Identification and Classification of the Dissolved Substances from Sludge Biochar and Their Effects on the Activity of Acid Phosphomonoesterase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Sludge Biomass and Biochar
2.2. Extraction and Characterization of Dissolved Substances
2.3. Exposure Testing Design
2.4. Statistical Analysis
3. Results and Discussion
3.1. Biochar Yield and Characterization for Particles and Dissolved Substances
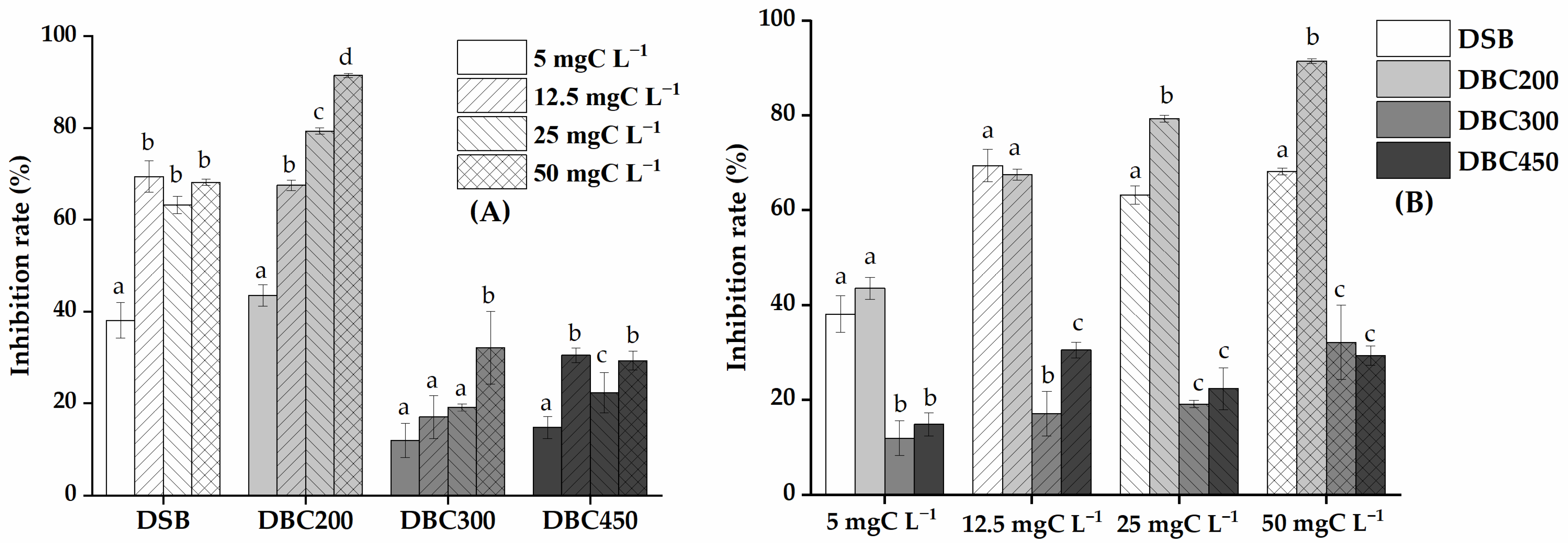
3.2. Activity of Acid Phosphomonoesterase as Affected by Dissolved Substances
3.3. Conformation of Acid Phosphomonoesterase as Affected by Dissolved Substances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Liu, B.; Zhang, W. Nutrients and heavy metals in biochar produced by sewage sludge pyrolysis: Its application in soil amendment. Pol. J. Environ. Stud. 2014, 23, 271–275. [Google Scholar] [CrossRef]
- Eurostat. Sewage Sludge Production and Disposal. Available online: http://epp.eurostat.ec.europa.eu (accessed on 28 May 2021).
- Mierzwa-Hersztek, M.; Gondek, K.; Baran, A. Effect of poultry litter biochar on soil enzymatic activity, ecotoxicity and plant growth. Appl. Soil Ecol. 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhai, Y.; Li, C.; Yang, F.; Chen, L.; Fan, X.; Peng, W.; Fu, Z. The fate of Cu, Zn, Pb and Cd during the pyrolysis of sewage sludge at different temperatures. Environ. Technol. 2010, 31, 567–574. [Google Scholar] [CrossRef]
- Lai, F.; Chang, Y.; Huang, H.; Wu, G.; Xiong, J.; Pan, Z.; Zhou, C. Liquefaction of sewage sludge in ethanol-water mixed solvents for bio-oil and biochar products. Energy 2018, 148, 629–641. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Influence of biochar on potential enzyme activities in two calcareous soils of contrasting texture. Geoderma 2012, 308, 149–158. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Baran, A.; Szostek, M.; Pieniążek, R.; Pieniążek, M.; Stanek-Tarkowska, J.; Noga, T. The Effect of low-temperature conversion of plant materials on the chemical composition and ecotoxicity of biochars. Waste Biomass Valorization 2017, 8, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Gu, J. Alteration of extracellular enzyme activity and microbial abundance by biochar addition: Implication for carbon sequestration in subtropical mangrove sediment. J. Environ. Manag. 2016, 182, 29–36. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Chmiel, M.J.; Dziedzic, K.; Taras, H. Assessment of soil quality after biochar application based on enzymatic activity and microbial composition. Int. Agrophys. 2019, 33, 331–336. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Yan, H.; Wang, N.; Weinfeld, M.; Cullen, W.R.; Le, X.C. Identification of arsenic-binding proteins in human cells by affinity chromatography and mass spectrometry. Anal. Chem. 2009, 81, 4144–4152. [Google Scholar] [CrossRef]
- Tan, W.F.; Koopal, L.K.; Norde, W. Interaction between humic acid and lysozyme, studied by dynamic light scattering and isothermal titration calorimetry. Environ. Sci. Technol. 2009, 43, 591. [Google Scholar] [CrossRef]
- Waters, C.L.; Janupala, R.R.; Mallinson, R.G.; Lobban, L.L. Staged thermal fractionation for segregation of lignin and cellulose pyrolysis products: An experimental study of residence time and temperature effects. J. Anal. Appl. Pyrolysis 2017, 126, 380–389. [Google Scholar] [CrossRef]
- Kusudo, T.; Sakaki, T.; Inouye, K. Purification and characterization of purple acid phosphatase pap1 from dry powder of sweet potato. Biosci. Biotechnol. Biochem. 2003, 67, 1609–1611. [Google Scholar] [CrossRef]
- Bhadouria, J.; Singh, A.P.; Mehra, P.; Verma, L.; Srivastawa, R.; Parida, S.K.; Giri, J. Identification of Purple Acid Phosphatases in Chickpea and Potential Roles of CaPAP7 in Seed Phytate Accumulation. Sci. Rep. 2017, 7, 11012. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dai, Q.; Jin, X.; Dong, X.; Peng, J.; Wu, M.; Liang, N.; Pan, B.; Xing, B. Negative Impacts of Biochars on Urease Activity: High pH, Heavy Metals, Polycyclic Aromatic Hydrocarbons, or Free Radicals? Environ. Sci. Technol. 2018, 52, 12740–12747. [Google Scholar] [CrossRef]
- He, C.; He, X.; Li, J.; Luo, Y.; Li, J.; Pei, Y.; Jiang, J. The spectral characteristics of biochar-derived dissolved organic matter at different pyrolysis temperatures. J. Environ. Chem. Eng. 2021, 9, 106075. [Google Scholar] [CrossRef]
- Beneireh, N.M.; Boakye, P.; Oduro-Kwarteng, S.; Sokama-Neuyam, Y.A. Valorization of faecal and sewage sludge via pyrolysis for application as crop organic fertilizer. J. Anal. Appl. Pyrolysis 2020, 151, 104903. [Google Scholar] [CrossRef]
- Song, X.D.; Xue, X.Y.; Chen, D.Z.; He, P.J.; Dai, X.H. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220. [Google Scholar] [CrossRef]
- Campisano, R.; Hall, K.; Griggs, J.; Willison, S.; Reimer, S.; Mash, H.; Magnuson, M.; Boczek, L.; Rhodes, E. Selected Analytical Methods for Environmental Remediation and Recovery (SAM); Environmental Protection Agency: Washington, DC, USA, 2017. [Google Scholar]
- Environmental Protection Agency. Method 8310: Polynuclear Aromatic Hydrocarbons; Office of Solid Waste and Emergency Response: Washington, DC, USA, 1986. [Google Scholar]
- Rajapaksha, A.U.; Ok, Y.S.; El-Naggar, A.; Kim, H.; Song, F.H.; Kang, S.; Tsang, Y.F. Dissolved organic matter characterization of biochars produced from different feedstock materials. J. Environ. Manag. 2019, 233, 393–399. [Google Scholar] [CrossRef]
- Li, M.; Zhang, A.; Wu, H.; Liu, H.; Lv, J. Predicting potential release of dissolved organic matter from biochars derived from agricultural residues using fluorescence and ultraviolet absorbance. J. Hazard. Mater. 2017, 334, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Schenk, G.; Carrington, L.E.; Gahan, L.R.; Hamilton, S.E.; de Jersey, J.; Guddat, L.W. Sweet Potato Purple Acid Phosphatase/Phosphate Complex. Worldwide Protein Data Bank 2020. Available online: https://www.uniprot.org/uniprot/Q9SE00 (accessed on 19 August 2021).
- Sander, M.; Tomaszewski, J.E.; Madliger, M.; Schwarzenbach, R.P. Adsorption of Insecticidal Cry1Ab Protein to Humic Substances. 1. Experimental Approach and Mechanistic Aspects. Environ. Sci. Technol. 2012, 46, 9923–9931. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Bulyáki, É.; Kun, J.; Moussong, É.; Lee, Y.; Goto, Y.; Réfrégiers, M.; Kardos, J. BeStSel: A web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res. 2018, 46, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Qu, W.; Yan, Y.; Ma, K. Influence of pyrolysis temperature on the properties and environmental safety of heavy metals in chicken manure-derived biochars. J. Environ. Sci. Health Part B 2020, 55, 941–950. [Google Scholar] [CrossRef]
- Li, B.; Ding, S.; Fan, H.; Ren, Y. Experimental investigation into the effect of pyrolysis on chemical forms of heavy metals in sewage sludge biochar (ssb), with brief ecological risk assessment. Materials 2021, 14, 447. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, X.; Adeosun, A.; Ruan, R.; Tan, H. Aggravated fine particulate matter emissions from heating-upgraded biomass and biochar combustion: The effect of pretreatment temperature. Fuel Process. Technol. 2018, 171, 1–9. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Wachenfeldt, E.V.; Koehler, B.; Tranvik, L.J. Selective loss and preservation of lake water dissolved organic matter fluorescence during long-term dark incubations. Sci. Total Environ. 2012, 433, 238–246. [Google Scholar] [CrossRef]
- Lin, H.; Guo, L. Variations in colloidal DOM composition with molecular weight within individual water samples as characterized by flow field-flow fractionation and EEM-PARAFAC analysis. Environ. Sci. Technol. 2020, 54, 1657–1667. [Google Scholar] [CrossRef]
- Perminova, I.V.; Frimmel, F.H.; Kudryavtsev, A.V.; Kulikova, N.A.; Abbt-Braun, G.; Hesse, S.; Petrosyan, V.S. Molecular weight characteristics of humic substances from different environments as determined by Size Exclusion chromatography and their statistical evaluation. Environ. Sci. Technol. 2003, 37, 2477–2485. [Google Scholar] [CrossRef]
- Lapierre, J.F.; Frenette, J.J. Effects of macrophytes and terrestrial inputs on fluorescent dissolved organic matter in a large river system. Aquat. Sci. 2009, 71, 15–24. [Google Scholar] [CrossRef]
- Cory, R.M.; Mcknight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in DOM. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Chen, M.; Price, R.M.; Yamashita, Y.; Jaffé, R. Comparative study of dissolved organic matter from groundwater and surface water in the Florida coastal Everglades using multi-dimensional spectrofluorometry combined with multivariate statistics. Appl. Geochem. 2010, 25, 872–880. [Google Scholar] [CrossRef]
- Yamashita, Y.; Maie, N.; Briceno, H.; Jaffe, R. Optical characterization of dissolved organic matter in tropical rivers of the Guayana Shield, Venezuela. J. Geophys. Res. 2010, 115, G00F10. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Cai, Z.; Chen, Z.; Mo, X.; Tian, J. A root-associated purple acid phosphatase, sgpap23, mediates extracellular phytate-p utilization in stylosanthes guianensis. Plant Cell Environ. 2018, 41, 2821–2834. [Google Scholar] [CrossRef]
- Juma, N.G.; Tabatabai, M.A. Effects of trace elements on phosphatase activity in soils. Soil Sci. Soc. Am. J. 1977, 41, 343–346. [Google Scholar] [CrossRef]
- Speir, T.W.; Kettles, H.A.; Parshotam, A.; Searle, P.L.; Vlaar, L.N.C. Simple kinetic approach to determine the toxicity of As[V] to soil biological properties. Soil Biol. Biochem. 1999, 31, 705–713. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Pan, B.; He, Y.; Li, B.; Zhou, D.; Xiao, Y.; Qiu, H.; Vijver, M.G.; Peijnenburg, W.J.G.M. The promoted dissolution of copper oxide nanoparticles by dissolved humic acid: Copper complexation over particle dispersion. Chemosphere 2020, 245, 125612. [Google Scholar] [CrossRef]
- Gagner, J.E.; Lopez, M.D.; Dordick, J.S.; Siegel, R.W. Effect of gold nanoparticle morphology on adsorbed protein structure and function. Biomaterials 2011, 32, 7241–7252. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, B.; Li, H.; Lang, D.; Zhao, Q.; Zhang, D.; Wu, M.; Steinberg, C.E.W.; Xing, B. Can the properties of engineered nanoparticles be indicative of their functions and effects in plants? Ecotoxicol. Environ. Saf. 2020, 205, 111128. [Google Scholar] [CrossRef]
- Jayaraman, S.; Gantz, D.L.; Haupt, C.; Gursky, O. Serum amyloid A forms stable oligomers that disrupt vesicles at lysosomal pH and contribute to the pathogenesis of reactive amyloidosis. Proc. Natl. Acad. Sci. USA 2017, 114, E6507–E6515. [Google Scholar] [CrossRef] [Green Version]

| Properties | SB | BC 200 | BC 300 | BC 450 |
|---|---|---|---|---|
| Biochar yield (%) | - | 88.7 ± 3.8 | 78.9 ± 2.1 | 70.5 ± 2.4 |
| pH | 8.5 ± 0.4 | 8.0 ± 0.3 | 8.3 ± 0.2 | 9.2 ± 0.3 |
| Specific surface area (m2 g−1) | 8.7 | 6.0 | 15.6 | 35.0 |
| C (%) | - | 26.4 | 23.1 | 22.0 |
| H (%) | - | 3.4 | 2.7 | 1.7 |
| O (%) | - | 26.0 | 24.4 | 23.2 |
| N (%) | - | 2.0 | 1.8 | 1.2 |
| S (%) | - | 0.47 | 0.51 | 0.62 |
| H/C | - | 1.6 | 1.4 | 0.8 |
| O/C | - | 0.80 | 0.79 | 0.80 |
| (O+N)/C | - | 0.87 | 0.86 | 0.85 |
| Mn (mg kg−1) | - | 697.4 ± 32.8 | 752.1 ± 36.6 | 925.7 ± 44.2 |
| Cr (mg kg−1) | - | 341.3 ± 16.1 | 391.0 ± 18.3 | 416.7 ± 20.1 |
| Fe (mg kg−1) | - | 6515.3 ± 225.7 | 16,241.4 ± 349.3 | 23,123.1 ± 756.1 |
| Cu (mg kg−1) | - | 269.4 ± 12.4 | 206.8 ± 9.34 | 295.5 ± 12.7 |
| Zn (mg kg−1) | - | 6402.9 ± 220.1 | 15,884.5 ± 594.2 | 13,267.0 ± 463.3 |
| Ni (mg kg−1) | - | 21.9 ± 0.9 | 89.9 ± 3.49 | 36.6 ± 1.2 |
| As (mg kg−1) | - | 11.5 ± 0.3 | 25.3 ± 0.8 | 41.4 ± 1.3 |
| Cd (mg kg−1) | - | 2.0 ± 0.1 | 3.7 ± 0.1 | 4.2 ± 0.1 |
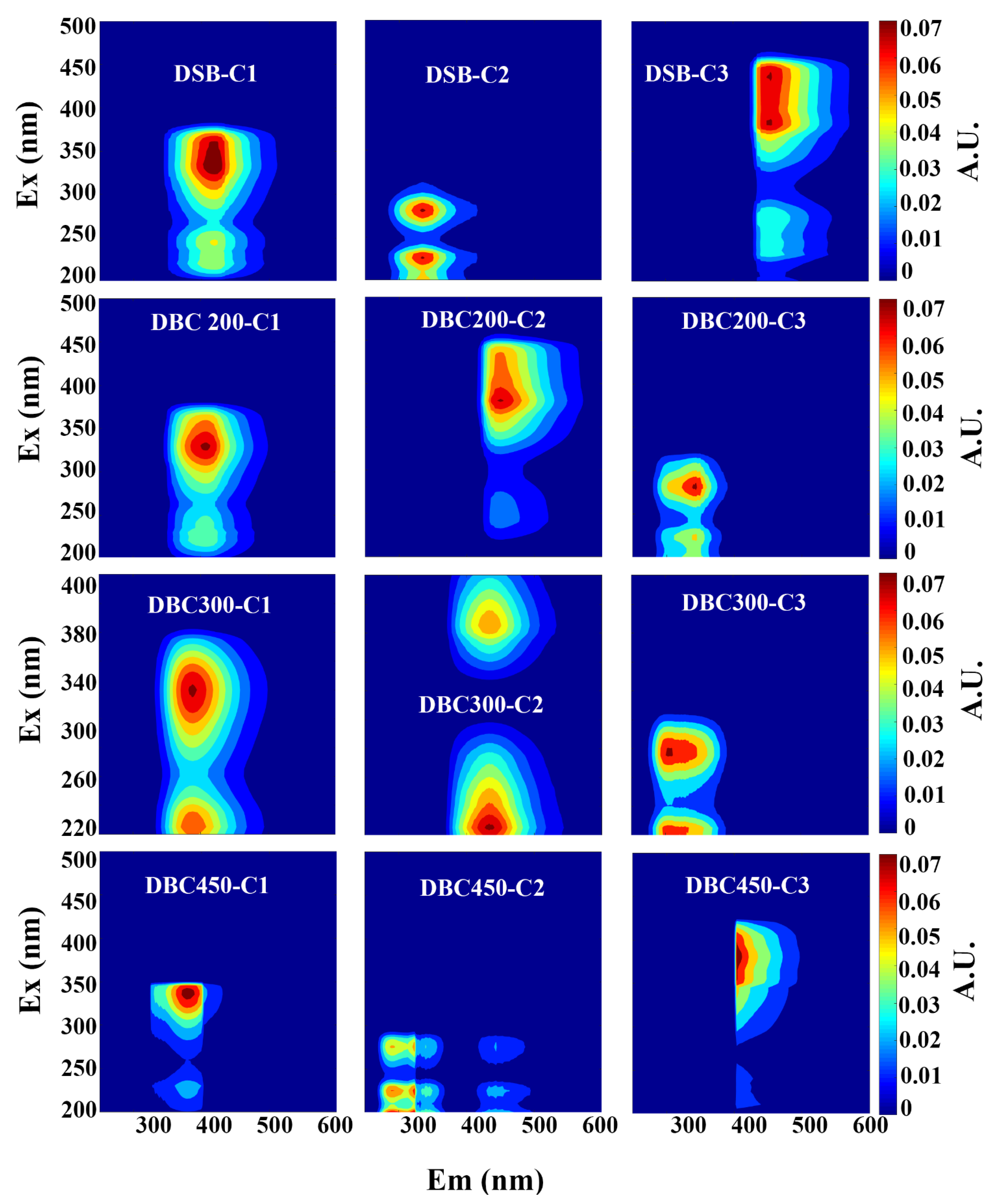
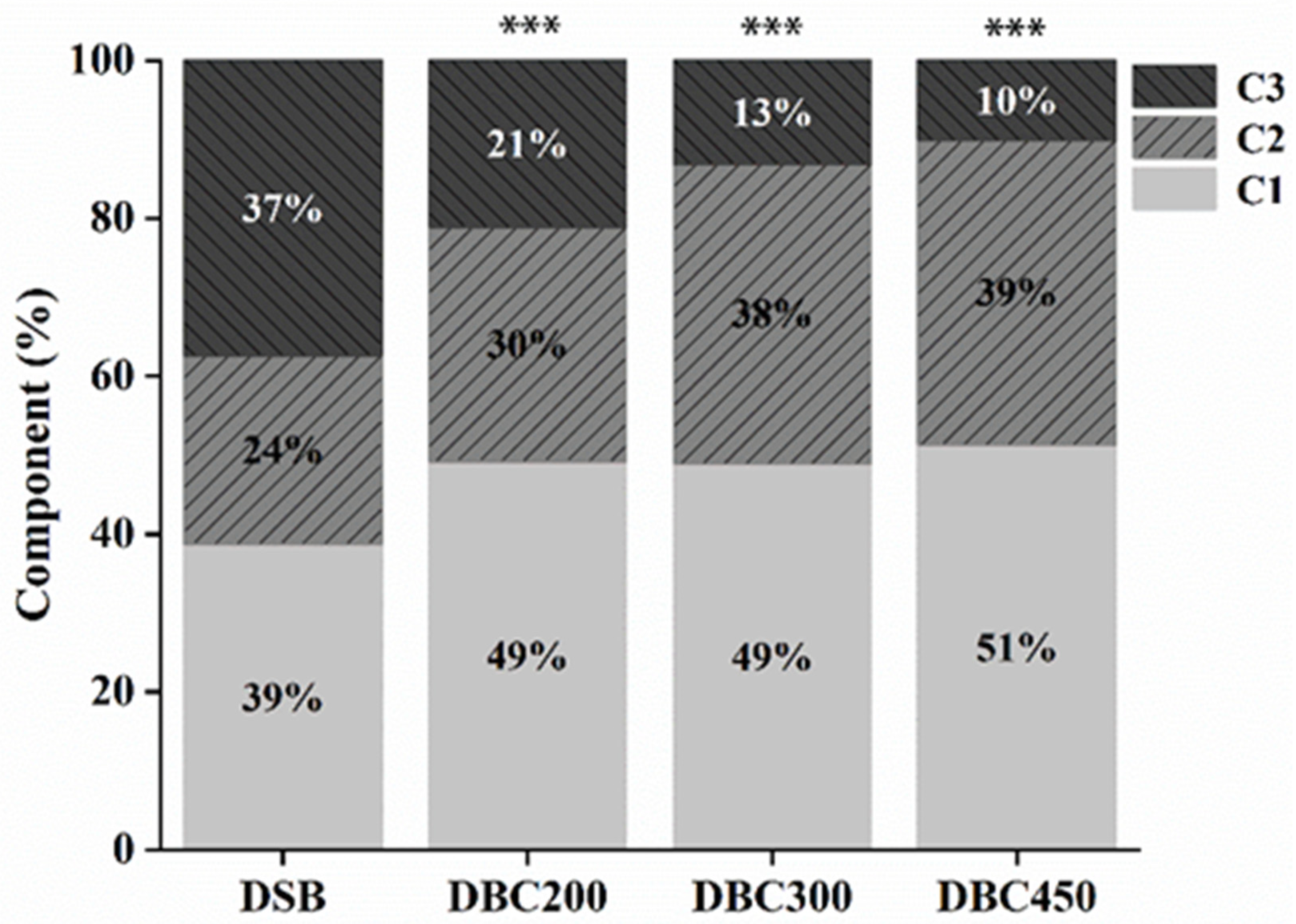
| Component | Ex (nm) | Em (nm) | Fluorescent Compound | References |
|---|---|---|---|---|
| DSB | ||||
| C1 | 250/335 | 410 | Humic-like | [34] |
| C2 | 240/405 | 470 | Humic-like | [31] |
| C3 | 225/280 | 336 | Protein-like (tyrosine) | [35] |
| DBC200 | ||||
| C1 | 220/335 | 410 | Humic-like | [36] |
| C2 | 250/370 | 460 | Humic-like | [34] |
| C3 | 225/285 | 350 | Protein-like (tryptophan) | [37] |
| DBC300 | ||||
| C1 | 225/320 | 390 | Humic-like (aliphatic carbon) | [36] |
| C2 | 225/365 | 438 | Humic-like (fulvic acid) | [38] |
| C3 | 225/280 | 304 | Protein-like (tyrosine) | [23] |
| DBC450 | ||||
| C1 | 225/320 | 390 | Humic-like | [36] |
| C2 | 220/365 | 402 | Humic-like (fulvic acid) | [38] |
| C3 | 225/280 | 310 | Protein-like (tryptophan) | [39] |
| Estimated Secondary Structure Content (%) | Enzyme | DSB | DBC200 | DBC300 | DBC450 |
|---|---|---|---|---|---|
| -Helix | 13.9 | 0.0 | 0.0 | 0.0 | 0.6 |
| -Sheet | 28.1 | 30.9 | 31.7 | 32.1 | 33.0 |
| -Turn | 5.2 | 13.1 | 13.7 | 13.8 | 11.6 |
| 52.8 | 56.0 | 54.5 | 54.1 | 54.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Y.; Li, B.; Tan, C.; Zhou, D.; Vijver, M.G.; Peijnenburg, W.J.G.M. Identification and Classification of the Dissolved Substances from Sludge Biochar and Their Effects on the Activity of Acid Phosphomonoesterase. Sustainability 2022, 14, 9749. https://doi.org/10.3390/su14159749
Zhang J, Liu Y, Li B, Tan C, Zhou D, Vijver MG, Peijnenburg WJGM. Identification and Classification of the Dissolved Substances from Sludge Biochar and Their Effects on the Activity of Acid Phosphomonoesterase. Sustainability. 2022; 14(15):9749. https://doi.org/10.3390/su14159749
Chicago/Turabian StyleZhang, Junyuan, Yang Liu, Bowen Li, Chunling Tan, Dandan Zhou, Martina G. Vijver, and Willie J. G. M. Peijnenburg. 2022. "Identification and Classification of the Dissolved Substances from Sludge Biochar and Their Effects on the Activity of Acid Phosphomonoesterase" Sustainability 14, no. 15: 9749. https://doi.org/10.3390/su14159749
APA StyleZhang, J., Liu, Y., Li, B., Tan, C., Zhou, D., Vijver, M. G., & Peijnenburg, W. J. G. M. (2022). Identification and Classification of the Dissolved Substances from Sludge Biochar and Their Effects on the Activity of Acid Phosphomonoesterase. Sustainability, 14(15), 9749. https://doi.org/10.3390/su14159749






