Pre-Harvest Application of Multi-Walled Carbon Nanotubes Improves the Antioxidant Capacity of ‘Flame Seedless’ Grapes during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MWCNTs
Characterization of MWCNTs
2.2. Plant Materials and Treatment
2.2.1. Postharvest Treatment
2.2.2. Grape Photos Taken at Maturity
2.3. Determination items and Methods
2.3.1. Physiochemical Properties of Grapes
2.3.2. Analysis of Malondialdehyde, H2O2 and O2− Production Rate
2.3.3. Assay of Antioxidant Enzyme Activity Content
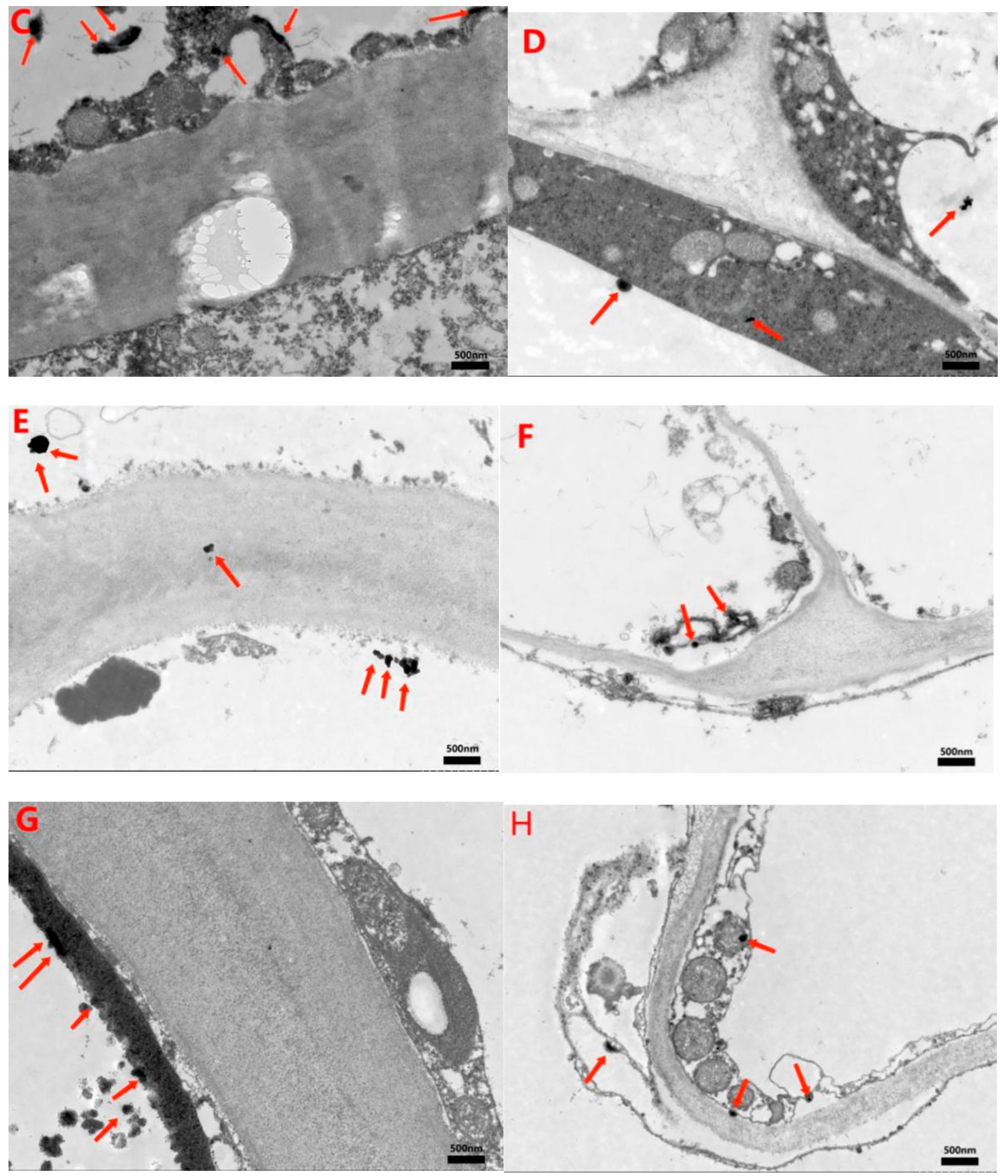
2.3.4. Morphological Observation by TEM
2.3.5. Statistical Analysis
3. Results
3.1. Biochemical and Physical Quality Characteristics of Grape in Storage
3.2. Production of MDA, O2−, and H2O2 during Storage
3.3. Antioxidant Enzyme Analysis
3.4. Detection of MWCNTs in Peel and Pulp
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Stefano, V.; Buzzanca, C.; Melilli, M.G.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol Characterization and Antioxidant Activity of Grape Seeds and Skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci Technol. 2015, 52, 3607–3616. [Google Scholar] [CrossRef] [PubMed]
- Nia, A.E.; Taghipour, S.; Siahmansour, S. Pre-harvest application of chitosan and postharvest Aloe vera gel coating enhances quality of table grape (Vitis vinifera L. cv. ‘Yaghouti’) during postharvest period. Food Chem. 2021, 347, 129012. [Google Scholar] [CrossRef]
- Eshghi, S.; Karimi, R.; Shiri, A.; Karami, M.; Moradi, M. The novel edible coating based on chitosan and gum ghatti to improve the quality and safety of ‘Rishbaba’ table grape during cold storage. J. Food Meas. Charac. 2021, 15, 3683–3693. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, P.; Zhang, P.; Su, L.; Jia, H.; Wei, X.; Fang, J.; Jia, H. Integrative transcriptomics and metabolomics data exploring the effect of chitosan on postharvest grape resistance to Botrytis cinerea. Postharvest Biol. Technol. 2020, 167, 111248. [Google Scholar] [CrossRef]
- Esparza, I.; Martinez-Inda, B.; Cimminelli, M.J.; Jimeno-Mendoza, M.C.; Moler, J.A.; Jimenez-Moreno, N.; Ancin-Azpilicueta, C. Reducing SO2 Doses in Red Wines by Using Grape Stem Extracts as Antioxidants. Biomolecules 2020, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Chen, W.; Wu, C.E.; Kou, X.; Fan, G.; Li, T.; Wu, Z. Preservation of Ginkgo biloba seeds by coating with chitosan/nano-TiO2 and chitosan/nano-SiO2 films. Int J. Biol. Macromol. 2019, 126, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Sun, X.; Marín, A.; dos Santos, L.C.; Plotto, A.; Bai, J.; Assis, O.B.G.; David Ferreira, M.; Baldwin, E. Nano- and micro-sized carnauba wax emulsions-based coatings incorporated with ginger essential oil and hydroxypropyl methylcellulose on papaya: Preservation of quality and delay of post-harvest fruit decay. Food Chem. X 2022, 13, 100249. [Google Scholar] [CrossRef]
- Castelo Branco Melo, N.F.; de MendonçaSoares, B.L.; Marques Diniz, K.; Ferreira Leal, C.; Canto, D.; Flores, M.A.P.; Henrique da Costa Tavares-Filho, J.; Galembeck, A.; Montenegro Stamford, T.L.; Montenegro Stamford-Arnaud, T.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Carrero-Sanchez, J.C.; Elias, A.L.; Mancilla, R.; Arrellin, G.; Terrones, H.; Laclette, J.P.; Terrones, M. Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Lett. 2006, 6, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Panessa-Warren, B.J.; Warren, J.B.; Wong, S.S.; Misewich, J.A. Biological cellular response to carbon nanoparticle toxicity. J. Phys. Condens. Matter 2006, 18, S2185–S2201. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B.; Matsuoka, M.; Akasaka, T.; Watari, F. Phytotoxicity of multi-walled carbon nanotubes assessed by selected plant species in the seedling stage. Appl. Sur. Sci. 2012, 262, 120–124. [Google Scholar] [CrossRef]
- Dela Rosa, G.; Garcia-Castaneda, C.; Vazquez-Nunez, E.; Alonso-Castro, A.J.; Basurto-Islas, G.; Mendoza, A.; Cruz-Jimenez, G.; Molina, C. Physiological and biochemical response of plants to engineered NMs: Implications on future design. Plant Physiol. Biochem. 2017, 110, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Teixeira da Silva, J.A. The effect of carbon nanotubes on the seed germination and seedling growth of four vegetable species. J. Crop Sci. Biotechnol. 2014, 17, 201–208. [Google Scholar] [CrossRef]
- Hossain, Z.; Mustafa, G.; Komatsu, S. Plant Responses to Nanoparticle Stress. Int. J. Mol. Sci. 2015, 16, 26644–26653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Estrada, A.; Silvestre-Albero, J.; Rodriguez, A.E.; Rodriguez-Reinoso, F.; Gomez-Tejedor, J.A.; Antolinos-Turpin, C.M.; Bataille, L.; Alio, J.L. Biocompatibility and Biomechanical Effect of Single Wall Carbon Nanotubes Implanted in the Corneal Stroma: A Proof of Concept Investigation. J. Ophthalmol. 2016, 2016, 4041767. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef]
- Hatami, M. Toxicity assessment of multi-walled carbon nanotubes on Cucurbita pepo L. under well-watered and water-stressed conditions. Ecotoxicol. Environ. Saf. 2017, 142, 274–283. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Z.; Su, M.Y.; Xiao, W.; Chao, L.; Qu, C.X.; Liang, C.; Hao, H.; Liu, X.Q.; Hong, F.S. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem Res. 2008, 121, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-l.; Liu, H.-n.; Wang, Z.-g.; Guo, L.-l.; Zhang, G.-h. Sodium dehydroacetate treatment prolongs the shelf-life of ‘Kyoho’ grape by regulating oxidative stress and DNA methylation. J. Integr. Agric. 2022, 21, 1525–1533. [Google Scholar] [CrossRef]
- Xu, M.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Li, J.; Zhang, Y.; Guo, Y.; Cheng, J.-H. Effects of dielectric barrier discharge cold plasma on the activity, structure and conformation of horseradish peroxidase (HRP) and on the activity of litchi peroxidase (POD). LWT 2021, 141, 111078. [Google Scholar] [CrossRef]
- Zheng, Y.; Fung, R.W.M.; Wang, S.Y.; Wang, C.Y. Transcript levels of antioxidative genes and oxygen radical scavenging enzyme activities in chilled zucchini squash in response to superatmospheric oxygen. Postharvest Biol. Technol. 2008, 47, 151–158. [Google Scholar] [CrossRef]
- Li, K.; Pang, C.H.; Ding, F.; Sui, N.; Feng, Z.T.; Wang, B.S. Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis chloroplasts enhances salt tolerance of plants. S. Afr. J. Bot. 2012, 78, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, Translocation, and Transmission of Carbon Nanomaterials in Rice Plants. Small 2009, 5, 1128–1132. [Google Scholar] [CrossRef]
- Kuchibhatla, S.V.N.T.; Karakoti, A.S.; Bera, D.; Seal, S. One dimensional nanostructured materials. Prog. Mater. Sci. 2007, 52, 699–913. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Samadi, S.; Saharkhiz, M.J.; Azizi, M.; Samiei, L.; Ghorbanpour, M. Multi-walled carbon nanotubes stimulate growth, redox reactions and biosynthesis of antioxidant metabolites in Thymus daenensis celak. in vitro. Chemosphere 2020, 249, 126069. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.Y.; Wu, R.Y.; Strik, B.C.; Zhao, Y.Y. Effect of edible coatings on the quality of fresh blueberries (‘Duke’ and ‘Elliott’) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Moriyama, A.; Nojiri, M.; Watanabe, G.; Enoki, S.; Suzuki, S. Exogenous allantoin improves anthocyanin accumulation in grape berry skin at early stage of ripening. J. Plant. Physiol. 2020, 253, 153253. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Wang, G.X.; Wang, X.D.; Guo, J.Y. Germination, osmotic adjustment, and antioxidant enzyme activities of gibberellin-pretreated Picea asperata seeds under water stress. New Forest. 2010, 39, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, N.; Radjabian, T.; Soltani, B.M. Impacts of foliar exposure to multi-walled carbon nanotubes on physiological and molecular traits of Salvia verticillata L., as a medicinal plant. Plant Physiol. Biochem. 2020, 150, 27–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Ntagkas, N.; Fanourakis, D.; Tsaniklidis, G.; Zhao, J.; Cheng, R.; Yang, Q.; Li, T. The role of light intensity in mediating ascorbic acid content during postharvest tomato ripening: A transcriptomic analysis. Postharvest Biol. Technol. 2021, 180, 111622. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ramachandraiah, K.; Hasan, R.; Chowdhury, R.I.; Kanan, K.A.; Ahmed, S.; Ali, M.A.; Islam, M.T.; Ahmed, M. Application of Oxalic Acid and 1-Methylcyclopropane (1-Mcp) with Low and High-Density Polyethylene on Post-Harvest Storage of Litchi Fruit. Sustainability 2021, 13, 3703. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, X.; Fu, Z.; Mu, W.; Zhang, X. Energy Conservation Potential Assessment Method for Table Grapes Supply Chain. Sustainability 2018, 10, 2845. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Chen, T.; Qin, G.; Li, B.; Tian, S. Synergistic action of antioxidative systems contributes to the alleviation of senescence in kiwifruit. Postharvest Biol. Technol. 2016, 111, 15–24. [Google Scholar] [CrossRef]
- Fan, X.; Xu, J.; Lavoie, M.; Peijnenburg, W.J.G.M.; Zhu, Y.; Lu, T.; Fu, Z.; Zhu, T.; Qian, H. Multiwall carbon nanotubes modulate paraquat toxicity in Arabidopsis thaliana. Environ. Pollut. 2018, 233, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, S.; Sensoy, S.; Karatas, A.; Tekin, O.; Islek, F.; Yilmaz, N.; Kipcak, S.; Ercisli, S.; Skrovankova, S.; Adamkova, A.; et al. Effect of Pre-Harvest Organic Cytokinin Application on the Post-Harvest Physiology of Pepper (Capsicum annuum L.). Sustainability 2021, 13, 8258. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E.K. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Lin, C.; Fugetsu, B.; Su, Y.; Watari, F. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J. Hazard. Mater. 2009, 170, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.-m.; Lin, C.; Fugetsu, B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 2009, 47, 3479–3487. [Google Scholar] [CrossRef]
- Ghosh, M.; Bhadra, S.; Adegoke, A.; Bandyopadhyay, M.; Mukherjee, A. MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat. Res. 2015, 774, 49–58. [Google Scholar] [CrossRef]
- Li, H.; Suo, J.; Han, Y.; Liang, C.; Jin, M.; Zhang, Z.; Rao, J. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest ‘Xuxiang’ kiwifruit during cold storage. Postharvest Biol. Technol. 2017, 124, 107–118. [Google Scholar] [CrossRef]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Zhao, L.; Li, H.; Zhang, Q.; Tan, J.; Huang, M.; He, S.; Li, L. Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J. Hazard. Mater. 2013, 246–247, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Gutowski, S.M.; Walters, K.S.; Yan, B.; Schnoor, J.L. Charge, size, and cellular selectivity for multiwall carbon nanotubes by maize and soybean. Environ. Sci. Technol. 2015, 49, 7380–7390. [Google Scholar] [CrossRef] [PubMed]
- Serag, M.F.; Kaji, N.; Gaillard, C.; Okamoto, Y.; Terasaka, K.; Jabasini, M.; Tokeshi, M.; Mizukami, H.; Bianco, A.; Baba, Y. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano 2011, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Majd, M.; Nejad, A.R.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and nanographene oxide improves rose keeping quality. Chem. Biol. Technol. Agric. 2022, 97, 346–360. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Mousavi-Fard, S.; Nejad, A.R.; Fanourakis, D. Carbon nanotubes in the holding solution stimulate flower opening and prolong vase life in carnation. J. Hortic. Sci. Biotech. 2022, 9, 15. [Google Scholar] [CrossRef]
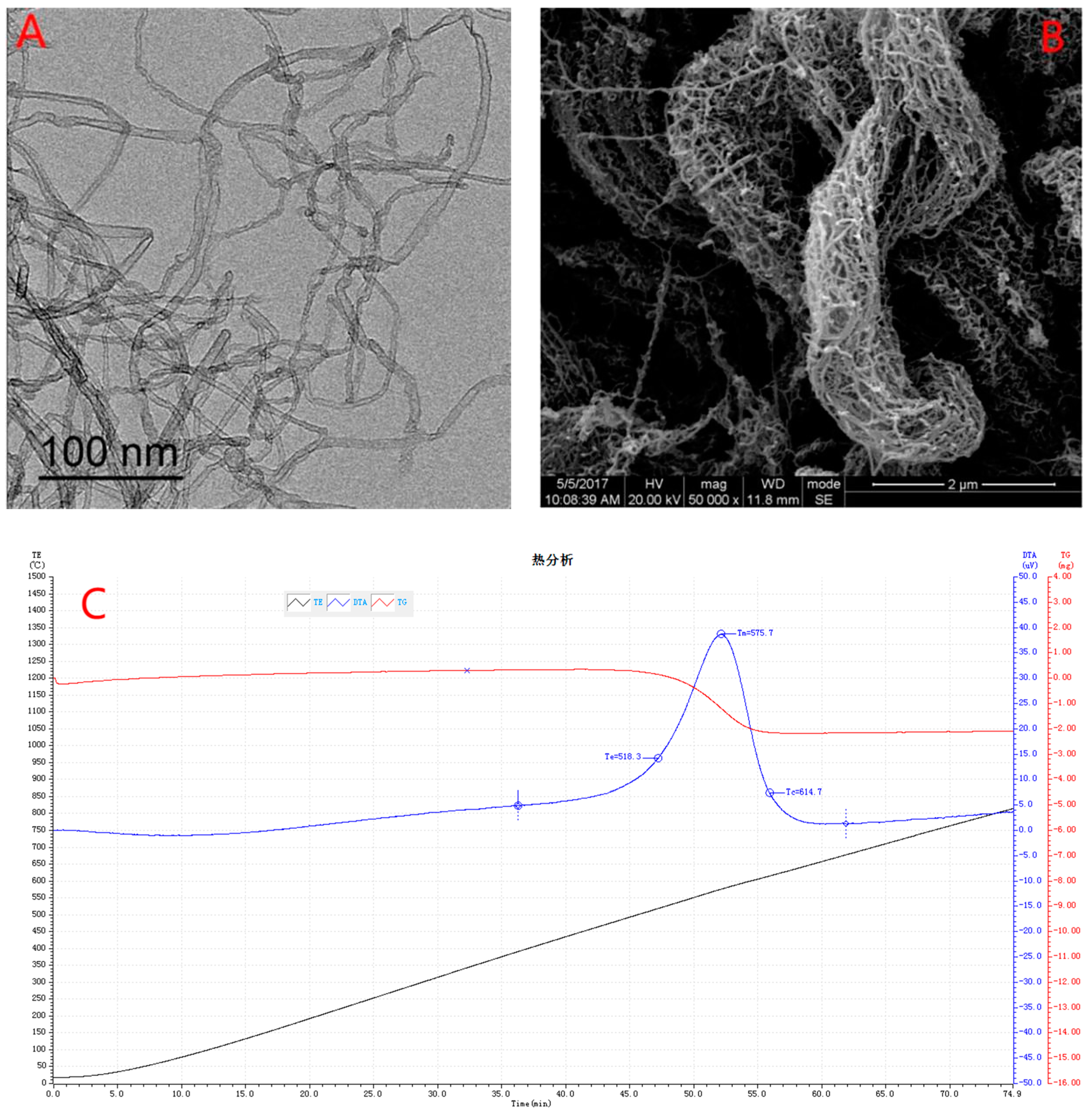


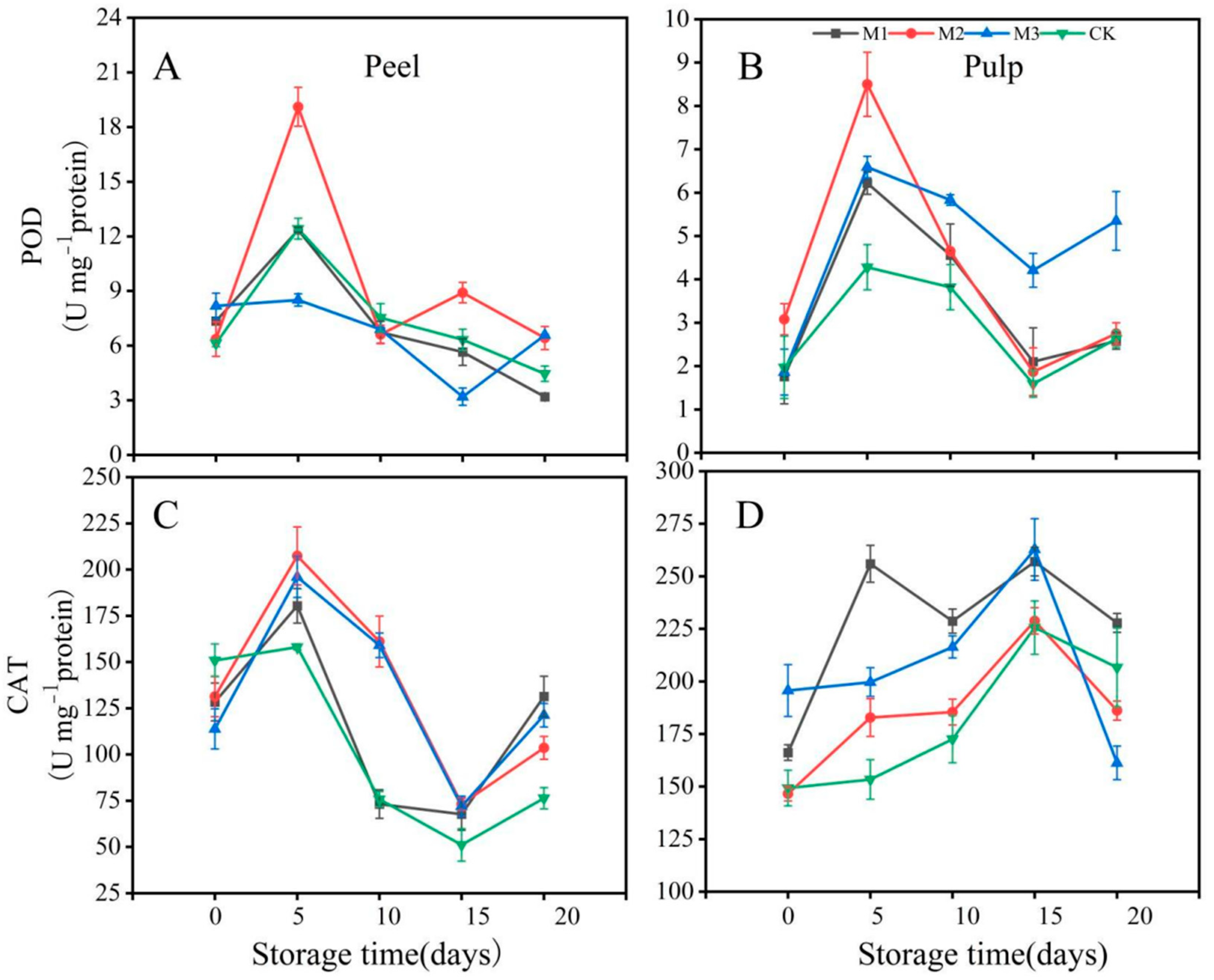
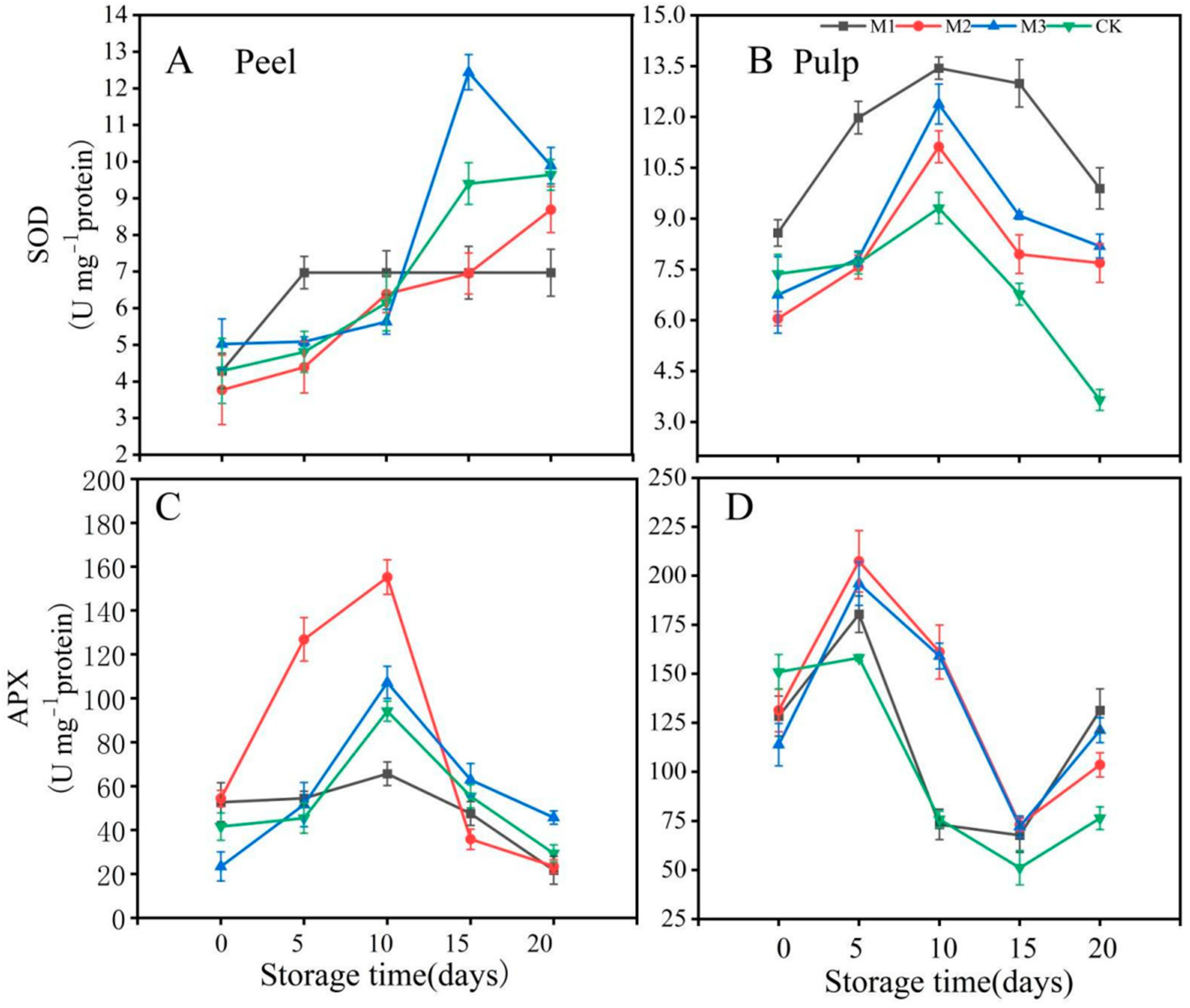
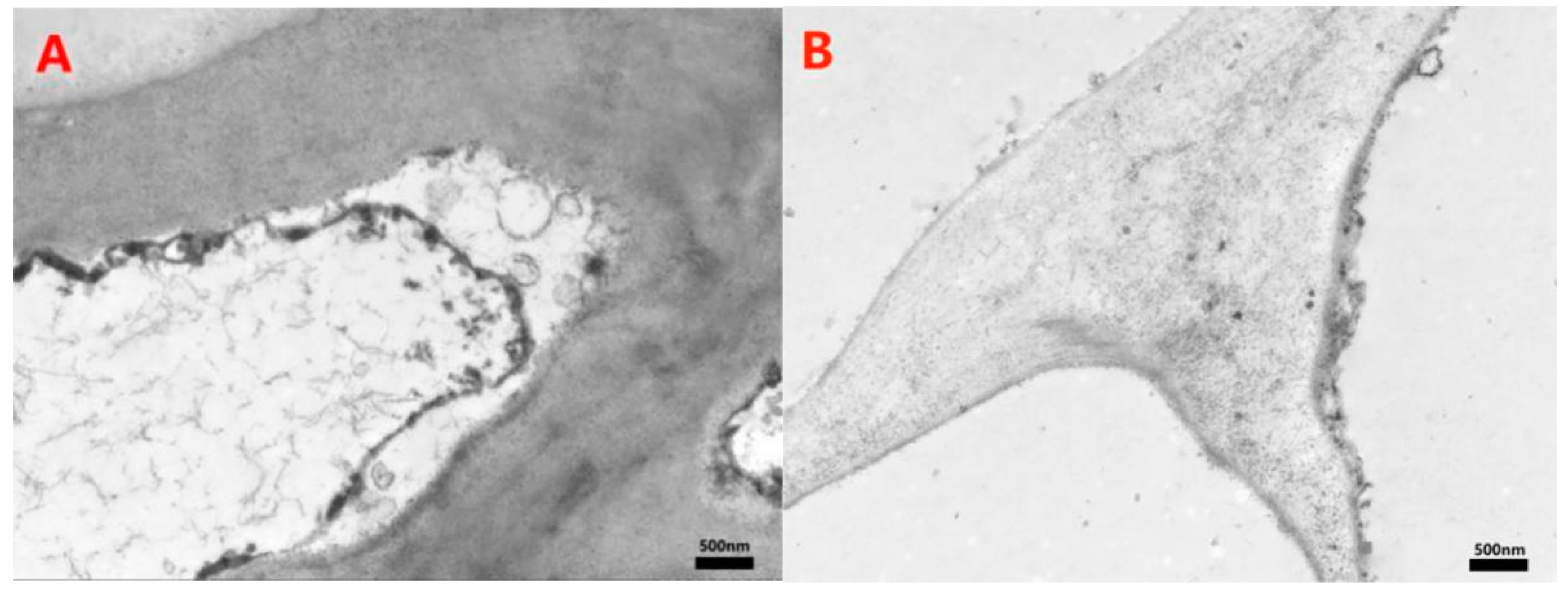

| Postharvest | Storage Time (Day) | ||||
|---|---|---|---|---|---|
| Quality | 0 | 5 | 10 | 15 | 20 |
| SSC (%) | |||||
| M1 | 21.43 ± 0.12 a | 21.87 ± 0.58 c | 21.93 ± 0.58 b | 22.50 ± 0.20 b | 23.70 ± 0.10 a |
| M2 | 21.23 ± 0.21 ab | 22.70 ± 0.10 a | 22.07 ± 0.58 ab | 23.20 ± 0.17 a | 23.43 ± 0.11 b |
| M3 | 21.10 ± 0.12 b | 22.30 ± 0.10 b | 22.37 ± 0.58 a | 22.40 ± 0.10 b | 23.07 ± 0.06 c |
| CK | 20.03 ± 0.06 c | 20.53 ± 0.11 d | 21.43 ± 0.32 c | 21.60 ± 0.10 c | 22.10 ± 0.10 d |
| TA (%) | |||||
| M1 | 0.59 ± 0.03 a | 0.43 ± 0.02 a | 0.36 ± 0.01 a | 0.34 ± 0.01 b | 0.29 ± 0.01 a |
| M2 | 0.54 ± 0.02 ab | 0.45 ± 0.07 a | 0.43 ± 0.01 a | 0.41 ± 0.04 a | 0.25 ± 0.01 a |
| M3 | 0.59 ± 0.05 a | 0.46 ± 0.06 a | 0.39 ± 0.02 a | 0.36 ± 0.04 ab | 0.33 ± 0.03 a |
| CK | 0.51 ± 0.01 b | 0.41 ± 0.01 a | 0.40 ± 0.02 a | 0.37 ± 0.03 ab | 0.30 ± 0.07 a |
| pH | |||||
| M1 | 3.37 ± 0.04 c | 3.43 ± 0.02 c | 3.56 ± 0.01 b | 3.58 ± 0.01 c | 3.75 ± 0.01 b |
| M2 | 3.43 ± 0.01 b | 3.46 ± 0.01 b | 3.52 ± 0.01 a | 3.55 ± 0.01 c | 3.76 ± 0.02 b |
| M3 | 3.48 ± 0.01 ab | 3.49 ± 0.01 a | 3.56 ± 0.01 b | 3.62 ± 0.02 b | 3.71 ± 0.01 c |
| CK | 3.49 ± 0.01 a | 3.46 ± 0.01 b | 3.60 ± 0.02 a | 3.75 ± 0.01 a | 3.83 ± 0.01 a |
| AsA (mg/kg) | |||||
| M1 | 4.75 ± 0.49 a | 12.29 ± 1.23 a | 13.92 ± 4.09 b | 15.91 ± 0.30 bc | 6.75 ± 0.12 a |
| M2 | 6.75 ± 0.59 a | 12.5 ± 0.90 a | 21.38 ± 1.04 a | 28.22 ± 8.18 a | 10.18 ± 0.10 a |
| M3 | 7.94 ± 0.67 a | 9.71 ± 2.27 a | 11.69 ± 2.76 b | 25.39 ± 5.09 ab | 11.06 ± 1.27 a |
| CK | 6.52 ± 0.10 a | 11.69 ± 0.79 a | 11.57 ± 1.57 b | 12.00 ± 3.29 c | 10.17 ± 1.74 a |
| Firmness (N) | |||||
| M1 | 3.93 ± 0.23 bc | 5.87 ± 0.32 b | 5.83 ± 0.32 a | 5.20 ± 0.20 a | 4.37 ± 0.15 a |
| M2 | 4.50 ± 0.35 a | 6.40 ± 0.10 a | 5.13 ± 0.21 b | 5.00 ± 0.17 a | 4.33 ± 0.32 a |
| M3 | 4.37 ± 0.15 ab | 6.20 ± 0.10 ab | 5.40 ± 0.17 ab | 4.33 ± 0.15 b | 3.70 ± 0.72 ab |
| CK | 3.80 ± 0.26 c | 5.07 ± 0.11 c | 5.60 ± 0.10 a | 4.10 ± 0.17 b | 3.17 ± 0.11 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, R.; Zhu, S.; Wu, L.; Li, X.; Zhang, H.; Yao, D.; Lv, Q.; Wang, F.; Zhao, F.; Li, P.; et al. Pre-Harvest Application of Multi-Walled Carbon Nanotubes Improves the Antioxidant Capacity of ‘Flame Seedless’ Grapes during Storage. Sustainability 2022, 14, 9568. https://doi.org/10.3390/su14159568
Sha R, Zhu S, Wu L, Li X, Zhang H, Yao D, Lv Q, Wang F, Zhao F, Li P, et al. Pre-Harvest Application of Multi-Walled Carbon Nanotubes Improves the Antioxidant Capacity of ‘Flame Seedless’ Grapes during Storage. Sustainability. 2022; 14(15):9568. https://doi.org/10.3390/su14159568
Chicago/Turabian StyleSha, Riye, Shuhua Zhu, Linnan Wu, Xujiao Li, Huanhuan Zhang, Dongdong Yao, Qi Lv, Fangxia Wang, Fengyun Zhao, Pengcheng Li, and et al. 2022. "Pre-Harvest Application of Multi-Walled Carbon Nanotubes Improves the Antioxidant Capacity of ‘Flame Seedless’ Grapes during Storage" Sustainability 14, no. 15: 9568. https://doi.org/10.3390/su14159568
APA StyleSha, R., Zhu, S., Wu, L., Li, X., Zhang, H., Yao, D., Lv, Q., Wang, F., Zhao, F., Li, P., & Yu, K. (2022). Pre-Harvest Application of Multi-Walled Carbon Nanotubes Improves the Antioxidant Capacity of ‘Flame Seedless’ Grapes during Storage. Sustainability, 14(15), 9568. https://doi.org/10.3390/su14159568







