catchers-traders-and-species (accessed on 28 May 2022).
- Simpfendorfer, C.A.; Dulvy, N.K. Bright spots of sustainable shark fishing. Curr. Biol. 2017, 27, R97–R98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiffman, D.S.; Hueter, R.E. A United States shark fin ban would undermine sustainable shark fisheries. Mar. Pol. 2017, 85, 138–140. [Google Scholar] [CrossRef]
- Shiffman, D.S.; Macdonald, C.C.; Wallace, S.S.; Dulvy, N.K. The role and value of science in shark conservation advocacy. Sci. Rep. 2021, 11, 16626. [Google Scholar] [CrossRef]
- Kroodsma, D.A.; Mayorga, J.; Hochberg, T.; Miller, N.A.; Boerder, K.; Ferretti, F.; Wilson, A.; Bergman, B.; White, T.D.; Block, B.A.; et al. Tracking the global footprint of fisheries. Science 2018, 359, 904–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.B. Environmental impact of trawling on the seabed: A review. N. Z. J. Mar. 1992, 26, 59–67. [Google Scholar] [CrossRef]
- Sumaila, R.U.; Rashid, K.; Ahmed, T.L.; Watson, R.; Tyedmers, P.; Pauly, D. Subsidies to high seas bottom trawl fleets and the sustainability of deep-sea demersal fish stocks. Mar. Pol. 2010, 34, 495–497. [Google Scholar] [CrossRef]
- Norse, E.A.; Brooke, S.; Cheung, W.W.L.; Clark, M.R.; Ekeland, I.; Froese, R.; Gjerde, K.M.; Haedrich, R.L.; Heppell, S.S.; Morato, T.; et al. Sustainability of deep-sea fisheries. Mar. Pol. 2011, 36, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Filmalter, J.D.; Capello, M.; Deneubourg, J.; Cowley, P.D.; Dagorn, L. Looking behind the curtain: Quantifying massive shark mortality in fish aggregating devices. Front. Ecol. Environ. 2013, 11, 291–296. [Google Scholar] [CrossRef]
- Hanich, Q.; Davis, R.; Holmes, G.; Amidjogbe, E.R.; Campbell, B. Drifting Fish Aggregating Devices (FADs) Deploying, Soaking and Setting—When Is a FAD ‘Fishing’? Int. J. Mar. Coast. 2019, 34, 731–754. [Google Scholar] [CrossRef] [Green Version]
- Blackford, M. A Tale of Two Fisheries: Fishing and Over-Fishing in American Waters. 2008. Available online: https://origins.osu.edu/article/tale-two-fisheries-fishing-and-over-fishing-american-waters?language_content_entity=en (accessed on 29 June 2022).
- Jackson, J.B.C.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; Hughes, T.P.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, R.U.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Campana, S.E. Transboundary movements, unmonitored fishing mortality, and ineffective international fisheries management pose risks for pelagic sharks in the Northwest Atlantic. Can. J. Fish. Aquat. Sci. 2016, 73, 1599–1607. [Google Scholar] [CrossRef]
- Fordham, S.; Fowler, S.L.; Coelho, R.P.; Goldman, K.; Francis, M.P. The IUCN Red List of Threatened Species: Squalus acanthias. J. Fish. Biol. 2016. [Google Scholar] [CrossRef]
- Myers, R.A.; Baum, J.K.; Shepherd, T.D.; Powers, S.P.; Peterson, C.H. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 2007, 315, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Heithaus, M.R.; Frid, A.; Wirsing, A.J.; Worm, B. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 2008, 23, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, P.S.; Triharyuni, S. Shark fisheries management as a sustainable development implementation in Indonesia fishery sector. IOP Conf. Ser. Earth Environ. Sci. 2021, 718, 012069. [Google Scholar] [CrossRef]
- Ferretti, F.; Myers, R.A.; Serena, F.; Lotze, H.K. Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 2008, 22, 952–964. [Google Scholar] [CrossRef]
- Amorim, A.F.; Arfelli, C.A.; Fagundes, L. Pelagic elasmobranchs caught by longliners off Southern Brazil during 1974–1997: An overview. Mar. Freshw. Res. 1998, 49, 621–632. [Google Scholar] [CrossRef]
- Balderson, S.D.; Martin, L.E.C. Environmental Impacts and Causation of ‘Beached’ Drifting Fish Aggregating Devices around Seychelles Islands: A Preliminary Report on Data Collected by Island Conservation Society. 2015. IOTC-2015-WPEB11-39. Available online: www.iotc.org/sites/default/files/documents/2015/09/IOTC-2015-WPEB11-39_-_FAD_beaching_Seychelles.pdf (accessed on 26 February 2022).
- Wang, Y.; Zhou, C.; Xu, L.; Wan, R.; Shi, J.; Wang, X.; Tang, H.; Wang, L.; Yu, W.; Wang, K. Degradability evaluation for natural material fibre used on fish aggregation devices (FADs) in tuna purse seine fishery. Aquacult. Fish. 2020, 6, 376–381. [Google Scholar] [CrossRef]
- Kriwet, J.; Benton, M.J. Neoselachian (Chondrichthyes, Elasmobranchii) diversity across the cretaceous-tertiary boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 214, 181–194. [Google Scholar] [CrossRef]
- Kriwet, J.; Kiessling, W.; Klug, S. Diversification trajectories and evolutionary life-history traits in early sharks and batoids. Proc. R. Soc. B 2009, 276, 945–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinot, G.; Cavin, L. ‘Fish’ (Actinopterygii and Elasmobranchii) diversification patterns through deep time. Biol. Rev. 2016, 91, 950–981. [Google Scholar] [CrossRef] [PubMed]
- Travis, J.; Coleman, F.C.; Auster, P.J.; Cury, P.M.; Estes, J.A.; Orensanz, J.; Peterson, C.H.; Power, M.E.; Steneck, R.S.; Wootton, T.J. Species interactions and fisheries management. Proc. Natl. Acad. Sci. USA 2014, 111, 581–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumby, P.J.; Dahlgren, C.P.; Harborne, A.R.; Kappel, C.V.; Micheli, F.; Brumbaugh, D.R.; Holmes, K.E.; Mendes, J.M.; Broad, K.; Sanchirico, J.N.; et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science 2006, 311, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Freire, K.D.; Christensen, V.; Pauly, D. Description of the East Brazil Large Marine Ecosystem using a trophic model. Scientia Marina. 2008, 72, 477–491. [Google Scholar] [CrossRef]
- Bascomte, J.; Melián, C.J.; Sala, E. Interaction strength combinations and the overfishing of a marine food web. Proc. Natl. Acad. Sci. USA 2005, 102, 5443–5447. [Google Scholar] [CrossRef] [Green Version]
- Okey, T.A.; Banks, S.; Born, A.F.; Bustamante, R.H.; Calvopiña, M.; Edgar, G.J.; Espinoza, E.; MiguelFariña, J.; Garske, L.E.; Reck, G.K.; et al. A trophic model of a Galápagos subtidal rocky reef for evaluating fisheries and conservation strategies. Ecol. Model. 2004, 172, 383–401. [Google Scholar] [CrossRef]
- Nadon, M.O.; Baum, J.K.; Williams, I.D.; McPherson, J.M.; Zgliczynski, B.J.; Richards, B.L.; Schroeder, R.E.; Brainard, R.E. Re-creating missing population baselines for Pacific reef sharks. Conserv. Biol. 2012, 26, 493–503. [Google Scholar] [CrossRef] [Green Version]
- MacNeil, M.A.; Chapman, D.D.; Heupel, M.; Simpfendorfer, C.A.; Heithaus, M.; Meekan, M.; Harvey, E.; Goetze, J.; Kiszka, J.; Bond, M.E.; et al. Global status and conservation potential of reef sharks. Nature 2020, 583, 801–806. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Williams, L.; Fallows, M.; Fallows, C. Disappearance of white sharks leads to the novel emergence of an allopatric apex predator, the sevengill shark. Sci. Rep. 2019, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Heithaus, M.R.; Frid, A.; Wirsing, A.J.; Dill, L.M.; Fourqurean, J.W.; Burkholder, D.; Thomson, J.; Bejder, L. State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J. Anim. Ecol. 2007, 6, 837–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcher, I.F. The Shark Sessions: My Sunset Rendezvous; Strategic Book Publishing: Traverse City, MI, USA, 2010; pp. 317–320. [Google Scholar]
- Porcher, I.F. The True Nature of Sharks; Independent: Chicago, IL, USA, 2017; p. 26. [Google Scholar]
- Mourier, J.; Vercelloni, J.; Planes, S. Evidence of social communities in a spatially structured network of a free-ranging shark species. Anim. Beh. 2012, 83, 389–401. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Bodey, T.W.; Caselle, J.E.; Bradley, D.; Freeman, R.; Friedlander, A.M.; Jacoby, D.M.P. Multiyear social stability and social information use in reef sharks with diel fission–fusion dynamics. Proc. R. Soc. B 2020, 287, 20201063. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, J. Personal Communication during Interview. 2013. Available online: https://xray-mag.com/content/deep-trust-sharks (accessed on 29 July 2022).
- Drumm, R. the Slick of the Cricket; BookSurge: Charleston, SC, USA, 1996. [Google Scholar]
- Muter, B.A.; Gore, M.L.; Gledhill, K.S.; Lamont, C.; Huveneers, C. Australian and US news media portrayal of sharks and their conservation. Cons. Biol. 2013, 27, 187–196. [Google Scholar] [CrossRef]
- Neff, C. The Jaws effect: How movie narratives are used to influence policy responses to shark bites in Western Australia. Austral. J. Pol. Sci. 2015, 50, 114–127. [Google Scholar] [CrossRef]
- Le Busque, B.; Litchfield, C. Sharks on film: An analysis of how shark-human interactions are portrayed in films. Hum. Dimens. Wildl. 2021, 27, 193–199. [Google Scholar] [CrossRef]
- Hasek, P. (Executive Producer and Senior Science Editor, Development & Production of ‘Shark Week’ for Discovery Channel, Silver Spring, MD, USA); Hasler, J. (Senior VP, Development & Production of ‘Shark Week’, Discovery Channel, Silver Spring, MD, USA). Personal communication during meeting with representatives of the Shark Group, 2010.
- Castro, J.I. The origins and rise of shark biology in the 20th century. Mar. Fish. Rev. 2017, 78, 1433. [Google Scholar]
- Gruber, H.; Myrberg, A.A. Approaches to the study of the behavior of sharks. Integr. Comp. Biol. 1977, 17, 471–486. [Google Scholar] [CrossRef] [Green Version]
- Coates, M.I.; Finarelli, J.A.; Sansom, I.J.; Andreev, P.S.; Criswell, K.E.; Tietjen, K.; Rivers, M.L.; La Riviere, P.J. An early chondrichthyan and the evolutionary assembly of a shark body plan. Proc. R. Soc. B 2018, 285, 20172418. [Google Scholar] [CrossRef]
- Andreev, P.S.; Zhao, W.; Wang, N.Z.; Smith, M.M.; Li, Q.; Cui, X.; Zhu, M.; Sansom, I.J. Early Silurian chondrichthyans from the Tarim Basin (Xinjiang, China). PLoS ONE. 2020, 15, e0228589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schluessel, V. Who would have thought that ‘Jaws’ also has brains? Cognitive functions in elasmobranchs. Anim. Cogn. 2015, 18, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, L.A.; Jefferson, R.; Glegg, G. Public perceptions of sharks: Gathering support for shark conservation. Mar. Pol. 2014, 47, 1–7. [Google Scholar] [CrossRef] [Green Version]
- PADI. 2011. Available online: www.diveagainstdebris.org/action/shark-awareness-campaign (accessed on 8 January 2022).
- NOAA Fisheries. Shark Finning Report to Congress. 2016. Available online: https://repository.library.noaa.gov/view/noaa/17060 (accessed on 14 April 2020).
- Queiroz, N.; Humphries, N.; Couto, A.; Vedor, M.; da Costa, I.; Sequeira, A.; Mucientes, G.; Santos, A.; Abascal, F.; Abercrombie, D.; et al. Global spatial risk assessment of sharks under the footprint of fisheries. Nature 2019, 572, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Musick, J.A.; Musick, S. Sharks; FAO Fisheries and Aquaculture Reviews and Studies; FAO: Rome, Italy, 2011; 13p, Available online: www.fao.org/fishery/docs/DOCUMENT/reviews%26studies/sharks.pdf (accessed on 28 May 2022).
- DeBruyn, P. Report of the 23rd Session of the IOTC Scientific Committee. In Proceedings of the 23rd Session of the IOTC Scientific Committee, Video-Conference, 7–11 December 2020; IOTC–2020–SC23–R[E]. Available online: www.iotc.org/sites/default/files/documents/2021/01/IOTC-2020-SC23-RE.pdf (accessed on 14 May 2021).
- Agnew, D.J.; Pearce, J.; Pramod, G.; Peatman, T.; Watson, R.; Beddington, J.R.; Pitcher, T.J. Estimating the worldwide extent of illegal fishing. PLoS ONE 2009, 4, e4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widjaja, S.; Long, T.; Wirajuda, H.; Van As, H.; Bergh, P.E.; Brett, A.; Copeland, D.; Fernandez, M.; Gusman, A.; Juwana, S.; et al. Illegal, Unreported and Unregulated Fishing and Associated Drivers; World Resources Institute: Washington, DC, USA, 2020; Available online: www.oceanpanel.org/iuu-fishing-and-associated-drivers (accessed on 9 April 2021).
- Meere, F.; Lack, M. Assessment of Impacts of Illegal, Unreported and Unregulated (IUU) Fishing in the Asia-Pacific. Asia-Pacific Economic Cooperation Fisheries Working Group APEC#208-FS-01.5 2008. Available online: www.apec.org/publications/2008/11/assessment-of-impacts-of-illegal-unreported-and-unregulated-iuu-fishing-in-the-asiapacific (accessed on 28 May 2022).
- Greenpeace. Choppy Waters: Forced Labour and Illegal Fishing in Taiwan’s Distant Water Fisheries. 2020. Available online: www.greenpeace.org/southeastasia/publication/3690/choppy-waters-forced-labour-and-illegal-fishing-in-taiwans-distant-water-fisheries (accessed on 28 May 2022).
- Chabrol, R.; Nouvian, C. The Hideous Price of Beauty. An Investigation into the Market of Deep-Sea Shark Liver Oil. Bloom. Assoc. 2012. Available online: www.bloomassociation.org/en/wp-content/uploads/2013/10/ENG_Squalene_4-pager.pdf (accessed on 4 June 2021).
- Cardeñosa, D. Genetic identification of threatened shark species in pet food and beauty care products. Conserv. Genet. 2019, 20, 1383–1387. [Google Scholar] [CrossRef]
- Hobbs, C.A.D.; Potts, R.W.A.; Bjerregaard, W.M.; Usher, J.; Griffiths, A.M. Using DNA barcoding to investigate patterns of species utilisation in UK shark products reveals threatened species on sale. Sci. Rep. 2019, 9, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxford University. OED Online: Oxford English Dictionary; Oxford University Press: Oxford, UK. Available online: www.oed.com (accessed on 28 December 2021).
- Guida, L. Why We Should #GiveFlakeABreak. Aust. Mar. Conserv. Soc. 2021. Available online: www.marineconservation.org.au/wp-content/uploads/2021/01/210120_Flake-Report-Full-Report.pdf (accessed on 18 April 2021).
- FAO. Database of measures on conservation and management of sharks. In Food and Agriculture Organization of the United Nation [online]; Database Version 1-2022; FAO: Rome, Italy, 2022; Available online: www.fao.org/ipoa-sharks/database-of-measures/en# (accessed on 18 May 2022).
- Ward-Paige, C.A.; Keith, D.M.; Worm, B.; Lotze, H.K. Recovery potential and conservation options for elasmobranchs. J. Fish. Biol. 2012, 80, 1844–1869. [Google Scholar] [CrossRef]
- Cardeñosa, D.; Fields, A.T.; Babcock, E.A.; Zhang, H.; Feldheim, K.; Shea, S.K.H.; Fischer, G.A.; Chapman, D.D. CITES-listed sharks remain among the top species in the contemporary fin trade. Conserv. Lett. 2018, 11, e12457. [Google Scholar] [CrossRef]
- CREMA. Costa Rica, Don’t Export that Pile of Hammerhead Shark Fins. 2018. Available online: www.cremacr.org/en/policy-advocacy/campaigns/costa-rica-dont-export-that-stockpile-of-hammerhead-shark-fins/ (accessed on 10 March 2021).
- Arauz, R. (Marine conservation policy advisor for Fins Attached Marine Research and Conservation, Costa Rica). Personal communication. 2021.
- Convention on the Conservation of Migratory Species of Wild Animals. 2020. Available online: www.cms.int/en/species?field_species_class_tid=1857 (accessed on 19 August 2020).
- Villate-Moreno, M.; Pollerspöck, J.; Kremer-Obrock, F.; Straube, N. Molecular analyses of confiscated shark fins reveal shortcomings of CITES implementations in Germany. Conserv. Sci. Pract. 2021, 3, e398. [Google Scholar] [CrossRef]
- Clarke, S.C.; Harley, S.J.; Hoyle, S.D.; Rice, J.S. Population trends in Pacific Oceanic sharks and the utility of regulations on shark finning. Conserv. Biol. 2013, 27, 197–209. [Google Scholar] [CrossRef]
- Cortés, E.; Neer, J.A. Preliminary reassessment of the validity of the 5% fin to carcass weight ratio for sharks. Collect. Vol. Sci. Pap. ICCAT. 2006, 59, 1025–1036. [Google Scholar]
- Fischer, J.; Erikstein, K.; D’Offay, B.; Guggisberg, S.; Barone, M. Review of the Implementation of the International Plan of Action for the Conservation and Management of Sharks. In FAO Fisheries and Aquaculture Circular; No. 1076; FAO: Rome, Italy, 2012; p. 65. Available online: www.fao.org/3/i3036e/i3036e00.htm (accessed on 12 August 2020).
- Biery, L.; Pauly, D. A global review of species specific shark fin to body mass ratios and relevant legislation. J. Fish Biol. 2012, 80, 1643–1677. [Google Scholar] [CrossRef]
- India: Humane Society International/India. ‘Fins Naturally Attached’ Policy Adopted to Protect Sharks. 2013. Available online: www.hsi.org/news-media/fins_attached_india_082613/ (accessed on 5 January 2022).
- Ward-Paige, C.A. A global overview of shark sanctuary regulations and their impact on shark fisheries. Mar. Pol. 2017, 82, 87–97. [Google Scholar] [CrossRef]
- Animal Welfare Institute. International Shark Finning Bans and Policies. Available online: https://awionline.org/content/international-shark-finning-bans-and-policies (accessed on 21 July 2022).
- Arauz, R. NGOs adverse MSC Sustainable Fisheries Certification granted to Western and Central Pacific Tuna Fishery. 2018. Available online: www.make-stewardship-count.org/ngos-adverse-msc-sustainable-fisheries-certification-granted-to-western-and-central-pacific-tuna-fishery (accessed on 28 December 2021).
- Ziegler, I. Shark Finning—A Case Study Highlighting the Lack of Best Practice and Application of a Risk Based Need for Data “Combating Shark Finning, an IUU Fishing Activity that Severely Undermines Conservation Efforts” Transparency and Monitoring to Combat IUU in MSC Certified Fisheries. 2019. Available online: www.make-stewardship-count.org/wp-content/uploads/2020/01/Iris-Ziegler-Discussion-Paper-Shark-Finning.pdf (accessed on 12 August 2020).
- Ziegler, A.H.; Millward, S.; Woodroffe, K.; Vail, C.; Guida, L.; Hofford, A.; Arauz, R. Analysis of the Marine Stewardship Council’s Policy on Shark Finning and the Opportunity for Adoption of a ‘Fins Naturally Attached’ Policy in the MSC. Fisheries Standard Review. 2021. Available online: www.sharkproject.org/wp-content/uploads/2021/02/Analyis-of-the-Marine-Stewardship-Councils-policy-on-shark-finning-February-2021.pdf (accessed on 4 June 2021).
- Porcher, I.F.; Darvell, B.W.; Cuny, G. Response to “A United States Shark Fin Ban Would Undermine Sustainable Shark Fisheries” Shiffman D.S.; Hueter, R.E. Mar. Pol. 2017, 85, 138–140. Mar. Pol. 2019, 104, 85–89. [Google Scholar] [CrossRef]
- Gehan, S.M. Testimony of the Sustainable Shark Alliance before the House Subcommittee on Water, Oceans, and Wildlife. 26 March 2019. Available online: https://naturalresources.house.gov/imo/media/doc/Gehan%20Testimony%20WOW%20Leg%20Hrg%2003.26.19.pdf (accessed on 28 May 2022).
- Florida Fish and Wildlife Conservation Commission. Available online: https://myfwc.com/fishing/saltwater/recreational/sharks/ (accessed on 14 April 2020).
- Florida Department of Health Florida Advisory on Fish Consumption. Available online: www.floridahealth.gov/programs-and-services/prevention/healthy-weight/nutrition/seafood-consumption/_documents/fish-advisory-big-book2019.pdf (accessed on 17 April 2020).
- Environmental Protection Agency, USA (US EPA). Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, 3rd ed.; Volume 2, Risk Assessment and Fish Consumption Limits 2000; EPA-823-B-00-008; U.S. Environmental Protection Agency, Office of Science and Technology, Office of Water: Washington, DC, USA, 2002. Available online: www.epa.gov/waterscience/fish/advice/volume2/index.html (accessed on 14 April 2020).
- Taylor, D.L.; Kutil, N.J.; Malek, A.J.; Collie, J.S. Mercury bioaccumulation in cartilaginous fishes from southern new England coastal waters: Contamination from a trophic ecology and human health perspective. Mar. Environ. Res. 2014, 99, 20–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maine Seafood Guide; University of Maine: Orono, ME, USA. Available online: seagrant.umaine.edu/maine-seafood-guide (accessed on 14 April 2020).
- Barcia, L.G.; Argiro, J.; Babcock, E.A.; Cai, Y.; Shea, S.K.H.; Chapman, D.D. Mercury and arsenic in processed fins from nine of the most traded shark species in the Hong Kong and China dried seafood markets: The potential health risks of shark fin soup. Mar. Pol. Bull. 2020, 157, 111281. [Google Scholar] [CrossRef]
- Wiersma, J.; Carroll, M. An Economic Analysis of Spiny Dogfish: Historical Trends, Future Markets, and Implications for Management Action. Massachusetts Division of Marine Fisheries, Seafood Marketing Program. Available online: www.mass.gov/files/documents/2018/12/05/AnEconomicAnalysisofSpinyDogfish.pdf (accessed on 14 April 2020).
- Walker, T.I. Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Mar. Freshw. Res. 1998, 49, 553–572. [Google Scholar] [CrossRef]
- Rago, P.J.; Sosebee, K.A.; Brodziak, J.K.; Murawski, S.A.; Anderson, E.D. Implications of recent increases in catches on the dynamics of Northwest Atlantic spiny dogfish (Squalus acanthias). Fish. Res. 1998, 39, 165–181. [Google Scholar] [CrossRef]
- Witkin, T.; Dissanayake, S.T.; McClenachan, L. Opportunities and barriers for fisheries diversification: Consumer choice in New England. Fish. Res. 2015, 168, 56–62. [Google Scholar] [CrossRef]
- St. Gelais, A.T.; Costa-Pierce, B.A. Mercury concentrations in Northwest Atlantic winter-caught, male spiny dogfish (Squalus acanthias): A geographic mercury comparison and risk-reward framework for human consumption. Mar. Poll. Bull. 2016, 102, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, B. Cod is Dead—Is Dogfish the Answer? Boston Newsmagazine. Available online: www.bostonmagazine.com/restaurants/2016/08/14/dogfish (accessed on 17 April 2020).
- New York Post. Fish Sticks for millennials! Seafood Industry Rebrands ‘Trash Fish’. Available online: https://nypost.com/2016/01/21/the-new-fish-sticks-for-millennials (accessed on 22 April 2020).
- Kowacki, E.B. Can Dogfish Save Cape Cod Fisheries? Christian Science Monitor. Available online: www.csmonitor.com/Environment/2018/0820/Can-dogfish-save-Cape-Cod-fisheries (accessed on 27 April 2020).
- NOAA Fisheries. Atlantic Spiny Dogfish. Available online: www.fisheries.noaa.gov/species/atlantic-spiny-dogfish (accessed on 6 May 2020).
- Atlantic States Marine Fisheries Commission Spiny Dogfish. Available online: www.asmfc.org/species/spiny-dogfish (accessed on 17 April 2020).
- Marine Stewardship Council. US Spiny Dogfish and Winter Skate. Available online: https://fisheries.msc.org/en/fisheries/us-atlantic-spiny-dogfish-and-winter-skate (accessed on 24 April 2020).
- Agrawal, A. Common property institutions and sustainable governance of resources. World Dev. 2001, 10, 1649–1672. [Google Scholar] [CrossRef]
- Byrne, M.E.; Cortés, E.; Jeremy, J.; Vaudo, J.J.; Harvey, G.C.M.; Sampson, M.; Wetherbee, B.M.; Shivji, M. Satellite telemetry reveals higher fishing mortality rates than previously estimated, suggesting overfishing of an apex marine predator. Proc. R. Soc. B 2017, 284, 20170658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Jabado, R.; Liu, K.M.; Marshall, A.; Pacoureau, N.; et al. Isurus oxyrinchus. IUCN Red List Threat. Species. Red List 2019, e.T39341A2903170. [Google Scholar] [CrossRef]
- ICCAT. Summary Report by the Commission Chair. Doc. No. PLE_150_A/2020. Available online: www.iccat.int/com2020/ENG/PLE_150A_ENG.pdf (accessed on 9 March 2021).
- ICCAT. ICCAT Press Release ICCAT Agreed a New Conservation Measure for the North Atlantic Shortfin Mako Shark. In Proceedings of the 27th Regular Meeting of the Commission, 23 November 2021. Available online: www.iccat.int/Documents/Meetings/COMM2021/PRESS_RELEASE_ENG.pdf (accessed on 4 December 2021).
- ICCAT. Report of the Standing Committee on Research and Statistics (SCRS). In Proceedings of the Standing Committee on Research and Statistics (SCRS), Madrid, Spain, 2–6 October 2017. Available online: www.iccat.int/Documents/Meetings/Docs/2017_SCRS_REP_ENG.pdf (accessed on 12 January 2022).
- ICCAT. Recommendation by ICCAT on the Conservation of the North Atlantic Stock of Shortfin Mako Caught in Association with ICCAT Fisheries. Rec. 17-08. Available online: www.iccat.int/Documents/Recs/compendiopdf-e/2017-08-e.pdf (accessed on 12 January 2022).
- CITES. Supplementary Information on CITES COP 18 Proposal 42: Confirming that Shortfin and Longfin Mako Sharks Fully Meet the Criteria for Inclusion on CITES Appendix II. Paper presented at Eighteenth Meeting of the Conference of the Parties, Geneva, Switzerland, June 17–28; CoP18 Inf. 40, 1 and 6. Available online: https://cites.org/sites/default/files/eng/cop/18/inf/E-CoP18-Inf-040.pdf (accessed on 12 December 2021).
- Wegner, N.C.; Lai, N.C.; Bull, K.B.; Graham, J.B. Oxygen utilization and the branchial pressure gradient during ram ventilation of the shortfin mako, Isurus oxyrinchus: Is lamnid shark-tuna convergence constrained by elasmobranch gill morphology? J. Exp. Biol. 2012, 215, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campana, S.E.; Joyce, W.; Fowler, M.; Showell, M. Discards, hooking, and post-release mortality of porbeagle (Lamna nasus), shortfin mako (Isurus oxyrinchus), and blue shark (Prionace glauca) in the Canadian pelagic longline fishery. ICES J. Mar. Sci. 2016, 73, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Kao, E. Hong Kong Shark Fin Traders ‘will be Hit Hard’ by Proposal to Protect Blue Sharks. South China Morning Post. 2017. Available online: www.scmp.com/news/hong-kong/health-environment/article/2108502/hong-kong-shark-fin-traders-will-be-hit-hard (accessed on 14 April 2020).
- Vedor, M.; Queiroz, N.; Mucientes, G. Climate-driven deoxygenation elevates fishing vulnerability for the ocean’s widest ranging shark. eLife 2021, 10, e62508. [Google Scholar] [CrossRef]
- Make Stewardship Count. Open Letter to MSC. Available online: www.make-stewardship-count.org/wp-content/uploads/2018/02/Open-Letter-to-MSC_FINAL_January-2018.pdf (accessed on 29 July 2022).
- Clarke, S. Use of shark fin trade data to estimate historic total shark removals in the Atlantic Ocean. Aquat. Living Resour. 2008, 21, 373–381. [Google Scholar] [CrossRef]
- ICCAT. Report of the Standing Committee on Research and Statistics (SCRS). In Proceedings of the Standing Committee on Research and Statistics (SCRS), Online, 27 September–2 October 2021. Available online: www.iccat.int/Documents/Meetings/Docs/2021/REPORTS/2021_SCRS_ENG.pdf (accessed on 4 December 2021).
- European Union Plenary sitting. Recommendation on the Draft Council Decision on the Conclusion, on Behalf of the European Union, of the Protocol to Amend the International Convention for the Conservation of Atlantic Tunas (13447/2019—C9-0187/2019—2019/0225(NLE)) 27 April 2020. Available online: www.europarl.europa.eu/doceo/document/A-9-2020-0089_EN.pdf (accessed on 7 August 2020).
- Tsikliras, A.C.; Froese, R. Maximum Sustainable Yield. In Encyclopedia of Ecology, 2nd ed.; Fath, B.D., Ed.; Elsevier: Oxford, UK, 2019; Volume 1, pp. 108–115. [Google Scholar]
- Sumaila, R.U.; Ebrahim, N.; Schuhbauer, A.; Skerritt, D.; Li, Y.; Sik Kim, H.; Mallory, T.G.; Lam, V.W.L.; Pauly, D. Updated estimates and analysis of global fisheries subsidies. Mar. Pol. 2019, 109, 103695. [Google Scholar] [CrossRef]
- Sala, E.; Mayorga, J.; Costello, C.; Kroodsma, D.; Palomares, M.L.D.; Pauly, D.; Sumaila, U.R.; Zeller, D. The economics of fishing the high seas. Sci. Adv. 2018, 4, eaat2504. [Google Scholar] [CrossRef] [Green Version]
- Arnason, R.; Kelleher, K.; Willmann, R. The Sunken Billions: The Economic Justification for Fisheries Reform; Joint publication of the World Bank and the FAO; World Bank: Washington, DC, USA; FAO: Rome, Italy, 2008; ISBN 978-0-8213-7790-1. [Google Scholar]
- Travis, W. Shark For Sale; Rand McNally: Chicago, IL, USA, 1961. [Google Scholar]
- Castro, J.I.; Woodley, C.M.; Brudek, R.L. A Preliminary Evaluation of Status of Shark Species; FAO Fisheries Technical Paper; Food and Agriculture Organization: Rome, Italy, 1999. [Google Scholar]
- Pauly, D.; Zeller, D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 2016, 7, 10244. [Google Scholar] [CrossRef] [PubMed]
- Vaness, B. Sustainable Shark Alliance: Setting the Record Straight on Sharks for Ocean Week. Available online: www.accesswire.com/547715/Sustainable-Shark-Alliance-Setting-the-Record-Straight-on-Sharks-for-Ocean-Week (accessed on 10 January 2022).
- Rutger, H. Sustainable Shark Trade Bill Offers Science Based Solutions for Overfishing. Mote Laboratories. 2018. Available online: https://mote.org/news/article/sustainable-shark-trade-bill-offers-science-based-solutions-for-overfishing (accessed on 10 January 2022).
- Ferretti, F.; Jacoby, D.M.P.; Pfleger, M.O.; White, T.D.; Dent, F.; Micheli, F.; Rosenberg, A.A.; Crowder, L.B.; Block, B.A. Shark fin trade bans and sustainable shark fisheries. Conserv. Lett. 2020, 13, e12708. [Google Scholar] [CrossRef] [Green Version]
- Human Rights at Sea. Fisheries Observer Deaths at Sea, Human Rights and the Role and Responsibilities of Fisheries Organisations. Available online: www.humanrightsatsea.org/2020/07/03/report-fisheries-observer-deaths-at-sea-human-rights-and-the-role-and-responsibilities-of-fisheries-organisations (accessed on 3 March 2021).
- McVeigh, K.; Firdaus, F. ‘Hold on Brother’: Final Days of Doomed Crew on Chinese Shark Finning Boat. The Guardian 7 July 2020. Available online: www.theguardian.com/environment/2020/jul/07/hold-on-brother-final-days-of-doomed-crew-on-chinese-shark-finning-boat (accessed on 12 August 2020).
- Convention on Biological Diversity (CBD). Available online: www.cbd.int/sustainable/introduction.shtml (accessed on 28 May 2022).
- Cooney, R. Sustainable Use: Concepts, Ambiguities, Challenges. In Proceedings of the IUCN Species Survival Commission’s Sustainable Use Specialist Group Strategic Planning Meeting, White Oak Plantation, Yulee, FL, USA, 10–13 July 2007; Available online: www.iucn.org/files/cooney-r-2007-sustainable-use-concepts-ambiguities-challenges (accessed on 28 May 2020).
- EU Monitor Explanatory Memorandum to COM(2002)185—Conservation and Sustainable Exploitation of Fisheries Resources under the Common Fisheries Policy. Available online: www.eumonitor.eu/9353000/1/j4nvhdfdk3hydzq_j9vvik7m1c3gyxp/vi8rm2yv7ezh (accessed on 5 January 2022).
- Magnuson-Stevens Fishery Conservation and Management Act (MSA) 16 USC Ch. 38: Fishery Conservation and Management. Available online: https://uscode.house.gov/view.xhtml?path=/prelim@title16/chapter38&edition=prelim (accessed on 28 May 2022).
- Preikshot, D.; Pauly, D. Global Fisheries and Marine Conservation: Is Coexistence Possible? In Marine Conservation Biology: The Science of Maintaining the Sea’s Biodiversity; Norse, E.A., Crowder, L.B., Eds.; Island Press: Washington, DC, USA, 2005; Chapter 11; pp. 185–197. [Google Scholar]
- Agrawal, A.; Ostrom, E. Political science and conservation biology: A dialog of the deaf. Conserv. Biol. 2006, 30, 681–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, J.M.; Myers, R.A.; Rosenberg, A.A. Wasted fishery resources: Discarded bycatch in the USA. Fish Fish. 2005, 6, 350–361. [Google Scholar] [CrossRef]
- Stelfox, M.; Bulling, M.; Sweet, M. Untangling the origin of ghost gear within the Maldivian archipelago and its impact on olive ridley (Lepidochelys olivacea) populations. Endang. Spec. Res. 2019, 40, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Make Stewardship Count. Available online: www.make-stewardship-count.org (accessed on 28 May 2022).
- Marine Stewardship Council. Echebastar Indian Ocean Purse Seine Skipjack Tuna. Available online: https://fisheries.msc.org/en/fisheries/echebastar-indian-ocean-purse-seine-skipjack-tuna (accessed on 10 March 2021).
- Kearns, M. IPNLF: Tuna Fishery Certification ‘Fatally Flawed’. Seafood Source. Available online: www.seafoodsource.com/news/environment-sustainability/ipnlf-tuna-fishery-certification-fatally-flawed (accessed on 24 April 2020).
- Edwards, S. WWF Statement on MSC Certification of Spanish Purse Seine “Echebastar” Fishery in the Indian Ocean. Available online: https://wwf.panda.org/wwf_news/press_releases/?337217/WWF-Statement-on-MSC-certification-of-Spanish-Purse-Seine-Echebastar-Fishery-in-the-Indian-Ocean%C2%A0 (accessed on 29 June 2022).
- Diggles, B.K.; Cooke, S.J.; Rose, J.D.; Sawynok, W. Ecology and welfare of aquatic animals in wild capture fisheries. Rev. Fish. Biol. Fish. 2011, 21, 739–765. [Google Scholar] [CrossRef]
- Meadows, D.H.; Meadows, D.L.; Randers, J.; Behrens, W.W. The Limits to Growth; Universe Books: New York, NY, USA, 1972. [Google Scholar]
- Lorenz, K. Das Sogenannte Böse, Zur Naturgeschichte der Aggression; Verlag Dr Borotha-Schoeler: Vienna, Austria, 1963. [Google Scholar]
- Barry, G. Terrestrial ecosystem loss and biosphere collapse. Manag. Environ. Qual. 2014, 25, 542–563. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, P. The Economics of Biodiversity: The Dasgupta Review; HM Treasury: London, UK, 2021. Available online: www.gov.uk/government/publications/final-report-the-economics-of-biodiversity-the-dasgupta-review (accessed on 29 June 2022).
- Mason, F. The Newfoundland cod stock collapse: A review and analysis of social factors. Electron. Green J. 2002, 1. [Google Scholar] [CrossRef]
- Dickey-Collas, M.; Nash, R.D.M.; Brunel, T.; van Damme, C.J.G.; Marshall, T.C.; Payne, M.R.; Corten, A.; Geffen, A.J.; Peck, M.A.; Hatfield, E.M.C.; et al. Lessons learned from stock collapse and recovery of North Sea herring: A review. ICES J. Mar. Sci. 2010, 67, 1875–1886. [Google Scholar] [CrossRef] [Green Version]
- Sea Shepherd. Operation Sola Stella: Combatting Illegal Fishing in Liberia, West Africa. Available online: https://seashepherd.org/campaigns/iuu-fishing-africa/iuu-campaigns/sola-stella (accessed on 22 August 2020).
- Sea Shepherd. Arrest of Poaching Vessel Shows Shark Liver Oil Production Could Drive Species to Extinction. Available online: www.seashepherdglobal.org/latest-news/shark-liver-oil-labiko2 (accessed on 4 June 2021).
- Vincent, A.C.J.; Sadovy de Mitcheson, Y.; Fowler, S.L.; Lieberman, S. The role of CITES in the conservation of marine fishes subject to international trade. Fish Fish. 2013, 15, 563–592. [Google Scholar] [CrossRef]
- Citizen’s Initiative: Stop Finning EU. Available online: https://europa.eu/citizens-initiative/initiatives/details/2020/000001_en (accessed on 29 June 2022).
- Alcala, A.C. Community-based coastal resource management in the Philippines: A case study. Ocean. Coast. Manag. 1998, 38, 179–186. [Google Scholar] [CrossRef]
- Diegues, A.C. Marine Protected Areas and Artisanal Fisheries in Brazil. 2008. Available online: https://aquadocs.org/bitstream/handle/1834/19431/Samudra_mon2.pdf (accessed on 28 May 2022).
- Espectato, L.N.; Monteclaro, H.M.; Arceo, H.O.; Catedrilla, L.C.; Baylon, C.C. Community perceptions on the role of inter-local government units alliance in coastal resource management: The case of Banate Bay alliance in Iloilo, Philippines. Ocean. Coast. Manag. 2022, 219, 106059. [Google Scholar] [CrossRef]
- Kaplan, I.C.; Cox, S.P.; Kitchell, J.F. Circle hooks for Pacific longliners: Not a panacea for marlin and shark bycatch, but part of the solution. Trans. Am. Fish. Soc. 2007, 136, 392–401. [Google Scholar] [CrossRef]
- Erickson, D.L.; Berkeley, S.A. Methods to reduce bycatch mortality in longline fisheries. In Sharks of the Open Ocean: Biology, Fisheries and Conservation; Camhi, M.D., Pikitch, E.K., Babcock, E.A., Eds.; Blackwell Publishing: Oxford, UK, 2008; pp. 462–471. [Google Scholar]
- Reid, D.G.; Graham, N.; Suuronen, P.; He, P.; Pol, M. Implementing balanced harvesting: Practical challenges and other implications. ICES J. Mar. Sci. 2016, 73, 1690–1696. [Google Scholar] [CrossRef]
- Zhou, S.; Kolding, J.; Garcia, S.M.; Plank, M.J.; Bundy, A.; Charles, A.; Hansen, A.; Heino, M.; Howell, D.; Jacobsen, N.S.; et al. Balanced harvest: Concept, policies, evidence, and management implications. Rev. Fish. Biol. Fish. 2019, 29, 711–733. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, B.C.; Winther-Janson, M.; Bainbridge, J.M.; Aitken, J.; Hawkins, J.P.; Roberts, C.M. Effective Coverage Targets for Ocean Protection. Conserv. Lett. 2016, 9, 398–404. [Google Scholar] [CrossRef]
- Klimley, A.P.; Arauz, R.; Bessudo, S.; Chávez, E.J.; Chinacalle, N.; Espinoza, E.; Green, J.; Hearn, A.R.; Hoyos-Padilla, M.E.; Nalesso, E.; et al. Studies of the movement ecology of sharks justify the existence and expansion of marine protected areas in the Eastern Pacific Ocean. Environ. Biol. Fish. 2022. [Google Scholar] [CrossRef]
- Baum, J. Industrial fishing boats leave few safe havens for sharks on the high seas. Nature 2019, 572, 449–450. [Google Scholar] [CrossRef]
- Dwyer, R.G.; Krueck, N.C.; Udyawer, V.; Heupel, M.R.; Chapman, D.; Pratt, H.L.; Garla, R.; Simpfendorfer, C.A. Individual and population benefits of marine reserves for reef sharks. Curr. Biol. 2020, 30, 480–489. [Google Scholar] [CrossRef]
- Sumaila, R.U.; Zeller, D.; Hood, L.; Palomares, M.L.D.; Li, Y.; Pauly, D. Illicit trade in marine fish catch and its effects on ecosystems and people worldwide. Sci. Adv. 2020, 6, eaaz3801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewell, C.; Hocevar, J.; Mitchell, E.; Snowden, S.J. An evaluation of Regional Fisheries Management Organization at-sea compliance monitoring and observer programs. Mar. Pol. 2020, 115, 103842. [Google Scholar] [CrossRef]
- Barnosky, A.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pievani, T. The sixth mass extinction: Anthropocene and the human impact on biodiversity. Rend. Lincei 2014, 25, 85–93. [Google Scholar] [CrossRef]
| Year | Nation | Comments |
|---|---|---|
| 2001 | Congo-Brazzaville | Shark fishing banned |
| 2004 | Ecuador | Shark fishing banned, but only enforced around Galapagos |
| 2006 | French Polynesia | Shark sanctuary |
| 2006 | Egypt | Shark fishing banned up to 12 NM from shore in the Red Sea |
| 2009 | Palau | Shark sanctuary |
| 2010 | Maldives | Shark sanctuary |
| 2011 | Tokelau | Shark sanctuary |
| 2011 | Marshall Islands | Shark sanctuary |
| 2011 | Bahamas | Shark sanctuary |
| 2011 | Honduras | Shark sanctuary |
| 2012 | Cook Islands | Shark sanctuary |
| 2013 | Brunei | In EEZ; ban on trade of shark products |
| 2013 | New Caledonia | Shark fishing, transportation, trade, and consumption banned |
| 2014 | United Arab Emirates | Shark fishing of CITES listed sharks banned; other species banned between 1 February and 30 June. Imports and exports banned |
| 2015 | Federated States of Micronesia | Shark sanctuary |
| 2015 | Cayman Islands | Shark sanctuary |
| 2015 | Kiribati | Shark sanctuary |
| 2015 | Bonaire | Shark sanctuary |
| 2015 | Sabah | Shark sanctuary |
| 2015 | British Virgin Islands | Commercial shark fishing banned |
| 2016 | St Maarten | Shark sanctuary |
| 2017 | Dominican Republic | Shark sanctuary |
| 2018 | American Samoa | Shark sanctuary |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcher, I.F.; Darvell, B.W. Shark Fishing vs. Conservation: Analysis and Synthesis. Sustainability 2022, 14, 9548. https://doi.org/10.3390/su14159548
Porcher IF, Darvell BW. Shark Fishing vs. Conservation: Analysis and Synthesis. Sustainability. 2022; 14(15):9548. https://doi.org/10.3390/su14159548
Chicago/Turabian StylePorcher, Ila France, and Brian W. Darvell. 2022. "Shark Fishing vs. Conservation: Analysis and Synthesis" Sustainability 14, no. 15: 9548. https://doi.org/10.3390/su14159548







