Abstract
Salt stress is one of the main abiotic factors affecting castor yield. Wild castor resources can provide important insights for cultivated castor breeding. However, little is known about how wild castor responds or adapts to salt stress. To understand the physiological mechanisms for salt tolerance in castor, the morphological and physiological responses of two varieties, wild and cultivated castor, with contrasted salt tolerance were characterized under salt stress. Seedlings were exposed to 0, 50, and 100 mM NaCl. The results showed that salt application significantly inhibited the increase in chlorophyll content and relative water content of cultivated castor. The degree of electrolyte leakage of wild castor under salt stress was significantly less than that of cultivated castor. In addition, the WT showed a lower content of reactive oxygen species (ROS) under the salt stress compared to CT. The activities of antioxidant enzymes like SOD, APX, GR, and MDHAR in the leaves of WT showed higher accumulation compared to those of CT under salt stress. The ratio of ASA/DHA and GSH/GSSG in leaves of WT showed a distinct increase compared to CT. In summary, our results revealed the salt stress resistance characteristics of wild castor. Wild castor also has the potential to be used as parental material in a breeding program. These results will be valuable for salt resistance breeding of cultivated castor.
1. Introduction
Castor (Ricinus communis L.) is an annual and perennial crop. As one of ten oil-bearing crops, it is cultivated around the world [1,2]. Additionally, it is also one of the three worldwide drought-tolerant crops (castor, safflower, chickpea) [3]. Due to the high oil content (45–55%) in its seed, castor has a high economic value as an alternative and effective biofuel [4,5,6]. In addition, castor has a relatively high tolerance for environmental stresses such as high temperature, drought, and salt [7,8,9].
In recent years, extensive studies on the molecular basis of salt tolerance and soil amelioration have been performed. The effects of some exogenous additives such as calcium ion and gibberellic acid, as well as some fungi and some transferred genes, on the salt-tolerance of cultivated castor have also been evaluated [10,11,12,13,14]. Wild castor growing along roadsides accumulates significant amounts of heavy metals and could be used to contribute to the containment of pollution along roadsides and other polluted areas [15]. Strategies for the improvement of salt-tolerance in castor, which is considered good research material for the stress-tolerance mechanism of the plant as well as an effective alternative crop for improvement of salt-alkali and uncultivated soil, have been studied extensively [12,16]. However, although the salt-tolerance of cultivated castor has been widely studied, the investigation on wild castor varieties still remains insufficient, particularly in terms of salt-tolerance.
Salt stress has become a serious environmental problem that can affect the development, growth, and productivity of plants [7]. To date, about 20% of land area worldwide has been affected by salinity as a major agricultural problem [17]; this percentage is predicted to reach about 50% of arable land in 2050 [18]. In the future, the frequency of salt stress will increase. Generally, salt stress can decrease the photosynthesis rate, interrupt ion imbalance of cells, and limit the yield of crop production [19]; moreover, salt stress can also abolish the balance of osmotic homeostasis and antioxidant systems in plants [20]. As the result, this imbalance will increase the content of reactive oxygen species (ROS) including superoxide anions (O2−) and hydrogen peroxide (H2O2) [21]. To cope with the adverse effects of salt stress, plants have developed some adaptive mechanisms, such as regulation of growth, osmotic adjustment, and the employment of various enzymatic antioxidants or non-enzymatic antioxidants [22].
In addition, to alleviate the adverse effects of salt, some varieties of salt-tolerant castor have been developed. Many studies have reported that wild plant varieties have stronger tolerance or adaptability to various environmental stresses, such as cold, salinity, and high temperature, in comparison to their relative cultivated species [23,24,25,26]. Wild castor varieties are potentially important for improving cultivated castor seed and oil quality [27,28]; therefore, these wild castor resources will be very valuable sources of potential use in castor breeding programs [29]. However, cultivated and wild castor have different growth characteristics, including adaptability and tolerance to various stresses [26], so investigation and research on salt-tolerant crops are vitally significant for the future development of agriculture and environmental management. Accordingly, to stabilize productivity, we should improve plant performance under salt stress. In this study, we evaluated some physiological characteristics of cultivated and wild castor under salt stress.
In this study, the morphological and physiological characteristics in the leaves of two castor varieties with contrasting salt tolerance were investigated under salt stress. Our results revealed the changes in stress biomarkers and gas exchange parameters in the two genotypes and their response to salt stress, which provided insight into the breeding and molecular mechanism underlying salt tolerance in castor.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The experiment was conducted in the greenhouse of the Institute of Life Sciences, Northeast Forestry University, China. Two castor varieties, including Tongbi No. 5 (Cultivated castor, CT), and wild castor varieties (WT) from Guangdong were used as experimental materials. We chose to test plantlets to verify whether castor may tolerate salinity and develop normally despite the salinity, even in very juvenile stages. Two castor varieties were grown in the greenhouse until three true leaves emerged [30,31]. The seedlings were transferred to a culture bottle after washing away soil dust with water. Seedlings were then treated with 0 mM NaCl (as a control), 50 mM NaCl, and 100 mM NaCl for 7 days. The solution was replaced once every 3 days. A week later, the leaves were collected to measure determination of the relative water content, electrolyte leakage, gas exchange parameters, chlorophyll content, ROS, and activity of antioxidant enzymes and non-enzymatic antioxidants. Five biological replicates were used for their photosynthesis and three biological replicates were used for physiology parameters. During the experiment, the plants were randomly divided into 3 groups with 10 plants in each group; three leaves of three plants in the treatment group and control group were randomly selected before each measurement.
2.2. Observation of Physiological and Biochemical Parameters
Leaf relative water content (RWC) was determined according to the Yildirim method [32]. The fresh weight (FW) of leaves was measured before they were immersed in distilled water for 3 h and the total weight (TW) was measured. Finally, the leaves were dried a 65 °C for 48 h and then their dry weight (DW) was measured. RWC was calculated using the following formula: RWC (%) = (FW − DW)/(TW − DW) × 100. (Three replications per treatment, with one leaf per plant).
Determination of electrolyte leakage was made using the Han method [33]. We cut 0.1 g fresh leaves into equal sizes with scissors and then put them into a test tube with 5 mL distilled water. The initial conductivity (E0) was measured using a DDS-307 type conductivity meter (Iinesa, Co., Ltd., Shanghai, China). The mixed solution was shaken for 1 h at 180 rpm, and the conductivity E1 was measured. Finally, the mixed solution was boiled for 15 min and then the conductivity E2 was measured. Electrolyte leakage was calculated using the following formula: electrolyte leakage conductivity (μS/cm) = E1 − E0/E2 − E0. (Three replications per treatment, with one leaf per plant.)
The gas exchange parameters were measured from 09:00 to 11:30 in the morning [34]. Measurements were repeated for six independent biological experiments for each treatment. Leaf transpiration rate (Tr), net photosynthesis rate (Pn), intercellular CO2 concentration (Ci), and stoma conductivity (Gs) were measured using the Li-6400 photosynthesis measurement system (Li-Cor, Lincoln, NE, USA). (Five replications per treatment, with one leaf per plant.)
The chlorophyll content of leaves was measured by the Agrawal method [35]. We added 4 mL of 80% acetone solution to 0.2 g fresh leaves, which were thoroughly smashed and centrifuged at 10,000 rpm for 10 min. The supernatant was collected and the absorbance was measured at 470 nm, 645 nm, and 663 nm using UV-1800 UV Spectrophotometer (Shimadzu, Japan). (Three replications per treatment, with one leaf per plant.)
2.3. Detection of ROS
Detection of ROS was determined on three leaves per treatment. The histochemical analyses of H2O2 and (O2−) in leaves were visualized by staining with 3,3-diaminobenzidine (DAB) and nitro blue tetrazolium chloride (NBT), respectively. The leaves were immersed in NBT solution (0.5 mg·mL−1) and DAB solution (2 mg·mL−1) overnight and bleached in a boiling water bath with bleaching solution for 10 min before the leaves were photographed [36,37].
The measurement of the H2O2 content was carried out according to the Velikova method [38]. We homogenized 0.5 g fresh leaves in 4 mL phosphate buffer saline (PBS, pH 7.8). The mixtures were centrifuged at 6000 rpm for 10 min and absorbance of the supernatant was measured at 390 nm.
Determination of O2− was performed according to the Cavalcanti method [39]. We ground 0.5 g fresh leaves to fine powder and mixed in a solution containing 0.1 mM ethylenediaminetetraacetic acid (EDTA), 1% PVP, and 0.2% Triton X-100. The mixture was centrifuged at 10,000 rpm for 15 min at 4 °C and the absorbance of the supernatant was measured at 530 nm.
2.4. Determination of the Activity of Antioxidant Enzymes and Non-Enzymatic Antioxidants
The determination of the Activity of Antioxidant Enzymes and Non-enzymatic Antioxidants was determined on three leaves per treatment. Leaves of seedlings (0.25 g fresh weight) were ground to a fine powder using liquid nitrogen and mixed with 50 mM phosphate buffer (4 mL, pH 7.5). Then, mixtures were centrifuged at 13,000 rpm for 10 min, and the supernatant was used for the quantification of four enzymes, including superoxide dismutase (SOD), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), and glutathione reductase (GR). Among these enzymes, SOD was evaluated by the Beauchamp method [40]. APX was determined by the Nazar method [34]. DHAR was assayed by the Dalton Method [41]. GR activity was determined according to the Spodary Method [42].
The determination of total ascorbic acid (AsA + DHA) was performed by the Kampfenkel method [43]. The ASA amount was measured by absorbance at 525 nm; acccordingly, total ascorbate acid was also determined. DHA content was calculated based on the difference between total ascorbate acid and AsA. Total glutathione (GSH + GSSG) was assayed according to the Edwards method [44]. GSH concentration was measured at 412 nm; accordingly, total glutathione was also obtained. GSSG content was obtained according to the difference between total glutathione and GSH.
2.5. Statistical Analysis
All parameters were performed using statistical software SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Homogeneity of variance was used to check the data distribution before running an analysis of variance (ANOVA). ANOVAs followed by Duncan’s post hoc tests were used to test the statistical significance of differences (p < 0.05) between the different groups.
3. Results
3.1. Observation on the Phenotypes and Determination of the Physiological and Biochemical Parameters in Leaves of Wild and Cultivated Castor
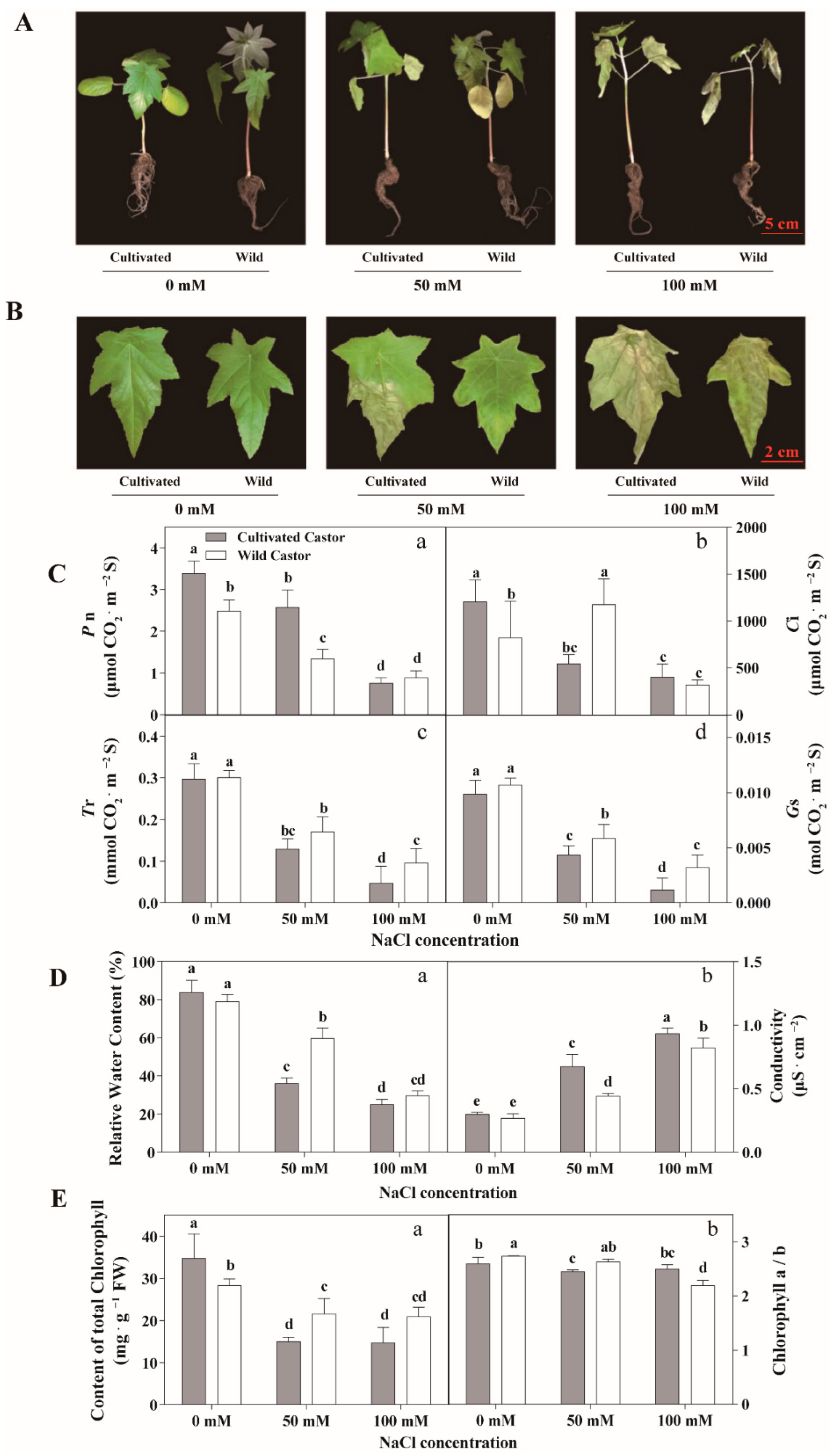
Results are summarized in Figure 1. When castor seedlings were grown under NaCl conditions (50 mM and 100 mM), the two varieties showed different phenotypes. WT showed relatively normal growth, whereas CT appeared to wilt and lose water (Figure 1A,B). Under high NaCl concentration (100 mM), CT wilted severely and relative water content decreased particularly (Figure 1A,B). At this time, although WT showed slow growth, CT had almost stopped growing (Figure 1A).
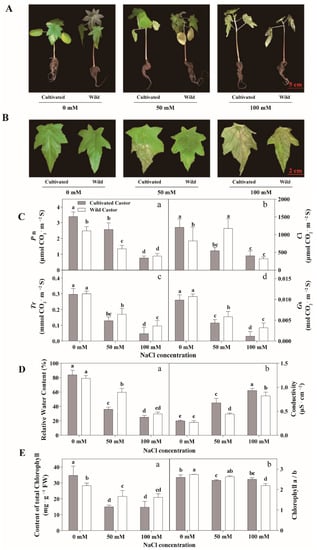
Figure 1.
Phenotypes of seedlings (A), phenotypes of leaves (B), gas exchange of leaves (C), relative water content and conductivity of leaves (D), and chlorophyll content of leaves (E) in three true leaves from cultivated and wild castor with or without NaCl treatment (0 mM, 50 mM and 100 mM NaCl). (C): a, Pn (Photosynthesis Rate) value; b, Ci (Internal CO2 Concentration) value; c, Tr (Transpiration Rate) value; d, Gs (Stomatal Conductance) value. (D): a, relative water content; b, conductivity. (E): a, content of total chlorophyll; b, chlorophyll a/b ratio. Values are means ± SD based on three biological experiments. Different lowercase letters mean significance of differences (p < 0.05) between the different groups.
In control conditions, CT had higher Pn value (Figure 1C). After exposure to 50 mM NaCl stress, a lower Pn value was observed in WT, while an increase in Pn value was found in WT under 100 mM NaCl. In the control condition, the values of Tr and Gs were similar for the two varieties, but higher values of Tr and Gs were detected in WT after NaCl treatment (Figure 1B). When exposed to NaCl stress, both varieties exhibited a reduction in Pn, while WT had higher Pn than CT under 100 mM NaCl (Figure 1C).
Under no salt treatment, there was no significant difference in relative water content and electrolyte leakage of leaves was observed between CT and WT. However, when subjected to NaCl stress, WT had a higher relative water content compared to CT. Under control conditions, the two varieties appeared to produce similar values of electrolyte leakage (Figure 1D). When they were subjected to NaCl stress, both varieties exhibited a decrease in electrolyte leakage value; however, CT appeared to have higher electrolyte leakage value than WT (Figure 1D).
Figure 1 shows that the content of total chlorophyll was low in WT at no NaCl condition. Surprisingly, the content of total chlorophyll in WT remained at a high level after NaCl treatment (50 mM and 100 mM) in comparison to CT (Figure 1E). In addition, our results showed that after low NaCl treatment (50 mM) and at the control condition, the chlorophyll a/b in wild castor showed a higher level than cultivated castor (Figure 1E).
3.2. Effects of NaCl Treatment on ROS Accumulates in Leaves of Wild and Cultivated Castor
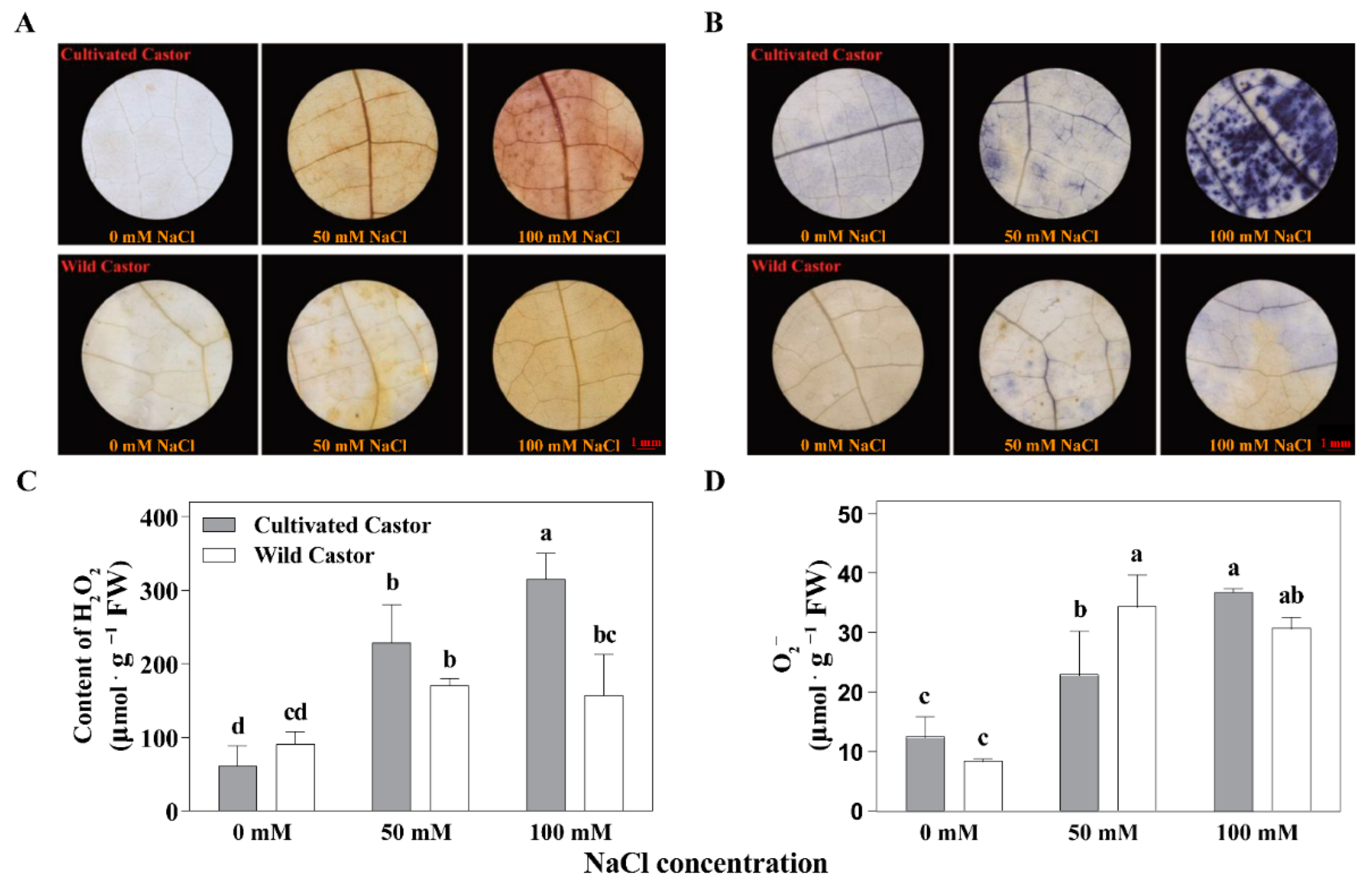
DAB suggested that WT contained less H2O2 under NaCl stress, whereas CT contained more (Figure 2). At the same time, we found that H2O2 content was also higher in CT but lower in WT under NaCl stress (50 mM and 100 mM) (Figure 2). In addition, the results of staining with NBT showed a similar pattern of DAB for the two varieties under NaCl stress (Figure 2).
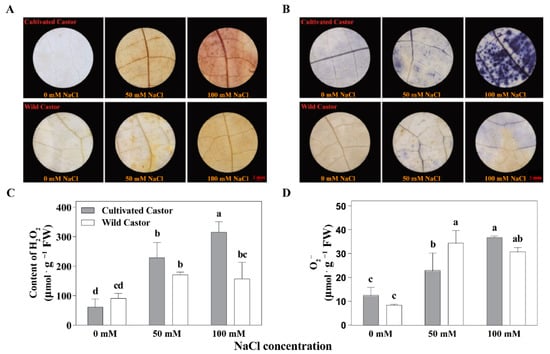
Figure 2.
ROS production of leaves in three true leaves from cultivated and wild castor with or without NaCl treatment. (A) Hydrogen peroxide (H2O2) content detected by 3, 3′-Diaminobenzidine (DAB) staining in leaves of three true leaves from cultivated and wild castor. (B) Superoxide anion (O2−) content detected by Nitro blue tetrazolium chloride (NBT) staining in leaves of three true leaves from cultivated and wild castor. Values are means ± SD based on three biological experiments. (C,D) H2O2 content and O2− content; (C,D), gray column belongs to cultivated castor and white column belongs to wild castor in the leaves of three true leaves from cultivated and wild castor. Different lowercase letters mean significance of differences (p < 0.05) between the different groups.
3.3. Effects of NaCl Treatment on Enzymes in Leaves of Wild and Cultivated Castor
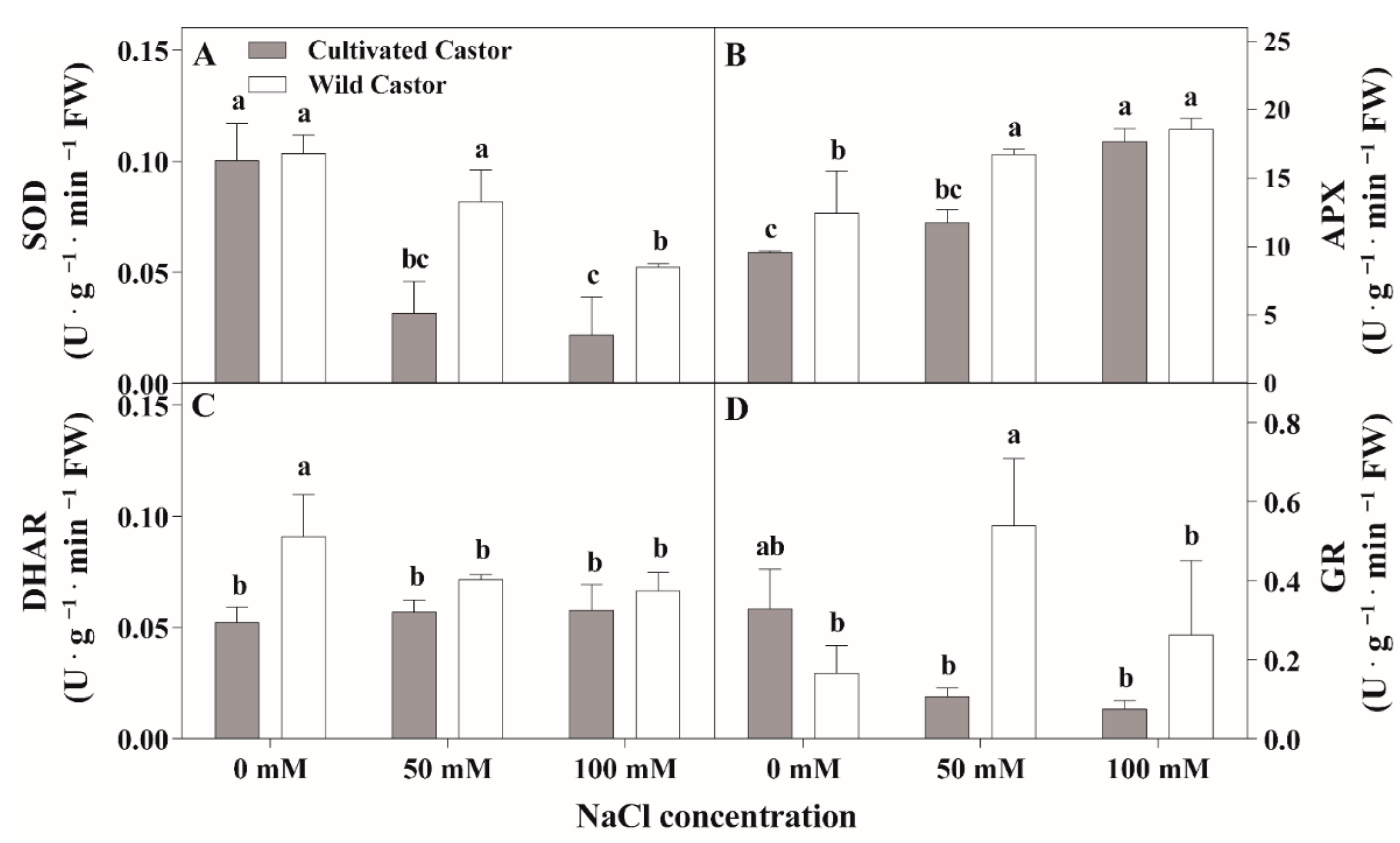
Under the control conditions, SOD activity showed no significant differences within the two varieties (Figure 3A). Under NaCl stress, SOD activity was decreased in both castor varieties compared with controls but higher SOD activity was observed in CT compared to WT. APX activity increased for the two varieties after NaCl treatment; however, WT had higher APX activity than cultivated castor (Figure 2C). DHAR activity responded differently across the treatments for the two castor varieties; it decreased in WT, but showed no significant change in CT in response to NaCl stress (Figure 2D). Further analysis of GR activity showed similar activity between the two varieties under control treatment (Figure 2B). At NaCl stress, GR activity was increased in WT but there was decreased GR activity in CT.
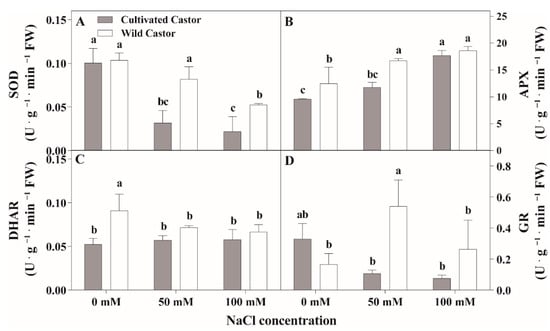
Figure 3.
SOD (A), APX (B), DHAR (C), and GR (D) activities of leaves in three true leaves from cultivated and wild castor with or without NaCl treatment (0 mM, 50 mM and 100 mM NaCl). Values are means ± SD based on three biological experiments. Different lowercase letters mean significance of differences (p < 0.05) between the different groups.
3.4. Effects of NaCl Treatment on ASA/DHA and GSH/GSSG in Leaves of Wild and Cultivated Castor
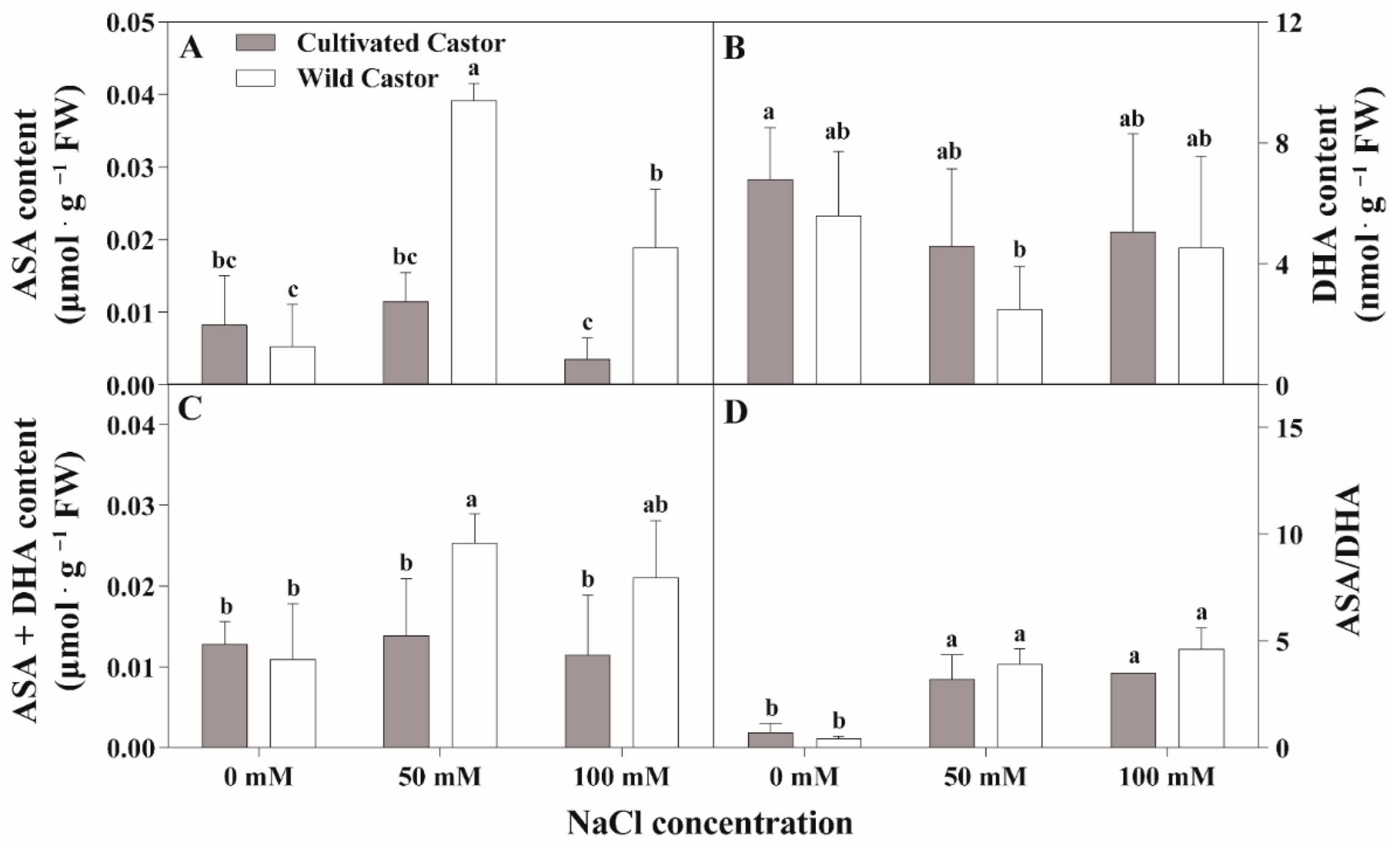
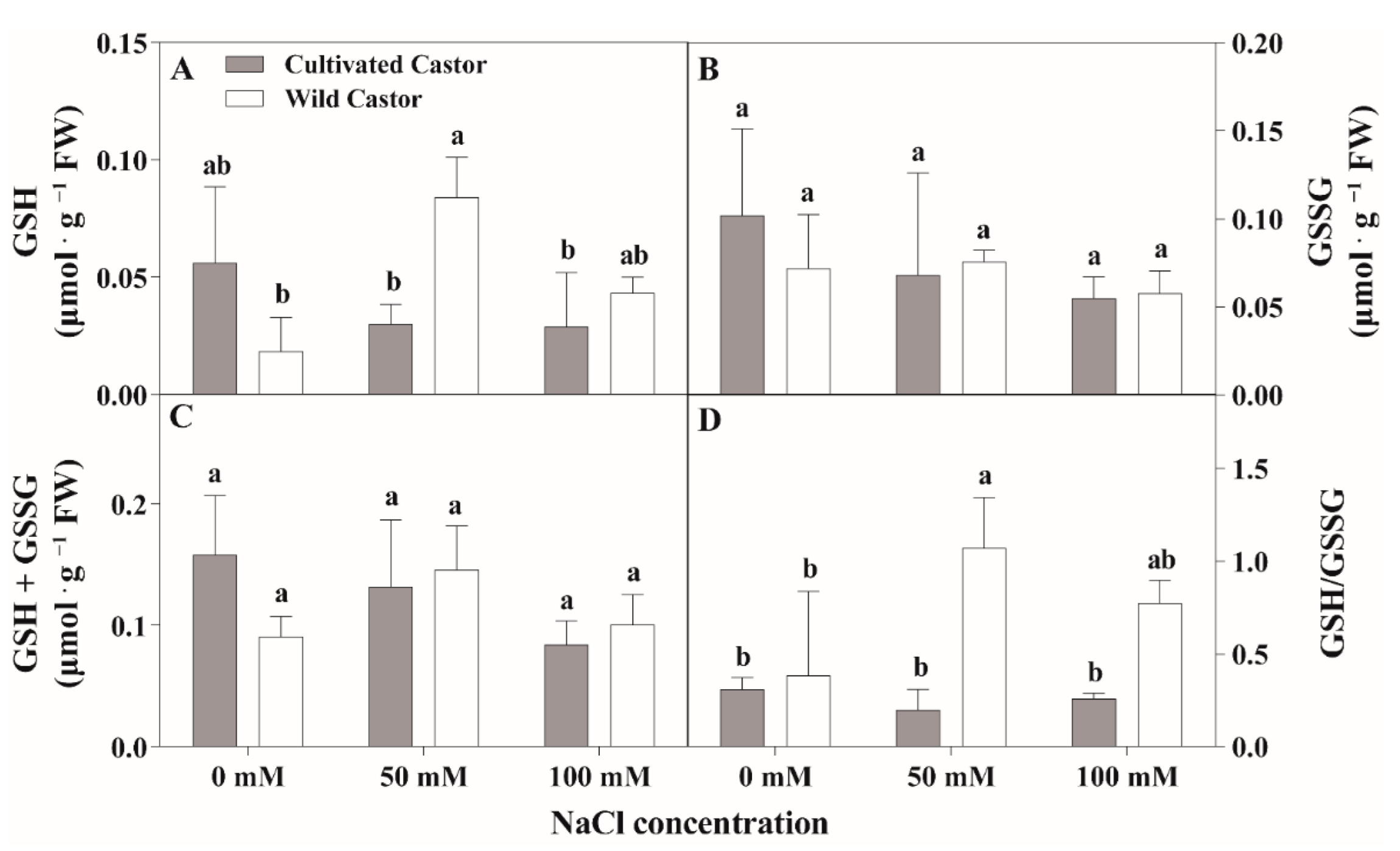
Results are summarized in Figure 4 and Figure 5. NaCl treatment induced a significant increase in ASA content in WT, which was maintained at a stable level in CT (Figure 4A). However, DHA appeared to decrease in the two varieties after NaCl treatment (Figure 4B).
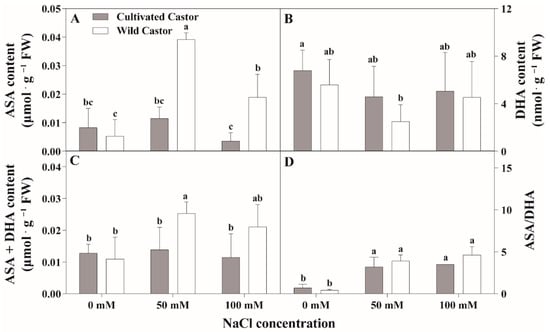
Figure 4.
The contents of ascorbic acid (AsA) and oxidized ascorbic acid (DHA) of leaves in three true leaves from cultivated and wild castor with or without NaCl treatment (0 mM, 50 mM and 100 mM NaCl). (A) ASA content; (B) DHA content; (C) ASA + DHA content; (D) ASA/DHA ratio. Values are means ± SD based on three biological experiments. Different lowercase letters mean significance of differences (p < 0.05) between the different groups.
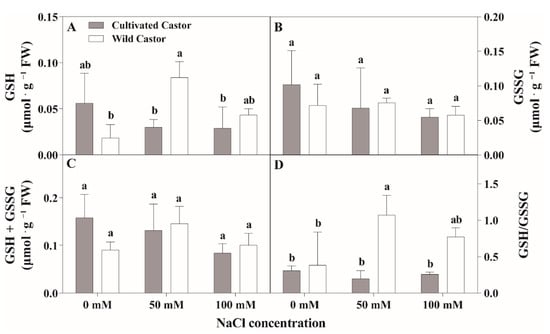
Figure 5.
The contents of reduced glutathione (GSH) and oxidized glutathione (GSSG) of leaves in three true leaves from cultivated and wild castor with or without NaCl treatment (0 mM, 50 mM and 100 mM NaCl). (A) GSH content; (B) GSSG content; (C) GSH + GSSG content; (D) GSH/GSSG ration. Values are means ± SD based on three biological experiments. Different lowercase letters mean significance of differences (p < 0.05) between the different groups.
After NaCl treatment, GSH content increased in WT, whereas it decreased in CT (Figure 5A). GSSG content had little effect on the two varieties after NaCl treatment (Figure 5B). Total ascorbic acid (ASA + DHA) content showed no significant fluctuations in CT, while a significant induction was observed under 50 mM NaCl stress (Figure 4C); conversely, NaCl stress stimulated the increase in the ASA/DHA ratio in two genotypes, which maintained a relatively higher level in WT compared to CT after 50 mM and 100 mM NaCl treatment (Figure 4D).
When seedlings were exposed to NaCl stress, no significant change in total glutathione content (GSH + GSSG) was observed in the two varieties, while a reduction was seen in CT and an increment was found in WT (Figure 5C). After NaCl treatment, the GSH/GSSG ratio content was significantly different between the two varieties; WT showed an increased tendency and there was no change in CT (Figure 5D).
4. Discussion
Salt stress is one of the major environmental threats that can abruptly hamper the growth of plants [45]. Severe salt stress reduced photosynthetic efficiency, leading to lower plant growth [46]. Generally, the most common characteristic plants under salt stress exhibited was growth inhibition [47]. In the present study, under salt stress condition, the morphology of seedlings and leaves of both varieties was significantly inhibited compared to the control; however, WT had better salinity tolerance in morphology than CT (Figure 1A,B). This improved plant growth under salt stress may be associated with net photosynthetic rate and chlorophyll content [48]; however, our present results are not in agreement with previously mentioned reports. We observed a lower value in Pn of WT when the concentration of NaCl was 50 mM (Figure 1C). This decrease can be explained by reducing the photosynthetic rate to minimize damage [49]. Furthermore, the RWC of WT was significantly higher than that of CT (Figure 1D). This suggests that WT is resistant to salt stress when enhancing the water absorption capability of roots, rather than when constraining the transpiration from stoma. These results are consistent with Ghaderi’s report [50]. Our study also showed that the chlorophyll content of both castor varieties decreased as the NaCl concentration increased, but the total chlorophyll content of the WT was significantly higher than that of CT under salt stress (Figure 1C). Rao [51] reported that the decrease in chlorophyll content under salt stress is mainly due to the degradation of chlorophyll by chlorophyllase. Therefore, in our study, the significant change of chlorophyll content in leaves showed that CT is more sensitive to salt stress than WT.
CO2 concentration in plants under abiotic stress is also reduced due to the stoma closure and continual activity of the photosynthetic system [52]. However, the Ci of WT had an increased tendency under the 50 mM NaCl compared to its control (Figure 1C). Brugnoli and Jacob [53,54] suggest that there were stomatal and non-stomatal limitations that reduced the photosynthesis rate. In the case of the stomatal limitation, when stoma closes and photosynthesis of leaf cells still continues, Ci decreases significantly; in contrast, in the non-stomatal limitations, the photosynthetic capacity of leaf cells is significantly reduced, causing the CO2 consumption to decrease and Ci to increase as a result. Here, it can be seen that the 50 mM NaCl treatment did not significantly affect the stoma closure and the photosynthetic capacity was first decreased in WT under 100 mM NaCl. This indicates that WT acquires salt tolerance by reducing the photosynthetic rate to minimize the damage caused by reactive oxygen species generated from the photosynthesis system.
In addition, salt stress can inhibit the photosynthetic process, which can lead to an accumulation of ROS such as H2O2 and O2− in plants [55]. The over accumulation of ROS may cause damage to plants. In general, electrolyte leakage is also used to indicate the degree of membrane lipid peroxidation caused by some environmental stress, including salt stress [56]. In this study, the level of electrolyte leakage significantly increased under salt stress in both castor varieties. However, WT seedlings exhibited an apparent reduction in level of electrolyte leakage compared to CT. An increase in H2O2 and O2− in CT under NaCl stress, especially 100 mM NaCl, suggested that salt stress resulted in an oxidative burst, thereby increasing ROS level and causing cellular damage. The steady level of H2O2 and O2− in WT seedlings exposed to salt stress may be attributed to the effects of both enzymatic and non-enzymatic systems. Our findings were similar to some studies; for example, the elevation of ROS production induced by salt stress has been previously reported in many plant varieties [57,58].
Plant adaptation to salt stress is often associated with ROS production [14]; hence, to remove excessive ROS, plants need an effective antioxidant defense system. Generally, this antioxidant defense system includes enzymatic and non-enzymatic constituents [59]. For the enzymatic system, SOD, APX, and GR are important defensive enzymes in plants [60]. In general, O2− can be translated into H2O2 by SOD. Accordingly, to prevent oxidative damage, H2O2 is also transformed into oxygen or water by CAT, APX, ascorbate, glutathione, and so on. Multiple studies on numerous plant varieties showed the positive effects of enzyme activities in response to stress [61]. After NaCl treatment, the activities of SOD, APX, DHAR, and GR in WT significantly enhanced in comparison to CT (Figure 3). These results indicate that these enzymes played positive roles in scavenging ROS in wild castor.
Similar to the enzymatic antioxidant system, non-enzymatic antioxidants such as ascorbate and glutathione play a key role against various types of stress in plants [62]. Our results showed that the contents of AsA and GSH were higher in WT than in CT in response to salt stress, but there was no significant difference in amounts of AsA + DHA and GSH + GSSG between the two varieties (Figure 4 and Figure 5). In general, the ratio of GSH/GSSG and AsA/DHA as redox signaling can reflect redox potential in the plant cell [20]. Higher GSH/GSSG and AsA/DHA ratios under environmental stress can maintain a high capability to protect the plants against various damages [63]. In our study, the ratios of GSH/GSSG and AsA/DHA in WT were significantly higher than those of CT under salt stress. These higher GSH/GSSG and ASA/DHA ratios in WT indicated that WT had a better redox potential under salt stress in comparison to CT.
The above results suggested that the antioxidant capacity of castor in response to salt stress is not only due to de novo synthesis of low-molecular antioxidants, but also mainly due to the change in activities of antioxidant enzymes. Previous studies also showed that stress-tolerant varieties have higher levels of antioxidant enzyme activities than susceptible varieties under different abiotic stress conditions [64]. Our results also showed that WT had superior salt tolerance in comparison to CT, and this characteristic was closely related to increased activities of antioxidant enzymes and low-molecular antioxidants in leaves under salt stress.
5. Conclusions
Physiological changes under NaCl stress showed that the electrolyte leakage and ROS content of wild castor was significantly lower than that of cultivated castor. The activities of SOD, APX, GR, and MDHAR antioxidant enzymes and the contents of ASA/DHA and GSH/GSSG non-enzymatic constituents of wild castor were significantly higher than those of cultivated castor. These results might give useful insights into the salt resistance breeding of castor.
Author Contributions
F.H. and Y.J. set up the experiment. F.H. and P.L. wrote the manuscript. S.L. and T.-J.E. edited the manuscript. S.Z. and Y.J. synthesized the study data. Y.J., F.M. and P.L. critically revised the draft and updated the manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Natural Science Foundation of China (31860071); Inner Mongolia Autonomous Region Grassland Talents Innovation Team—Castor Molecular Breeding Research Innovative Talent Team Rolling Support Project (2022); Natural Science Foundation of Inner Mongolia autonomous region (2021MS03008); Fundamental Research Funds in Higher Education Institutions of Inner Mongolia (237); Inner Mongolia Autonomous Region Castor Industry Collaborative Innovation Center Construction Project (MDK2021011); Open fund project in State Key Laboratory of Castor Breeding of China’s National Ethnic Affairs Commission (MDK2021006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the reviewers and editors for their work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kallamadi, P.R.; Nadigatla, V.P.R.G.R.; Mulpuri, S. Molecular diversity in castor (Ricinus communis L.). Ind. Crops Prod. 2015, 66, 271–281. [Google Scholar] [CrossRef]
- Thatikunta, R.; Siva Sankar, A.; Sreelakshmi, J.; Palle, G.; Leela, C.; Durga Rani, C.V.; Gouri Shankar, V.; Lavanya, B.; Narayana Reddy, P.; Dudhe, M.J.P.; et al. Utilization of in silico EST–SSR markers for diversity studies in castor (Ricinus communis L.). Physiol. Mol. Biol. Plants 2016, 22, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Yang, W.; Cong, J.; Zhang, L.; Liu, X.; Zhang, J. Comparison of Salt Tolerance and Salt-responsive Genes Expression in Two Different Varieties of Castor (Ricinus communis) at Germination Stage. J. Biotechnol. 2018, 16, 7473–7480. [Google Scholar] [CrossRef]
- Jeong, G.T.; Park, D.H. Optimization of Biodiesel Production from Castor Oil Using Response Surface Methodology. Appl. Biochem. Biotechnol. 2009, 156, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Severino, L.S.; Auld, D.L.; Baldanzi, M.; Candido, M.J.D.; Chen, G.; Crosby, W.; Tan, D.; He, X.H.; Lakshmamma, P.; Lavanya, C.; et al. A Review on the Challenges for Increased Production of Castor. Agron. J. 2012, 104, 853–880. [Google Scholar] [CrossRef]
- Gajera, B.B.; Kumar, N.; Singh, A.S.; Punvar, B.S.; Ravikiran, R.; Subhash, N.; Jadeja, G.C. Assessment of genetic diversity in castor (Ricinus communis L.) using RAPD and ISSR markers. Ind. Crops Prod. 2010, 32, 491–498. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, X.; Salvato, F.; Wang, Y.; Yan, X.; Zhou, Z.; Lin, J. Salt-adaptive strategies in oil seed crop Ricinus communis early seedlings (cotyledon vs. true leaf) revealed from proteomics analysis. Ecotoxicol. Env. Saf. 2019, 171, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.R.F.; Silva, E.N.; Moura, R.D.; dos Anjos, D.C.; Hernandez, F.F.F.; Viegas, R.A. Physiological adjustment to salt stress in R-communis seedlings is associated with a probable mechanism of osmotic adjustment and a reduction in water lost by transpiration. Ind. Crops Prod. 2014, 54, 233–239. [Google Scholar] [CrossRef]
- Han, B.; Xu, W.; Ahmed, N.; Yu, A.; Wang, Z.; Liu, A. Changes and Associations of Genomic Transcription and Histone Methylation with Salt Stress in Castor Bean. Plant Cell Physiol. 2020, 61, 1120–1133. [Google Scholar] [CrossRef]
- Zhou, G.S.; Nimir, N.; Lu, S.Y.; Zhai, F.Y.; Wang, Y.H. Gibberellic Acid and Salinity Affected Growth and Antioxidant Enzyme Activities in Castor Bean Plants at Early Growth Stage. Agron. J. 2014, 106, 1340–1348. [Google Scholar] [CrossRef]
- Guo, X.Q.; Zhou, G.S.; Zhu, G.L.; Jiao, X.R. Effects of calcium on emergence and seedling growth of castor bean under salinity stress. Curr. Sci. 2019, 116, 2028–2035. [Google Scholar] [CrossRef]
- Jiao, X.; Zhi, W.; Liu, G.; Zhu, G.; Feng, G.; Eltyb Ahmed Nimir, N.; Ahmad, I.; Zhou, G.J.A. Responses of foreign GA3 application on seedling growth of castor bean (Ricinus communis L.) under salinity stress conditions. Agronomy 2019, 9, 274. [Google Scholar] [CrossRef]
- Joshi, S.V.; Patel, N.T.; Pandey, I.B.; Pandey, A.N. Effect of supplemental Ca2+ on NaCl-stressed castor plants (Ricinus communis L.). Acta Bot. Croat. 2012, 71, 13–29. [Google Scholar] [CrossRef]
- Lei, P.; Liu, Z.; Hu, Y.; Kim, H.; Liu, S.; Liu, J.; Xu, L.; Li, J.; Zhao, Y.; Yu, Z.; et al. Transcriptome analysis of salt stress responsiveness in the seedlings of wild and cultivated Ricinus communis L. J. Biotechnol. 2021, 327, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Altaf, R.; Altaf, S.; Hussain, M.; Shah, R.U.; Ullah, R.; Ullah, M.I.; Rauf, A.; Ansari, M.J.; Alharbi, S.A.; Alfarraj, S.; et al. Heavy metal accumulation by roadside vegetation and implications for pollution control. PLoS ONE 2021, 16, e0249147. [Google Scholar] [CrossRef]
- Olivares, A.R.; Carrillo-Gonzalez, R.; Gonzalez-Chavez, M.D.A.; Hernandez, R.M.S. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production. J. Environ. Manag. 2013, 114, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N. Mechanisms of high salinity tolerance in plants. Methods Enzym. 2007, 428, 419–438. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Hao, S.H.; Wang, Y.R.; Yan, Y.X.; Liu, Y.H.; Wang, J.Y.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, Z.; Wu, H.; Tian, Z.; Zhao, Z. Redox regulation of plant stem cell fate. EMBO J. 2017, 36, 2844–2855. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Ansari, W.A.; Jaiswal, D.K.; Singh, A.K.; Prasad, R.; Verma, J.P.; Singh, M. Overexpression of AtDREB1 and BcZAT12 genes confers drought tolerance by reducing oxidative stress in double transgenic tomato (Solanum lycopersicum L.). Plant Cell Rep. 2021, 40, 2173–2190. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, S.; Zhao, Y.; Chen, D.; Chong, K.; Xu, Y. Overexpression of a homopeptide repeat-containing bHLH protein gene (OrbHLH001) from Dongxiang Wild Rice confers freezing and salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2010, 29, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.U.; Prasannalaxmi, K. Water stress induced physiological and biochemical changes in Piper betle L. and Ricinus communis L. plants and their effects on Spodoptera litura. Allelopath. J. 2014, 33, 25–41. [Google Scholar]
- Jiang, W.; Shi, W.; Ma, X.; Zhao, J.; Wang, S.; Tan, L.; Sun, C.; Liu, F. Identification of microRNAs responding to cold stress in Dongxiang common wild rice. Genome 2019, 62, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Bao, J.G.; Zheng, J.; Hu, H.Q.; Du, J.K. Distribution and chemical forms of copper in the root cells of castor seedlings and their tolerance to copper phytotoxicity in hydroponic culture. Environ. Sci. Pollut. Res. 2015, 22, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Fernandez-Cuesta, A.; Pascual-Villalobos, M.J.; Fernandez-Martinez, J.M. Variability of seed quality traits in wild and semi-wild accessions of castor collected in Spain. Ind. Crops Prod. 2015, 65, 203–209. [Google Scholar] [CrossRef]
- Lu, J.; Pan, C.; Fan, W.; Liu, W.; Zhao, H.; Li, D.; Wang, S.; Hu, L.; He, B.; Qian, K.J.G.; et al. A Chromosome-level Assembly of a Wild Castor Genome Provides New Insights into the Adaptive Evolution in a Tropical Desert. Genom. Proteom. Bioinform. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Vollmann, J.; Rajcan, I. Oil crop breeding and genetics. In Oil Crops; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–30. [Google Scholar]
- Jeschke, W.D.; Wolf, O. Effect of NaCI salinity on growth, development, ion distribution, and ion translocation in castor bean (Ricinus communis L.). J. Plant Physiol. 1988, 132, 45–53. [Google Scholar] [CrossRef]
- Niu, M.; Xie, J.; Chen, C.; Cao, H.; Sun, J.; Kong, Q.; Shabala, S.; Shabala, L.; Huang, Y.; Bie, Z. An early ABA-induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J. Exp. Bot. 2018, 69, 4945–4960. [Google Scholar] [CrossRef]
- Yildirim, E.; Karlidag, H.; Turan, M. Mitigation of salt stress in strawberry by foliar K, Ca and Mg nutrient supply. Plant Soil Environ. 2009, 55, 213–221. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Cheng, H.; Fan, X.-J.; Zhang, H.; Cheng, E.-M.; Tian, J. Assay on electrolyte leakage rate of walnut shoots of Jinboxiang Series. Acta Agric. Boreali-Sin. 2007, 22, 56–58. [Google Scholar]
- Lei, P.; Liu, Z.; Li, J.X.; Jin, G.Z.; Xu, L.P.; Ji, X.M.; Zhao, X.Y.; Tao, L.; Meng, F.J. Integration of the Physiology, Transcriptome and Proteome Reveals the Molecular Mechanism of Drought Tolerance in Cupressus gigantea. Forests 2022, 13, 401. [Google Scholar] [CrossRef]
- Agrawal, S.B.; Rathore, D. Changes in oxidative stress defense system in wheat (Triticum aestivum L.) and mung bean (Vigna radiata L.) cultivars grown with and without mineral nutrients and irradiated by supplemental ultraviolet-B. Environ. Exp. Bot. 2007, 59, 21–33. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Daudi, A.; O’Brien, J.A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A.J.P.S. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Cavalcanti, F.R.; Lima, J.P.; Ferreira-Silva, S.L.; Viegas, R.A.; Silveira, J.A. Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J. Plant Physiol. 2007, 164, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Dalton, D.A.; Russell, S.A.; Hanus, F.J.; Pascoe, G.A.; Evans, H.J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc. Natl. Acad. Sci. USA 1986, 83, 3811–3815. [Google Scholar] [CrossRef] [PubMed]
- Spodaryk, K. The red blood cell glutathione reductase activity in anaemic rats. Mech. Ageing Dev. 1990, 52, 255–261. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.; Dixon, D.P.; Walbot, V. Plant glutathione S-transferases: Enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000, 5, 193–198. [Google Scholar] [CrossRef]
- Challabathula, D.; Analin, B.; Mohanan, A.; Bakka, K. Differential modulation of photosynthesis, ROS and antioxidant enzyme activities in stress-sensitive and-tolerant rice cultivars during salinity and drought upon restriction of COX and AOX pathways of mitochondrial oxidative electron transport. J. Plant Physiol. 2022, 268, 153583. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Mahouachi, J. Long-term salt stress influence on vegetative growth and foliar nutrient changes in mango (Mangifera indica L.) seedlings. Sci. Hortic. 2018, 234, 95–100. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Liu, L.; Meng, F. Physiological and proteomic responses of diploid and tetraploid black locust (Robinia pseudoacacia L.) subjected to salt stress. Int. J. Mol. Sci. 2013, 14, 20299–20325. [Google Scholar] [CrossRef]
- Mauromicale, G.; Lo Monaco, A.; Longo, A.M.G. Effect of branched broomrape (Orobanche ramosa) infection on the growth and photosynthesis of tomato. Weed Sci. 2008, 56, 574–581. [Google Scholar] [CrossRef]
- Ghaderi, N.; Hatami, M.R.; Mozafari, A.; Siosehmardeh, A. Change in antioxidant enzymes activity and some morpho-physiological characteristics of strawberry under long-term salt stress. Physiol. Mol. Biol. Plants 2018, 24, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.G.; Rao, G.R. Pigment composition and chlorophyllase activity in pigeon pea (Cajanus indicus Spreng) and Gingelley (Sesamum indicum L.) under NaCl salinity. Indian J. Exp. Biol. 1981, 19, 768–770. [Google Scholar]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.J.P.B. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, E.; Bjorkman, O. Growth of cotton under continuous salinity stress: Influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta 1992, 187, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Lawlor, D.W. Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat plants. J. Exp. Bot. 1991, 42, 1003–1011. [Google Scholar] [CrossRef]
- Yang, W.; Wang, F.; Liu, L.N.; Sui, N. Responses of Membranes and the Photosynthetic Apparatus to Salt Stress in Cyanobacteria. Front. Plant Sci. 2020, 11, 713. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Liu, L.; Ueda, A.; Nagaoka, T.; Saneoka, H. Triveni Enterprises. J. Environ. Biol. 2016, 37, 107–114. [Google Scholar]
- Leshem, Y.; Seri, L.; Levine, A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007, 51, 185–197. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, J.A.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of Antioxidants to Paraquat in Pea Leaves (Relationships to Resistance). Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef]
- Orendi, G.; Zimmermann, P.; Baar, C.; Zentgraf, U.J.P.S. Loss of stress-induced expression of catalase3 during leaf senescence in Arabidopsis thaliana is restricted to oxidative stress. Plant Sci. 2001, 161, 301–314. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, F.; Liu, J.; Guan, F.; Quan, H.; Meng, F.J.B.G. The adaptation strategies of Herpetospermum pedunculosum (Ser.) Baill at altitude gradient of the Tibetan plateau by physiological and metabolomic methods. BMC Genom. 2019, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, Y.; Jin, G.; Lei, P.; Sang, L.; Luo, Q.; Liu, Z.; Guan, F.; Meng, F.; Zhao, X. Physiological and Proteomic Responses to Drought in Leaves of Amygdalus mira (Koehne) Yü et Lu. Front. Plant Sci. 2021, 876. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).