Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar and Nanobiochar Preparation
2.2. Biochar and Nanobiochar Characterization
2.3. Sorption Experiments
2.3.1. Mono-Metal Sorption System
2.3.2. Competitive Sorption System
2.3.3. Quality Control and Statistical Analysis
3. Results and Discussion
3.1. Biochar and Nanobiochar Characterization
3.1.1. Physiochemical Properties and Metal Concentrations of Biochar and Nanobiochar
3.1.2. Biochar and Nanobiochar Characterization by TEM
3.1.3. Biochar Functional Groups Characterization by FTIR
3.2. Adsorption Efficiency and Isotherm Models’ Characteristics
3.2.1. Removal Efficiency
3.2.2. Partition Coefficient (PC)
3.2.3. Freundlich and Langmuir Isotherm Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F. Sustainable and Green Management of Wastewater Under Climate Change Conditions. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Padilla-Ortega, E.; Regules-Martínez, M.C.; Leyva-Ramos, R.; Ocampo-Pérez, R.; Carranza-Alvarez, C. Single and competitive adsorption of Cd (II) and Pb (II) ions from aqueous solutions onto industrial chili seeds (Capsicum annuum) waste. Sustain. Environ. Res. 2017, 27, 61–69. [Google Scholar] [CrossRef]
- Darweesh, M.; Zedan, A.M.G.; El-Banna, A.; Elbasiuny, H.; Elbehiry, F. Biotechnology for Green Future of Wastewater Treatment. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Elbehiry, F.; Alshaal, T.; Elhawat, N.; Elbasiouny, H. Environmental-Friendly and Cost-Effective Agricultural Wastes for Heavy Metals and Toxicants Removal from Wastewater. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Pal, D.; Maiti, S.K. Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J. Clean. Prod. 2019, 212, 986–996. [Google Scholar] [CrossRef]
- Sedeño-Díaz, J.E.; López-López, E.; Mendoza-Martínez, E.; Rodríguez-Romero, A.J.; Morales-García, S.S. Distribution coefficient and metal pollution index in water and sediments: Proposal of a new index for ecological risk assessment of metals. Water 2020, 12, 29. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Darweesh, M.; Elbltagy, H.; Abo-Alhamd, F.G.; Amer, A.A.; Elsegaiy, M.A.; Khattab, I.A.; Elsharawy, E.A.; Elbehiry, F.; El-Ramady, H.; et al. Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: A review. Environ. Monit. Assess. 2021, 193, 542. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, F.; Elbasiouny, H.; El-Ramady, H.; Brevik, E.C. Mobility, distribution, and potential risk assessment of selected trace elements in soils of the Nile Delta, Egypt. Environ. Monit. Assess. 2019, 191, 713. [Google Scholar] [CrossRef]
- Ma, J.; Huang, W.; Zhang, X.; Li, Y.; Wang, N. The utilization of lobster shell to prepare low-cost biochar for high-efficient removal of copper and cadmium from aqueous: Sorption properties and mechanisms. J. Environ. Chem. Eng. 2021, 9, 104703. [Google Scholar] [CrossRef]
- Bognár, S.; Putnik, P.; Šojić Merkulov, D. Sustainable Green Nanotechnologies for Innovative Purifications of Water: Synthesis of the Nanoparticles from Renewable Sources. Nanomaterials 2022, 12, 263. [Google Scholar] [CrossRef]
- Han, Y.; Tang, Z.; Sun, J.; Xing, X.; Zhang, M.; Cheng, J. Heavy metals in soil contaminated through e-waste processing activities in a recycling area: Implications for risk management. Process Saf. Environ. Prot. 2019, 125, 189–196. [Google Scholar] [CrossRef]
- Chakraborty, S.C.; Qamruzzaman, M.; Zaman, M.W.U.; Alam, M.M.; Hossain, M.D.; Pramanik, B.K.; Nguyen, L.N.; Nghiem, L.D.; Ahmed, M.F.; Zhou, J.L.; et al. Metals in e-waste: Occurrence, fate, impacts and remediation technologies. Process Saf. Environ. Prot. 2022, 162, 230–252. [Google Scholar] [CrossRef]
- Kotb, M.S. Effect of surfactant on adsorption and mobility of lead and cadmium in soils. Egypt. J. Soil Sci. 2017, 57, 155–165. [Google Scholar] [CrossRef]
- Abdin, Y.; Usman, A.; Ok, Y.S.; Tsang, Y.F.; Al-Wabel, M. Competitive sorption and availability of coexisting heavy metals in mining-contaminated soil: Contrasting effects of mesquite and fishbone biochars. Environ. Res. 2020, 181, 108846. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Caracciolo, A.B.; Mariani, L.; Cardoni, M.C.; Riccucci, M.; Elhaes, H.; Ibrahim, M.A. Effectiveness of a new green technology for metal removal from contaminated water. Microchem. J. 2019, 147, 1010–1020. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, C.; Li, Y.; Ke, K.; Cheng, G. Efficient removal of tetracycline hydrochloride from aqueous solution by mesoporous cage MOF-818. SN Appl. Sci. 2019, 2, 669. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.A.; Amare, E. Summary on adsorption and photocatalysis for pollutant remediation: Mini review. J. Encapsul. Adsorpt. Sci. 2018, 8, 225–255. [Google Scholar] [CrossRef]
- Abebe, B.; Ananda Murthy, H.C. Synthesis and characterization of Ti-Fe oxide nanomaterials for lead removal. J. Nanomater. 2018, 2018, 9651030. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.; Ashour, M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials 2022, 15, 3922. [Google Scholar] [CrossRef]
- Elbehiry, F.; Elbasiouny, H.; Ali, R.; Brevik, E.C. Enhanced Immobilization and Phytoremediation of Heavy Metals in Landfill Contaminated Soils. Water Air Soil Pollut. 2020, 231, 204. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Yin, D.; Peng, B.; Tan, C.; Liu, Y.; Tan, X.; Wu, S. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J. Environ. Manag. 2015, 153, 68–73. [Google Scholar] [CrossRef]
- Shamim, S. Biosorption of heavy metals. Biosorption 2018, 2, 21–49. [Google Scholar] [CrossRef]
- Liu, C.; Ye, J.; Lin, Y.; Wu, J.; Price, G.W.; Burton, D.; Wang, Y. Removal of Cadmium (II) using water hyacinth (Eichhornia crassipes) biochar alginate beads in aqueous solutions. Environ. Pollut. 2020, 264, 114785. [Google Scholar] [CrossRef] [PubMed]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Dhaveethu Raja, J.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A complete review on biochar: Production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdelfattah, A.M.; Tharwat, R.M.; Nabil, G.M. Adsorption of negatively charged food tartrazine and sunset yellow dyes onto positively charged triethylenetetramine biochar: Optimization, kinetics and thermodynamic study. J. Mol. Liq. 2020, 318, 114297. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing efficiency of particles in heavy metal removal processes under various inlet conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef]
- Karvelas, E.G.; Lampropoulos, N.K.; Karakasidis, T.E.; Sarris, I.E. A computational tool for the estimation of the optimum gradient magnetic field for the magnetic driving of the spherical particles in the process of cleaning water. Desalination Water Treat. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.; Ashour, M. Dried brown seaweed’s phytoremediation potential for methylene blue dye removal from aquatic environments. Polymers 2022, 14, 1375. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Ashour, M.; Ramadan, K.M.; Alhajji, A.H.; Abualnaja, K.M. Do Red Seaweed Nanoparticles Enhance Bioremediation Capacity of Toxic Dyes from Aqueous Solution? Gels 2022, 8, 310. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Vithanage, M.; Alessi, D.S.; Liu, W.J.; Jayasundera, A.C.; Ok, Y.S. Nanobiochar: Production, properties, and multifunctional applications. Environ. Sci. Nano 2020, 7, 3279–3302. [Google Scholar] [CrossRef]
- Ramadan, M.M.; Abd-Elsalam, K.A. Micro/nano biochar for sustainable plant health: Present status and future prospects. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 323–357. [Google Scholar] [CrossRef]
- Walkley, A. A Critical Examination of a Rapid Method for Determining Organic Carbon in Soils—Effect of Variations in Digestion Conditions and of Inorganic Soil Constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar]
- Soltanpour, P.N.; Schwab, A.P. A new soil test for simultaneous extraction of macro- and micro-nutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Taha, A.A.; Shreadah, M.A.; Ahmed, A.M.; Heiba, H.F. Multi-component adsorption of Pb (II), Cd (II), and Ni (II) onto Egyptian Na-activated bentonite; equilibrium, kinetics, thermodynamics, and application for seawater desalination. J. Environ. Chem. Eng. 2016, 4, 1166–1180. [Google Scholar] [CrossRef]
- Schaumann, G.E.; Philippe, A.; Bundschuh, M.; Metreveli, G.; Klitzke, S.; Rakcheev, D.; Grün, A.; Kumahor, S.K.; Kühn, M.; Baumann, T.; et al. Understanding the fate and biological effects of Ag-and TiO2-nanoparticles in the environment: The quest for advanced analytics and interdisciplinary concepts. Sci. Total Environ. 2015, 535, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean. Techn. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Çelebi, H.; Gök, G.; Gök, O. Adsorption capability of brewed tea waste in waters containing toxic lead(II), cadmium (II), nickel (II), and zinc(II) heavy metal ions. Sci. Rep. 2020, 10, 17570. [Google Scholar] [CrossRef]
- Jha, M.K.; Joshi, S.; Sharma, R.K.; Kim, A.A.; Pant, B.; Park, M.; Pant, H.R. Surface Modified Activated Carbons: Sustainable Bio-Based Materials for Environmental Remediation. Nanomaterials 2021, 11, 3140. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, Y.T.; Hong, D.Y.; Sharma, Y.C.; Chen, S.C.; Tripathi, K. Effective removal of copper ions from aqueous solution using base treated black tea waste. Ecol. Eng. 2014, 67, 127–133. [Google Scholar] [CrossRef]
- Munene, J.M.; Onyatta, J.O.; Yusuf, A.O. Characterization of water hyacinth powder using FTIR spectroscopy and the adsorption behaviour of Pb2+, Cd2+, Zn2+, Ni2+ and Cr2+ in Aqueous Solution. Asian J. Appl. Chem. Res. 2020, 6, 47–55. [Google Scholar] [CrossRef]
- Herath, A.; Layne, C.A.; Perez, F.; Hassan, E.B.; Pittman Jr, C.U.; Mlsna, T.E. KOH-activated high surface area Douglas Fir biochar for adsorbing aqueous Cr (VI), Pb (II) and Cd (II). Chemosphere 2021, 269, 128409. [Google Scholar] [CrossRef]
- Ouyang, D.; Zhuo, Y.; Hu, L.; Zeng, Q.; Hu, Y.; He, Z. Research on the Adsorption Behavior of Heavy Metal Ions by Porous Material Prepared with Silicate Tailings. Minerals 2019, 9, 291. [Google Scholar] [CrossRef]
- Peng, H.; Wang, Y.; Tan, T.L.; Chen, Z. Exploring the phytoremediation potential of water hyacinth by FTIR Spectroscopy and ICP-OES for treatment of heavy metal contaminated water. Int. J. Phytoremediat. 2020, 22, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Alyemeni, M.N.; Wijaya, L. Adsorption-reduction performance of tea waste and rice husk biochars for Cr (VI) elimination from wastewater. J. Saudi Chem. Soc. 2020, 24, 799–810. [Google Scholar] [CrossRef]
- Neris, J.B.; Luzardo, F.H.; Santos, P.F.; de Almeida, O.N.; Velasco, F.G. Evaluation of single and tri-element adsorption of Pb2+, Ni2+ and Zn2+ ions in aqueous solution on modified water hyacinth (Eichhornia crassipes) fibers. J. Environ. Chem. Eng. 2019, 7, 102885. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H. Nanomaterials for the adsorptive treatment of Hg(II) ions from water. Chem. Eng. J. 2019, 358, 264–282. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Palanivell, P.; Ahmed, O.H.; Latifah, O.; Abdul Majid, N.M. Adsorption and desorption of nitrogen, phosphorus, potassium, and soil buffering capacity following application of chicken litter biochar to an acid soil. Appl. Sci. 2020, 10, 295. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Montané, D.; Celzard, A. Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater. 2008, 111, 276–284. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Fierro, V.; Rogaume, Y.; Rogaume, C.; Zoulalian, A.; Celzard, A. Activated carbons prepared from wood particleboard wastes: Characterisation and phenol adsorption capacities. J. Hazard. Mater. 2009, 166, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Feng, Y.; Zhu, C.; Liu, F.; Li, A. Enhanced synergistic removal of Cr (VI) and Cd (II) with bi-functional biomass-based composites. J. Hazard. Mater. 2020, 388, 121776. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Elbltagy, H.; Elbasiouny, H.; Almuhamady, A.; Gamal El-Dein, H. Low Cost and Eco-Friendly Removal of Toxic Heavy Metal from Industrial Wastewater. Egypt. J. Soil Sci. 2021, 61, 181–190. [Google Scholar] [CrossRef]
- Xiao, Y.; Xue, Y.; Gao, F.; Mosa, A. Sorption of heavy metal ions onto crayfish shell biochar: Effect of pyrolysis temperature, pH and ionic strength. J. Taiwan Inst. Chem. Eng. 2017, 80, 114–121. [Google Scholar] [CrossRef]

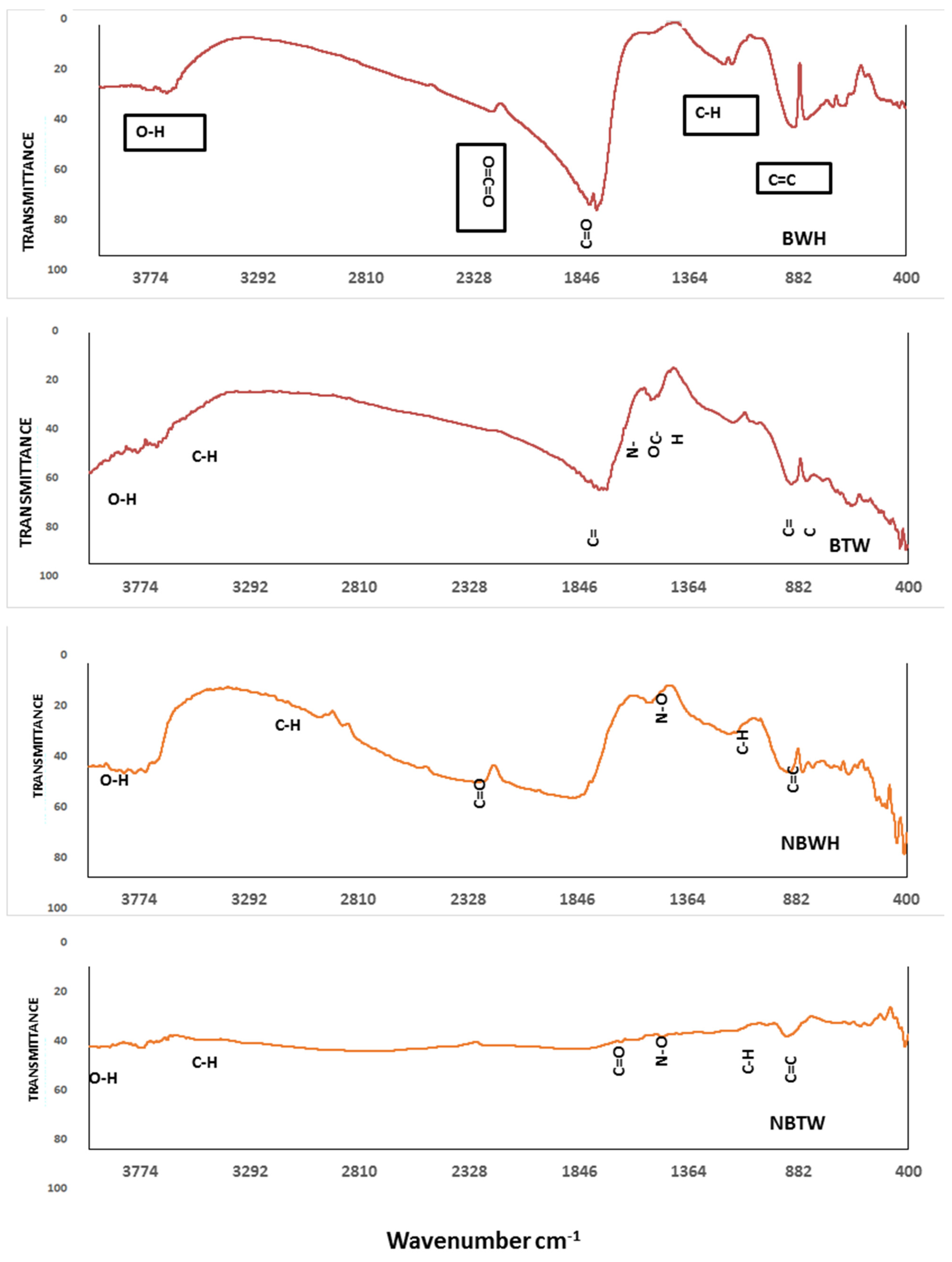

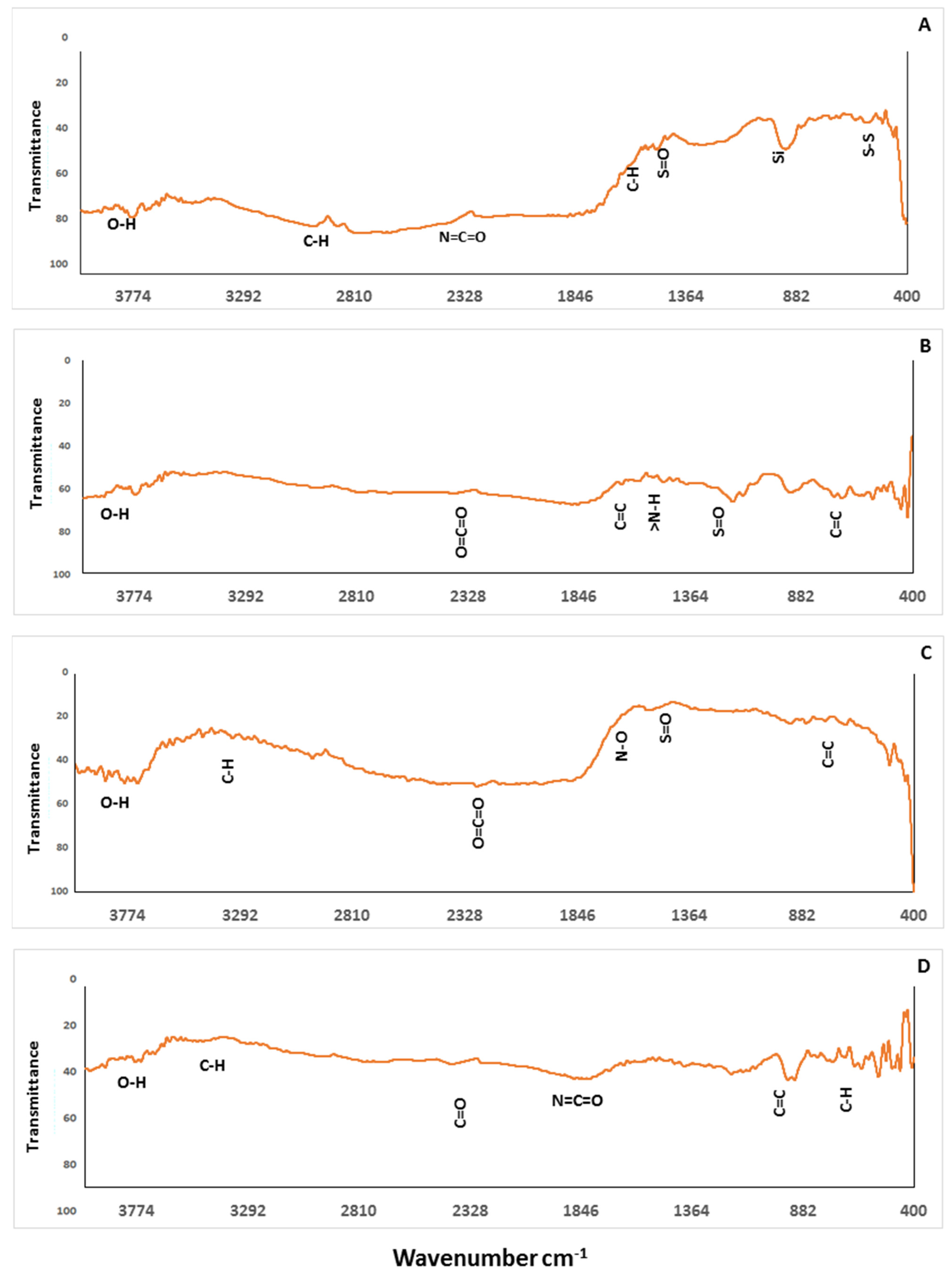
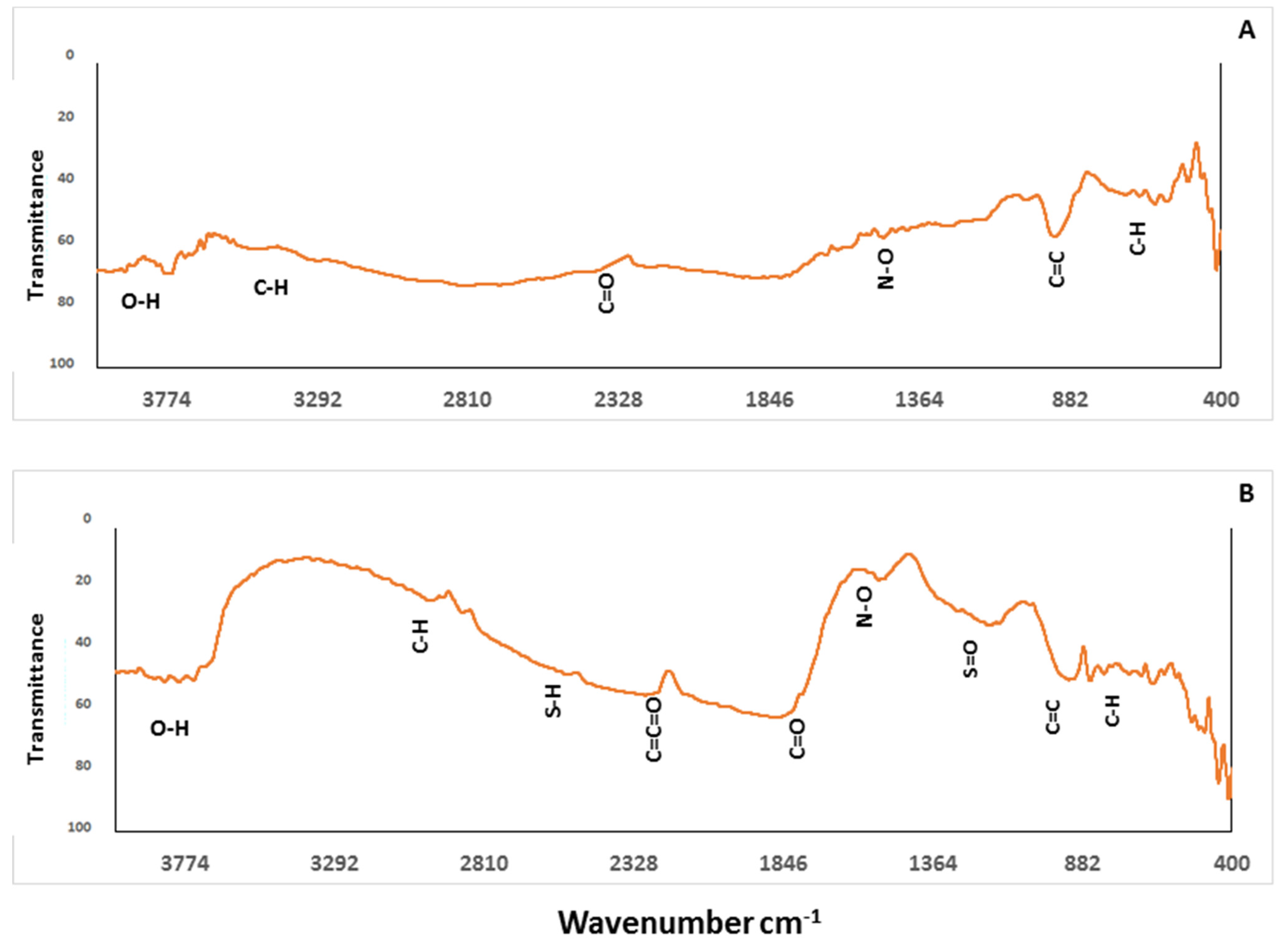

| pH | EC dS m−1 | TDS (g L−1) | OM (g kg−1) | Total Cations (mg kg−1) | Available Heavy Metals (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | Cd | Cr | Ni | |||||
| BWH | 9.90 | 8.10 | 4.00 | 682 | 57.3 | 9.0 | 19.8 | 13.4 | <0.01 | <0.02 | <0.03 |
| BTW | 10.71 | 3.31 | 1.6 | 430 | 23.5 | 7.2 | 10.4 | 8.8 | <0.01 | <0.02 | <0.03 |
| NBWH | 9.95 | 7.8 | 3.7 | 611 | 48.8 | 10.2 | 16.3 | 12.8 | <0.01 | <0.02 | <0.03 |
| NBTW | 10.2 | 2.8 | 1.4 | 402 | 22.4 | 6.6 | 9.4 | 7.2 | <0.01 | <0.02 | <0.03 |
| Adsorbent Martial | Initial Metal Conc. mg L−1 | Cd | Cr | Ni | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mono | Competitive | Mono | Competitive | Mono | Competitive | ||||||||
| RE (%) | PC | RE (%) | PC | RE (%) | PC | RE (%) | PC | RE (%) | PC | RE (%) | PC | ||
| BWH | 5 | 97.4 | 0.95 | 99.5 | 4.98 | 98.4 | 1.54 | 98.2 | 1.36 | 98.8 | 2.02 | 96.2 | 0.63 |
| 10 | 95.8 | 0.56 | 99.2 | 2.92 | 97.8 | 1.09 | 97.5 | 0.96 | 93.1 | 0.33 | 94.6 | 0.44 | |
| 20 | 96.3 | 0.65 | 99.4 | 4.14 | 96.8 | 0.75 | 97.1 | 0.82 | 94.6 | 0.43 | 94.8 | 0.45 | |
| 40 | 97.2 | 0.86 | 99.4 | 4.48 | 97.0 | 0.81 | 97.3 | 0.89 | 95.0 | 0.47 | 95.2 | 0.49 | |
| BTW | 5 | 88.4 | 0.19 | 99.7 | 9.23 | 95.5 | 0.53 | 97.0 | 0.81 | 95.6 | 0.45 | 87.9 | 0.18 |
| 10 | 85.8 | 0.15 | 99.3 | 3.31 | 88.4 | 0.19 | 96.9 | 0.78 | 93.0 | 0.27 | 85.9 | 0.15 | |
| 20 | 85.3 | 0.14 | 99.4 | 3.82 | 91.3 | 0.26 | 96.4 | 0.67 | 94.7 | 0.46 | 87.1 | 0.17 | |
| 40 | 85.9 | 0.15 | 99.5 | 4.93 | 91.7 | 0.28 | 96.6 | 0.71 | 93.8 | 0.39 | 87.7 | 0.18 | |
| NBWH | 5 | 99.8 | 26.27 | 99.7 | 18.47 | 98.6 | 3.52 | 99.2 | 6.20 | 98.3 | 2.94 | 98.2 | 2.76 |
| 10 | 99.6 | 12.77 | 99.3 | 6.62 | 97.6 | 2.03 | 98.5 | 3.28 | 98.0 | 2.39 | 97.3 | 1.80 | |
| 20 | 99.6 | 14.03 | 99.4 | 7.64 | 98.5 | 3.23 | 98.6 | 3.52 | 98.3 | 2.94 | 98.3 | 2.81 | |
| 40 | 99.7 | 17.23 | 99.5 | 9.39 | 98.8 | 3.97 | 98.9 | 4.33 | 98.6 | 3.61 | 98.7 | 3.78 | |
| NBTW | 5 | 99.8 | 24.95 | 99.8 | 24.95 | 98.5 | 3.28 | 98.5 | 3.35 | 97.0 | 1.62 | 95.9 | 1.17 |
| 10 | 99.7 | 15.33 | 99.8 | 26.98 | 98.2 | 2.71 | 98.2 | 2.65 | 95.4 | 1.04 | 94.9 | 0.92 | |
| 20 | 99.5 | 10.65 | 99.8 | 20.36 | 98.5 | 3.34 | 98.6 | 3.59 | 97.0 | 1.63 | 87.0 | 0.33 | |
| 40 | 99.6 | 11.90 | 99.8 | 22.75 | 98.7 | 3.74 | 98.8 | 4.01 | 97.3 | 1.83 | 88.4 | 0.38 | |
| Treatments | Initial Conc. mg L−1 | Cd | Cr | Ni | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Langmuir | Freundlich | Langmuir | Freundlich | Langmuir | Freundlich | ||||||||||||||
| Q Max | KL | R2 | 1/n | KF | R2 | Q Max | KL | R2 | 1/n | KF | R2 | Q Max | KL | R2 | 1/n | KF | R2 | ||
| BWH | Mono | 1.28 | 0.80 | 0.96 | 0.92 | 0.69 | 0.93 | 0.98 | 1.75 | 0.98 | 0.74 | 0.76 | 0.99 | 0.47 | 5.67 | 0.85 | 0.53 | 0.46 | 0.83 |
| Comp. | 1.34 | 3.98 | 0.94 | 0.94 | 3.51 | 0.94 | 1.14 | 1.31 | 0.98 | 0.82 | 0.82 | 0.99 | 1.33 | 0.51 | 0.97 | 0.89 | 0.48 | 0.98 | |
| BTW | Mono | 1.58 | 0.13 | 0.99 | 0.90 | 0.17 | 0.99 | 0.61 | 1.05 | 0.89 | 0.73 | 0.30 | 0.89 | 1.40 | 0.33 | 0.94 | 0.98 | 0.38 | 0.94 |
| Comp. | 0.66 | 16.95 | 0.91 | 0.71 | 2.29 | 0.89 | 3.72 | 0.22 | 0.99 | 0.92 | 0.70 | 0.99 | 3.37 | 0.05 | 0.99 | 1.00 | 0.17 | 0.99 | |
| NBWH | Mono | 1.65 | 18.19 | 0.93 | 0.80 | 8.87 | 0.93 | 3.18 | 1.17 | 0.93 | 1.02 | 3.17 | 0.89 | 19.62 | 0.15 | 0.98 | 1.10 | 3.41 | 0.97 |
| Comp. | 1.31 | 17.03 | 0.91 | 0.71 | 4.48 | 0.90 | 1.79 | 3.91 | 0.94 | 0.82 | 3.06 | 0.94 | 6.62 | 0.42 | 0.94 | 1.12 | 3.18 | 0.89 | |
| NBTW | Mono | 1.71 | 0.02 | 0.97 | 0.71 | 6.19 | 0.98 | 18.33 | 0.18 | 0.98 | 1.08 | 3.65 | 0.98 | 4.48 | 0.37 | 0.94 | 1.05 | 1.56 | 0.92 |
| Comp. | 11.18 | 2.32 | 0.99 | 0.92 | 17.60 | 0.99 | 23.51 | 0.14 | 0.98 | 1.11 | 3.97 | 0.97 | 1.54 | 0.89 | 0.99 | 0.58 | 0.62 | 0.96 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbehiry, F.; Darweesh, M.; Al-Anany, F.S.; Khalifa, A.M.; Almashad, A.A.; El-Ramady, H.; El-Banna, A.; Rajput, V.D.; Jatav, H.S.; Elbasiouny, H. Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions. Sustainability 2022, 14, 10118. https://doi.org/10.3390/su141610118
Elbehiry F, Darweesh M, Al-Anany FS, Khalifa AM, Almashad AA, El-Ramady H, El-Banna A, Rajput VD, Jatav HS, Elbasiouny H. Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions. Sustainability. 2022; 14(16):10118. https://doi.org/10.3390/su141610118
Chicago/Turabian StyleElbehiry, Fathy, Marwa Darweesh, Fathia S. Al-Anany, Asmaa M. Khalifa, Aliaa A. Almashad, Hassan El-Ramady, Antar El-Banna, Vishnu D. Rajput, Hanuman Singh Jatav, and Heba Elbasiouny. 2022. "Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions" Sustainability 14, no. 16: 10118. https://doi.org/10.3390/su141610118
APA StyleElbehiry, F., Darweesh, M., Al-Anany, F. S., Khalifa, A. M., Almashad, A. A., El-Ramady, H., El-Banna, A., Rajput, V. D., Jatav, H. S., & Elbasiouny, H. (2022). Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions. Sustainability, 14(16), 10118. https://doi.org/10.3390/su141610118









