1. Introduction
Scientists believe reducing greenhouse and pollutant gas emissions at industrial companies is one of the most important problems of the 21st century due to their negative impact on the environment [
1]. A major source of greenhouse gases and other pollutants in the atmosphere is the burning of co-products of gas- and petroleum-based products. The use of flares to burn these dangerous gaseous products improves safety at the plants, but it has a negative impact on the environment [
2]. Alongside heat and noise emissions which are normal consequences of flaring, it causes other problems which can be concluded as follows: (1) By burning low-quality gas in flares, many impurities and toxic particles and gases (such as CO and NO
x) are released into the atmosphere, which can be very harmful to human health, especially at high concentration. (2) The waste gases resulting from flares contain chemical substances such as SO
x, CO
2, and H
2S featured as weak acids which are corrosive in the presence of rainfall and result in acidic rain. Acidic rain inflicts damage to crops and buildings and can also cause problems for the ecosystem. (3) CO
2, which is one of the main products of gas burning in flares, is a major greenhouse gas, and its accumulation in the atmosphere is the main reason for the global warming phenomenon in recent years [
3].
Flare gas recovery units have many advantages, both from an economic and environmental standpoint such as enhancing production efficiency, reducing maintenance and operating costs, and decreasing noise and flare emissions. Recently, the environmental and economic regulations and policies have made the industrial parties implement Flare Gas Recovery Systems (FGRS) due to the fact that it provokes the reduction in burnt gas in flares [
4]. Nevertheless, after more than a decade of the implementation of FGRSs in developed nations, these systems are considered as a new method of waste management in industrial plants. By applying this new technology, the gases which are burnt in flares can be recovered to be used in other units of the plants. The compression section is the most important of the various parts of a flare gas recovery system. This compression section normally includes either a compressor or an ejector.
The design of a compressor for the compression section of an FGRS is influenced by several elements listed as archetypal such as initial cost and size and function such as process requirements, efficiency, operating, and maintenance requirements. In recent years many different types of compressors such as Dry Screw Compressors (DSCs), Sliding Vane Compressors (SVCs), Reciprocating Compressors (RCs), Liquid Ring Compressors (LRCs), and oil-injected compressors have been used in FGRSs. Generally, LRC or RC are the favorite types of compressors for use in an FGRS. An advantage that this type has over other kinds of compressors is that in the LRCs, the gas is cooled down during compression by losing its heat to a cooling fluid (usually water) inside the compressor [
5].
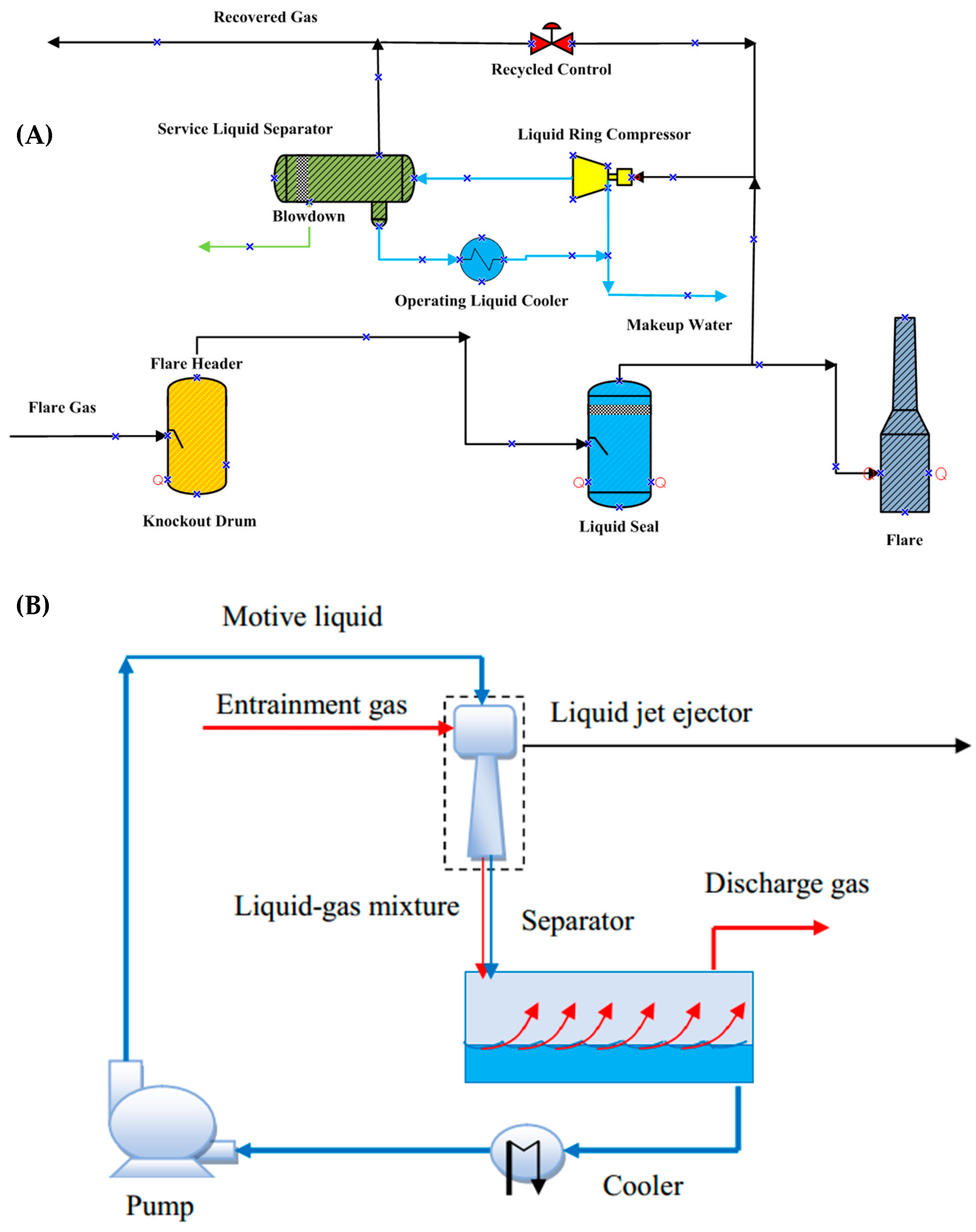
Figure 1A depicts a schematic of an FGRS with LRC. After collecting flare gas from the flare header into a knock-out drum, it is then directed towards the compressor. Afterward, the gas is compressed and discharged into a dual-phase separator in which more liquid is separated from it. The liquid is then pumped through a heat exchanger and is sent back to the compressor as the inlet service liquid. The compressed gas also exits in the separator drum to be sent to the plant fuel gas header or other parts of the plant [
5]. However, this system also has some disadvantages for example, compressors are expensive, and they have many rotating parts, which mean their maintenance and repair costs are high. They also produce a great deal of noise [
6].
Another design currently being used in the industry for flare gas recovery is to employ ejectors instead of compressors in the compression section. An ejector unit is a long-lasting device for pumping dirty or harmful gases due to its simplicity and lack of moving parts [
7]. A schematic design of an FGRS with an ejector is shown in
Figure 1B. In these systems, ejectors are used to increase the pressure of flare gases to up to 150 times at which they can be recovered. Ejectors are considered as equipment with high reliability and at the same time simple in order to compress fluids. The operation of this device is based on Bernoulli’s principle, which implies reduction in pressure when the speed of the motive fluid increases in the nozzle. This low-pressure section draws the entrained gas towards it. Although flare gas recovery systems using ejectors have simple designs, they have some issues too; for instance, they have a high degree of susceptibility to change in operating conditions and in order to compress a small amount of secondary fluid, a large amount of motive fluid is required.
Many studies have been performed so far to evaluate flare gas recovery from technical, environmental, and economic standpoints. Mousavi et al. investigated three main FGR methods to optimize the level of energy consumption and prevent pollution by exploiting the abilities of environmental flow diagrams as well as a package of simulation software. They found that when gas flare recovery methods are used, the amount of CO
2, CO, and NO
X in the furnaces, dehumidifier, and flare reduces by 100%, 100%, and about 57%, respectively [
8]. Studies by Ojijiagwo et al. found that gas to wire (GTW) technology can be economically viable as part of gas flare management. The investigation conducted a cost-benefit analysis of the GTW technology and its potential impact [
9]. An experimental study was conducted to determine the flare gas’s composition and flow rate by Comodi et al. [
6]. It was estimated how much energy can be recovered each year and an economic evaluation was performed. Khalili-Garakani et al. reviewed different flare gas recovery technologies to assess the potential of their implementation in the gas and petroleum industries in Iran. According to their findings, flare gas recovery systems can both reduce emissions and generate a significant profit [
10]. Asadi et el. investigated and optimized the performance of a novel flare gas recovery process in which compression and treatment of flare gas are carried out simultaneously [
11]. Exergy analysis attributes as a sound thermodynamic analysis technique which is evolved from the Second Law of Thermodynamics and also provides a rational and meaningful setting to evaluate and scrutinize processes and systems [
12]. Exergy analysis has been widely used in various processes such as sweet gas production [
13,
14,
15], Liquefied Natural Gas (LNG), and Natural Gas Liquids (NGL) production processes [
16,
17], Gas to Liquid (GTL) [
18], and power generation [
19,
20,
21].
This study proposes a new system for flare gas recovery in which energy consumption is at an optimum level. The novelty of this work lies in the utilization of both compressors and ejectors being used at the same time and parallel to each other for the purpose of gas compression. From the Abadan Oil Refining Company, we took the flare gas process data. The Aspen Hysys software was employed to simulate this novel system. After completing the simulation, an exergy analysis was applied to investigate the performance of all the equipment of the process from the perspective of energy conversion quality and deviation from the ideal condition. Exergy and exergoeconomic analyses were used to identify possible ways to optimize the system technically and economically. The parallel ejector-compressor system arrangement can increase the performance and profitability of the flare gas recovery system. From a technical point of view, refinery inlet natural gas has a limited, constant volume flow rate. Therefore, it is desirable to consume less natural gas. In this parallel ejector-compressor system (compared with ejector FGRS), the amount of flare gas, as the secondary fluid, that enters the ejector reduces significantly and as a result less natural gas, as the motive fluid, is required. Moreover, compared with compressor FGRS, the flare gas flow rate that enters the compressors in this parallel system remarkably decreases and therefore smaller (and less expensive) compressors with less noise are needed. This can help the system to save more energy efficiency and have more flexibility.
2. Process Description
The main equipment of the designed flare gas recovery system (FGRS) in this study includes compressors, ejectors, air coolers, and separators. The flare gas process data (flow rate, gas composition, and gas condition) was taken from the Abadan refinery. The process flow diagram of the designed FGRS is demonstrated in
Figure 2. In this Figure, stream number 1, the flare gas is divided into streams 1a and 1b by the TE-1 header. Flow 1b burns in the flare after passing through the drum and separation of its liquid depending on the operating conditions. The stream that enters the designed recovery system is current 1a and its flow rate stream is about 5000 kg/h. Liquids in stream 1a are separated by the D-2 flash tank, then stream 2a is sent to the compression system, which consists of multiple compressors and ejectors. After each compressor, an air cooler, AC, is installed which reduces the temperature of the compressed gas.
The only energy consuming devices in this system are compressors and air coolers. The first, second, and third compressors consume 297, 97, and 55 kW electrical energy, respectively. Moreover, the first, the second, and the third air coolers consume 32, 26, and 21 kW, respectively. The ejectors’ motive gas is natural gas injected into all three ejectors to create a vacuum region and entrain secondary gas streams. The first, the second, and the third air ejectors use 66,000, 132,000, and about 265,000 kg/h of natural gas, respectively. Air coolers are designed to reduce the compressors’ outlet temperatures to 40 °C. This decrease in temperature causes the flare gas to be compressed to the desired pressure in three stages. The temperature and pressure of stream 8 are 40 °C and 60 bar, respectively, which is suitable for use in gas turbine systems for power generation. Properties of all streams in this system including temperatures, pressures, entropies, enthalpies, and flow rates are presented in
Table 1. Simplicity and low energy requirement are important characteristics of this process.
3. Numerical Implementation
The thermodynamic analyses are founded on implementing a process model and executing simulation using common software packages. In this study, the simulation of the designed FGRS is performed by Aspen HYSYS [
22]. Every process simulation begins with selecting a suitable equation of state to simulate the process. A successful simulation relies heavily on choosing the right equation of state. The state equation of Peng–Robinson (PR) is frequently recommended for simulating systems containing oil and gas [
23,
24,
25,
26]. In this research, the Peng–Robinson (PR) equation was used for the simulation of FGRS. The Peng–Robinson (PR) equation was used for petrochemical, petroleum, and gas applications within a wide range of temperature and pressure values. It adequately describes both single phase and multi-phase systems. In addition, several cases and applications of flare gas recovery system similar to our case and within the range of applications of PR equation (T and P) were performed using the quoted equation, which leads us to say that the PR equation is the most appropriate one for our case. The main equations are provided below [
27]:
where
is molar volume. The Peng–Robinson constants are determined from the critical properties
,
as [
27]:
3.1. Exergy Analysis
The first and second laws of thermodynamics are recognized as robust toolkits in energy consumption processes that enable the exergy analysis in qualitative and quantitative considerations and assessments. Exergy analysis is a technique in thermodynamic analysis to determine the maximum useful work that can be accomplished with a given amount of input energy. In fact, exergy analysis discusses the most efficient and usable part to manage the effective part in the system’s performance. The efficiencies expressed around exergy analysis are very effective criteria for system improvement. In the current work, exergy analysis aims to ascertain the FGR system elements’ efficiency and compare it with each equipment’s ideal state. Streams with much higher exergy are much more expensive than energies with less exergy.
Table 2 presents the process’s descriptions of ejectors, air coolers, and compressors.
The state of the system and its environment are a constructive component of exergy. The system at the temperature of T0 and pressure P0 must exchange heat with the environment. This means that when a system has no difference in temperature, pressure, etc., with its surroundings, it does not have the power to go through the process. Accordingly, the dead state is defined as a system in balance with its surrounding environment, where the system is in balance in density, heat, and mechanics with its surroundings.
3.1.1. Exergy Efficiency
For each element, the ratio of ideal product exergy to consumed fuel exergy is the definition of the exergy efficiency. In each element, the product and fuel are chosen based on the desired outcome as well as the resources required to achieve that outcome. The major important parameters in exergy analysis are exergy efficiency and exergy destruction rate and ratio. The following is the equation for the exergy balance of a control volume under steady-state conditions [
12]:
where E
F,K is exergy rates of fuel and E
P,K and E
D,K are the exergy rates of product and destruction, respectively. As previously stated, an element’s exergy efficiency is defined as the product-to-fuel ratio. This means that both exergy efficiency and exergy destruction can be calculated simultaneously.
3.1.2. Physical Exergy
Physical exergy is equivalent to the theoretical work that the system reaches from the initial point (T and P) to the second point with the ambient state (T
0 and P
0) [
12]:
3.1.3. Chemical Exergy
When the flow of material from the ambient state reaches the dead state (T
0 and P
0), by a reversible process that includes only heat and mass transfer, and contraction and expansion, the amount of work obtained is equal to chemical exergy. According to what has been said, in order to obtain chemical exergy, in addition to the physical condition of the material stream (temperature and pressure), its chemical composition should also be determined. Chemical exergy for a flow is obtained from the following equation [
12]:
3.1.4. Irreversibility
Irreversibility is defined as the difference between reversible work and useful work. When the process is reversible, irreversibility is zero. Irreversibility indicates the amount of energy that can be transformed into work, but this has not happened. Irreversibility shows the degree of inefficiency of a system. To improve the performance of a complex system, the irreversibility associated with each device should be determined and then reduced [
12].
3.1.5. Exergy Balance
The exergy balance equation for a control volume is as follows [
12]:
In this equation, the difference between input and output exergy indicates the degree of irreversibility in the control volume. To evaluate the efficiency and to optimize the designed system, exergy efficiency and an optimal process to determine the cost of different equipment in the system are required.
Table A1 shows the equations for exergy efficiency and irreversibility for each device. Moreover, the thermodynamic and calculated exergy of process streams are presented in
Table 3.