Evaluation of the Effects of Water and Salinity Stress on the Growth and Biochemistry of Alfalfa (Medicago sativa L.) at the Branching Stage
Abstract
:1. Introduction
2. Materials and Methods
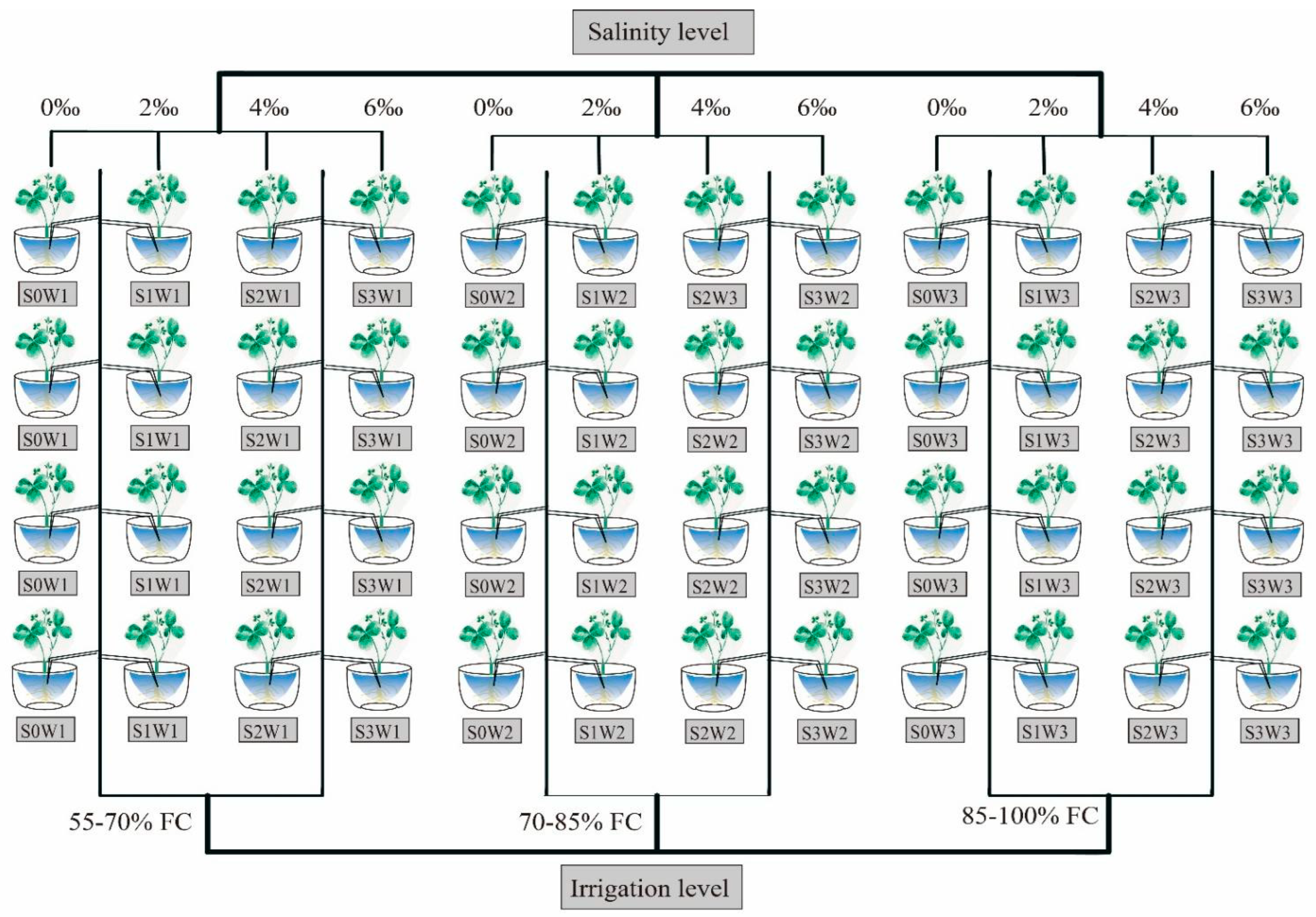
2.1. Experimental Design
2.2. Sampling and Measurements
2.2.1. The Aboveground and Root Biomass of Alfalfa
2.2.2. Soil Electrical Conductivity
2.2.3. Biochemical Assays on Shoot
2.2.4. Stress Resistance Index (RIs)
2.3. Statistical Analysis
3. Results
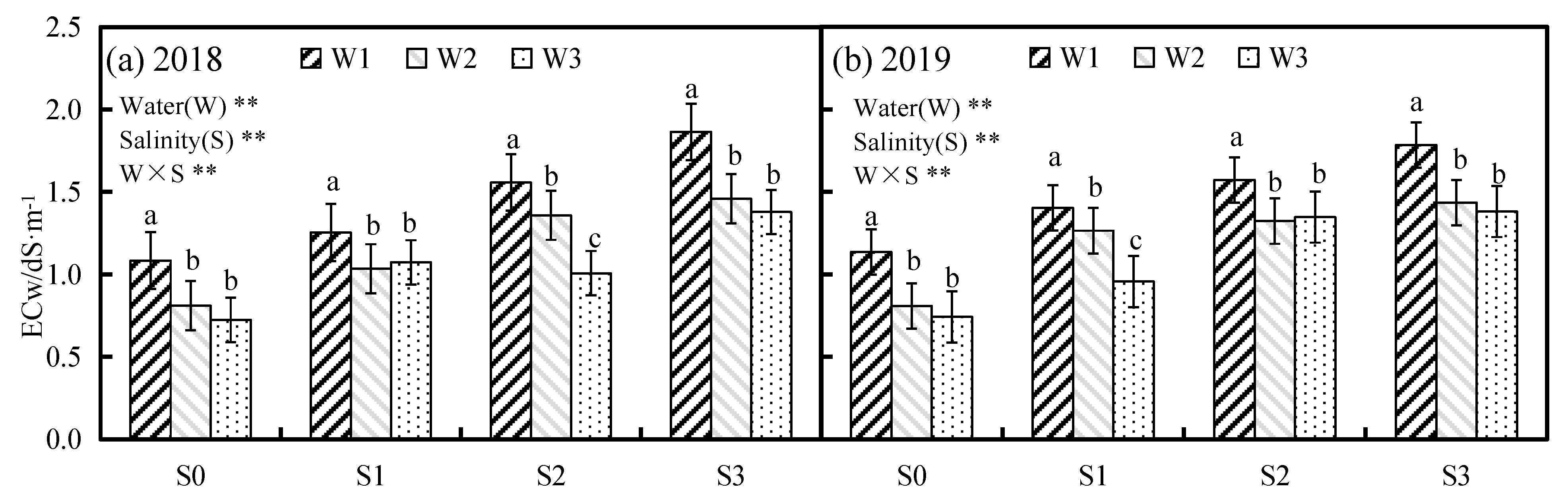
3.1. Effects of Water and Salinity Stress on Soil Salt and the Water Consumption of Alfalfa
3.2. Effects of Water and Salinity Stress on the Growth and Biomass of Alfalfa
3.3. Effects of Water and Salinity Stress on Proline, Na+, SOD and POD in the Shoots of Alfalfa
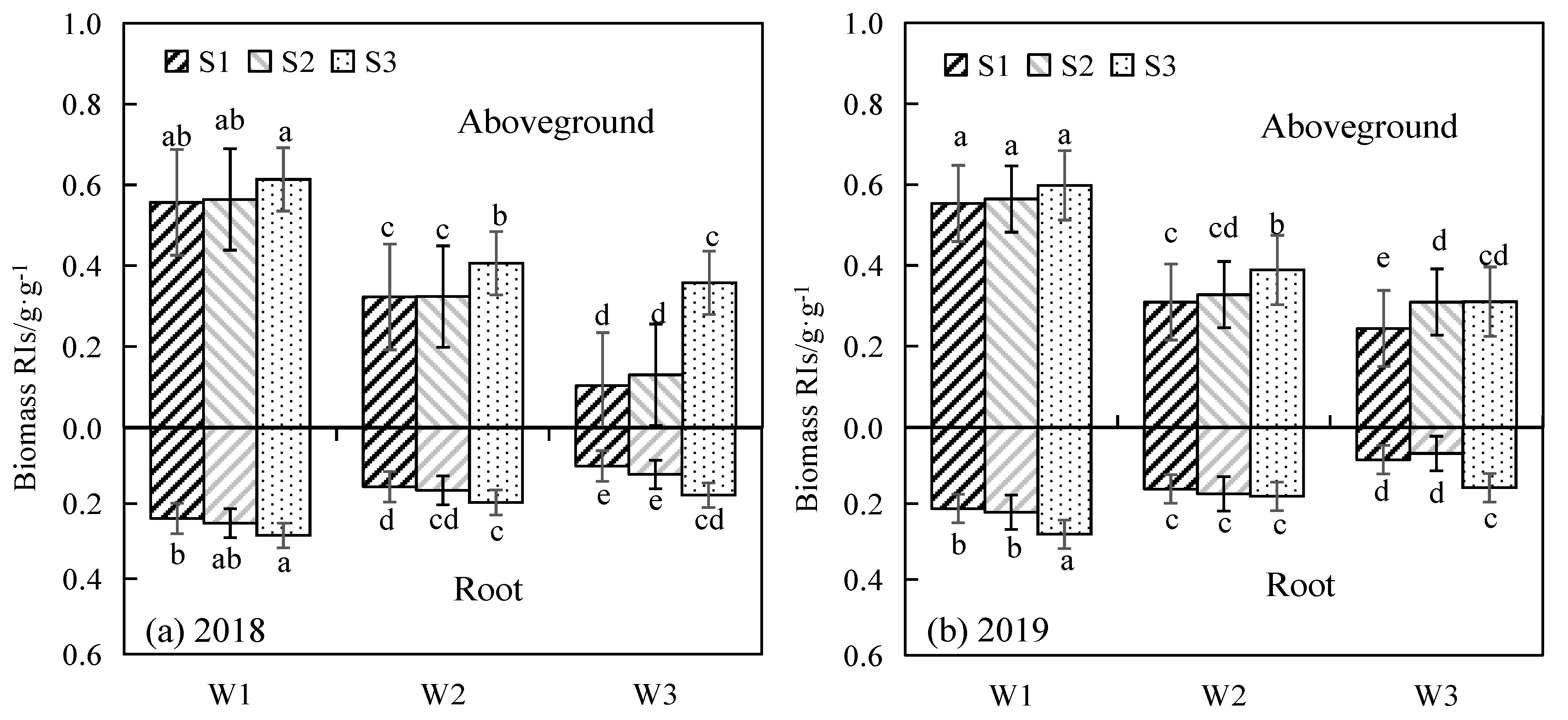
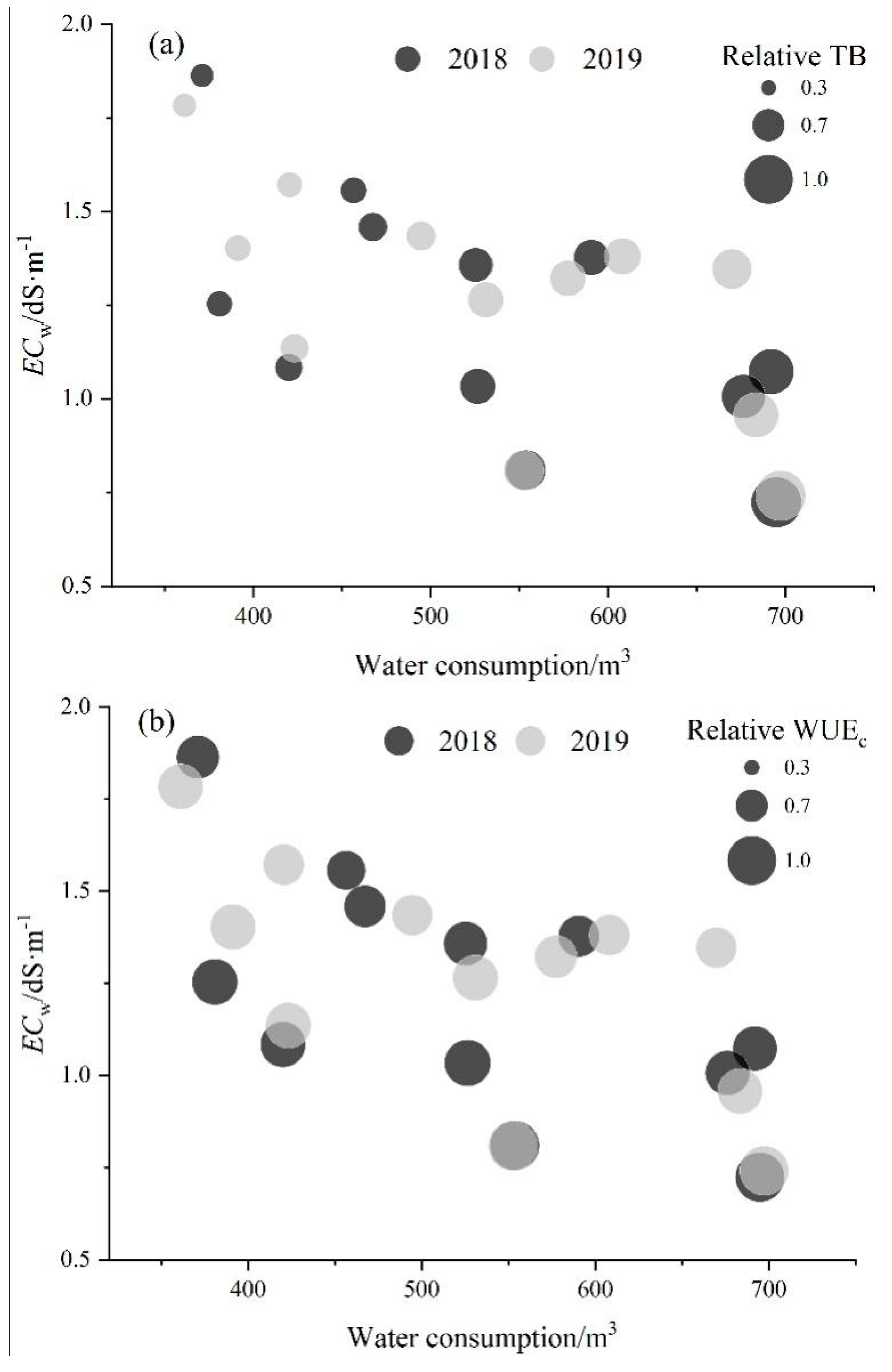
3.4. Sensitivity of Shoot and Root Biomass of Alfalfa to Water and Salinity Stress
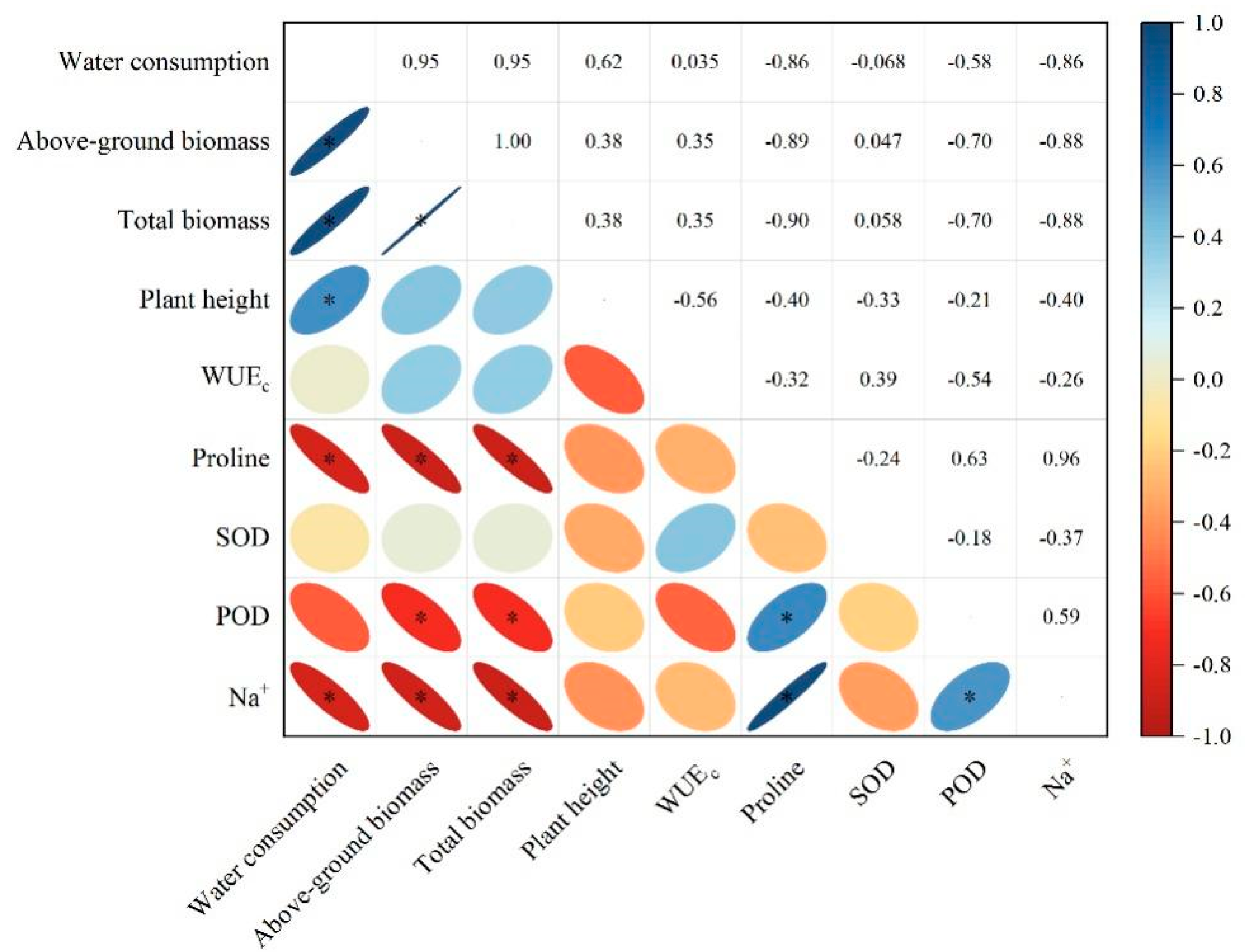
3.5. Relationship between the Growth and Biochemical Characteristics of Alfalfa
4. Discussion
4.1. Effects of Water and Salt Stress on the Growth and Water Consumption of Alfalfa
4.2. Response of Osmolytes and Antioxidant Enzyme Activities of Alfalfa to Water and Salinity Stress
4.3. Relationship between Growth and the Biochemical Characteristics of Alfalfa
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scasta, J.D.; Trostle, C.L.; Foster, M.A. Evaluating alfalfa (medicago sativa L.) cultivars for salt tolerance using laboratory, greenhouse and field methods. J. Agric. Sci. 2012, 4, 90. [Google Scholar] [CrossRef]
- Díaz, F.J.; Grattan, S.R.; Reyes, J.A.; Roza-Delgado, B.; Tejedor, M. Using saline soil and marginal quality water to produce alfalfa in arid climates. Agric. Water Manag. 2018, 199, 11–21. [Google Scholar] [CrossRef]
- Wang, C.; Che, G.; Li, Y.Q. Experimental study on effect of drying process on crude protein content of alfalfa. Trans. CSAE 2006, 9, 225–228. [Google Scholar]
- Elgharably, A.; Benes, S. Alfalfa biomass yield and nitrogen fixation in response to applied mineral nitrogen under saline soil conditions. J. Soil Sci. Plant Nutr. 2021, 21, 744–755. [Google Scholar] [CrossRef]
- Yang, H.; Unkovich, M.; McNeill, A.; Wang, X.Z. Symbiotic N2 fixation and nitrate utilisation in irrigated lucerne (Medicago sativa) systems. Biol. Fertil. Soils 2011, 47, 377–385. [Google Scholar] [CrossRef]
- Xiong, J.; Sun, Y.; Yang, Q.; Tian, H.; Zhang, H.; Liu, Y. Proteomic analysis of early salt stress responsive proteins in alfalfa roots and shoots. Proteome Sci. 2017, 15, 19. [Google Scholar] [CrossRef]
- Hou, C.L.; Tian, D.L.; Xu, B.; Ren, J.; Hao, L.; Chen, N.; Li, X.Y. Use of the stable oxygen isotope method to evaluate the difference in water consumption and utilization strategy between alfalfa and maize fields in an arid shallow groundwater area. Agric. Water Manag. 2021, 256, 107065. [Google Scholar] [CrossRef]
- Bansal, S.; Yin, X. Effects of salinity, growth condition, and soil type on shoot dry matter of alfalfa (medicago sativa)–a meta-analysis. Commun. Soil Sci. Plant Anal. 2020, 52, 201–211. [Google Scholar] [CrossRef]
- Qiu, Y.; Fan, Y.; Chen, Y.; Hao, X.; Li, S.; Kang, S. Response of dry matter and water use efficiency of alfalfa to water and salinity stress in arid and semiarid regions of northwest china. Agric. Water Manag. 2021, 254, 106934. [Google Scholar] [CrossRef]
- Djaman, K.; Smeal, D.; Koudahe, K.; Allen, S. Hay yield and water use efficiency of alfalfa under different irrigation and fungicide regimes in a semiarid climate. Water 2020, 12, 1721. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S.; Wang, B.; Zhao, J. Physiological and biochemical changes in different drought-tolerant alfalfa (Medicago sativa L.) varieties under PEG-induced drought stress. Acta Physiol. Plant 2018, 40, 25. [Google Scholar] [CrossRef]
- Akcay, U.C.; Ercan, O.; Kavas, M.; Yildiz, L.; Yilmaz, C.; Oktem, H.A. Drought-induced oxidative damage and antioxidant responses in peanut (arachis hypogaea L.) seedlings. Plant Growth Regul. 2010, 61, 21–28. [Google Scholar] [CrossRef]
- Yang, C.; Luo, Y.; Sun, L.; Na, W.U. Effect of deficit irrigation on the growth, water use characteristics and yield of cotton in arid northwest china. Pedosphere 2015, 25, 910–924. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirbyste 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Gao, L.; Su, J.; Tian, Q.; Shen, Y. Contrasting strategies of nitrogen absorption and utilization in alfalfa plants under different water stress. J. Soil Sci. Plant Nutr. 2020, 20, 1515–1523. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Modarressanavy, S.A.M.; Ghanati, F. Effect of salinity on growth, xylem structure and anatomical characteristics of soybean. Not. Sci Biol. 2011, 3, 41–45. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Chen, X.; Xie, W.; Lu, Z. Effects of salt stress and nitrogen application on growthand ion accumulation of Suaeda salsa plants. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011; pp. 8268–8272. [Google Scholar]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Maas, E.V.; Grattan, S.R. Crop yields as affected by salinity. Agric. Drain. 1999, 38, 55–108. [Google Scholar]
- Yang, H.; Du, T.; Mao, X.; Shukla, M.K. Modeling tomato evapotranspiration and yield responses to salinity using different macroscopic reduction functions. Vadose Zone J. 2020, 19, e20074. [Google Scholar] [CrossRef]
- Xie, W.H.; Ma, S.J.; Qi, L.; Zhang, Z.H.; Bai, X.F. The mitigating effects of Na+ accumulation on the drought-induced damage to photosynthetic apparatus in cotton seedlings. Acta Ecol. Sinica. 2015, 35, 6549–6556. [Google Scholar]
- Weng, Y.W.; Zhang, L.; Zhang, S.; Tian, Z.W.; Jin, X.Y.; Li, M.Y.; Yu, Z.Y.; Jiang, D.; Dai, T.B. Effects of salt with drought stress on growth and water uptake of wheat seedlings. Acta Ecol. Sinica. 2017, 37, 2244–2252. [Google Scholar]
- Albacete, A.A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Hormonal and metabolic regulation of source–sink relations under salinity and drought: From plant survival to crop yield stability. Biotechnol. Adv. 2014, 32, 12–30. [Google Scholar] [CrossRef]
- Atikur, R.M.; Yong-Goo, K.; Iftekhar, A.; Liu, G.S.; Hyoshin, L.; Joo, L.J. Proteome analysis of alfalfa roots in response to water deficit stress. J. Agric. Sci. 2016, 6, 1275–1285. [Google Scholar]
- Al-Farsi, S.M.; Al-Sadi, A.M.; Ullah, A.; Farooq, M. Salt tolerance in alfalfa landraces of omani origin: Morpho-biochemical, mineral, and genetic diversity assessment. J. Soil Sci. Plant Nutr. 2021, 1–16. [Google Scholar] [CrossRef]
- Wang, B.S. Plant Biology under Stress; Higher Education Press: Beijing, China, 2010. [Google Scholar]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar]
- Karatas, I.; Ozturk, L.; Demir, Y.; Unlukara, A.; Kurunc, A.; Duzdemir, O. Alterations in antioxidant enzyme activities and proline content in pea leaves under long-term drought stress. Toxicol. Ind. Health 2012, 30, 693–700. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment—A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Parish, R.W.; Phan, H.A.; Iacuone, S.; Li, S.F. Tapetal development and abiotic stress: A centre of vulnerability. Funct. Plant Biol. 2012, 39, 553–559. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.K.; Kaur, N. Differential responses of antioxidative defencesystem to long-term field drought in wheat (Triticum aestivum L.) genotypesdiffering in drought tolerance. J. Agron. Crop Sci. 2012, 198, 185–195. [Google Scholar] [CrossRef]
- Cao, X.S.; Feng, Y.Y.; Li, H.P.; Zheng, H.X.; Wang, J.; Tong, C.F.; Wang, J.W. Effects of subsurface drip irrigation on water consumption and yields of alfalfa under different water and fertilizer conditions. J. Sens. 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Z.; White, P.J. The effect of supplemental irrigation after jointing on leaf senescence and grain filling in wheat. Field Crops Res. 2013, 151, 35–44. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Mu, L.; Xu, R.; Yang, H. Effect of regulated deficit irrigation on alfalfa performance under two irrigation systems in the inland arid area of midwestern china. Agric. Water Manag. 2021, 248, 106764. [Google Scholar] [CrossRef]
- Szabolcs, I. Salt Affected Soils in Europe; Martinus Nijhoff: The Hague, The Netherlands, 1974; p. 63. [Google Scholar]
- Yuan, C.; Feng, S.; Wang, J.; Huo, Z.; Ji, Q. Effects of irrigation water salinity on soil salt content distribution, soil physical properties and water use efficiency of maize for seed production in arid northwest china. Int. J. Agric. Biol. Eng. 2018, 11, 137–145. [Google Scholar] [CrossRef]
- Bates, S.; Waldren, R.P.; Teare, I.D. Rapid determination of the free proline in water stress studies. Plant Soil 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Messedi, D.; Hessini, K.; Labidi, N.; Savoure, A.; Abdelly, C. Effect of sodium chloride on the response of the halophyte species Sesuvium portulacastrum grown in mannitol-induced water stress. J. Plant Res. 2007, 120, 291–299. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions - sciencedirect. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Angelini, R.; Manes, F.; Federico, R. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon deetiolation and wounding in chick-pea stems. Planta 1990, 182, 89–96. [Google Scholar] [CrossRef]
- Fisher, R.A.; Maurer, R. Drought resistance in wheat cultivars I Grain yield response. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Rejili, M.; Vadel, A.M.; Guetet, A.; Neffatti, M. Effect of nacl on the growth and the ionic balance K+/Na+ of two populations of lotus creticus (l.) (papilionaceae). S. Afr. J. Bot. 2007, 73, 623–631. [Google Scholar] [CrossRef]
- Chen, C.X.; Xie, X.H.; Wang, Y.P.; Li, J.P.; Xin, J.; Zhu, T.T.; Liu, J.; Chen, M. Effects of salt and drought on the physiological characteristics of Elaeagnus angustifolia L. seedlings. Acta Ecol. Sinica. 2019, 39, 4540–4550. [Google Scholar]
- Ors, S.; Ekinci, M.; Yildirim, E.; Sahin, U.; Turan, M.; Dursun, A. Interactive effects of salinity and drought stress on photosynthetic characteristics and physiology of tomato (Lycopersicon esculentum L.) seedlings. S. Afr. J. Bot. 2021, 137, 335–339. [Google Scholar] [CrossRef]
- Paez-Garcia, A.; Christy, M.; Wolf-Rüdiger Scheible, M.; Chen, R.; Elison, B.; Blancaflor, M.; Monteros, J. Root traits and phenotyping strategies for plant improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.P.; Sui, F.G.; Da, G.T.; Sun, Z.H.; Lu, Y.Y.; Zhou, G.S. Effect of Soil Drought Stress on Leaf Water Status, Membrane Permeability and Enzymatic Antioxidant System of Maize. Pedosphere 2006, 16, 326–332. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef]

| Treatments | 2018 | 2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | S0 | S1 | S2 | S3 | |||
| ΔSWS (mm) | Mean | Mean | ||||||||
| W1 | −12.40 | −8.48 | −16.04 | −7.50 | −11.11c | −5.64 | −6.25 | −9.84 | −4.11 | −6.46b |
| W2 | −9.76 | −7.04 | −11.93 | −1.13 | −7.47b | −8.66 | −7.13 | −8.49 | −2.59 | −6.72b |
| W3 | −9.39 | −7.10 | −5.52 | 3.04 | −4.74a | −9.71 | −9.51 | −4.89 | 1.77 | −5.59a |
| Mean | −10.52c | −7.54b | −11.16c | −1.86a | −8.00c | −7.63b | −7.74b | −1.64a | ||
| ETc max (mm) | Mean | Mean | ||||||||
| W1 | 7.89 | 8.05 | 10.54 | 8.93 | 8.85c | 7.73 | 7.25 | 9.84 | 6.52 | 7.84c |
| W2 | 10.85 | 11.11 | 11.98 | 11.48 | 11.36b | 10.61 | 10.23 | 10.13 | 9.80 | 10.19b |
| W3 | 15.84 | 15.27 | 15.14 | 16.03 | 15.57a | 12.64 | 12.75 | 12.76 | 11.67 | 12.46a |
| Mean | 11.53b | 11.48b | 12.55a | 12.15a | 10.33ab | 10.08b | 10.91a | 9.33c | ||
| ETc min (mm) | Mean | Mean | ||||||||
| W1 | 6.14 | 5.18 | 3.93 | 3.04 | 4.57c | 5.70 | 6.09 | 3.36 | 5.10 | 5.06c |
| W2 | 6.34 | 6.89 | 5.13 | 4.88 | 5.81b | 6.85 | 6.53 | 6.56 | 6.25 | 6.55b |
| W3 | 8.25 | 7.63 | 7.98 | 4.24 | 7.03a | 10.19 | 9.84 | 9.01 | 8.74 | 9.45a |
| Mean | 6.91a | 6.57a | 5.68b | 4.05c | 7.58a | 7.49a | 6.31b | 6.70b | ||
| ETc sum (mm) | ||||||||||
| W1 | 42.00 | 38.08 | 45.64 | 37.10 | 40.71c | 40.31 | 39.11 | 42.04 | 35.11 | 39.14c |
| W2 | 55.36 | 52.64 | 57.53 | 46.73 | 53.07b | 55.26 | 53.09 | 52.73 | 49.45 | 52.63b |
| W3 | 71.49 | 69.20 | 67.62 | 59.06 | 66.84a | 69.74 | 68.35 | 66.99 | 60.82 | 66.48a |
| Mean | 56.28a | 53.31b | 56.93a | 47.63c | 55.10a | 53.52b | 53.92b | 48.46c | ||
| WCI (mm·d−1) | Mean | Mean | ||||||||
| W1 | 2.33 | 2.12 | 2.54 | 2.06 | 2.26c | 2.24 | 2.17 | 2.34 | 1.95 | 2.18c |
| W2 | 3.08 | 2.92 | 3.20 | 2.60 | 2.95b | 3.07 | 2.95 | 2.93 | 2.75 | 2.93b |
| W3 | 3.97 | 3.84 | 3.76 | 3.28 | 3.71a | 3.87 | 3.80 | 3.72 | 3.38 | 3.69a |
| Mean | 3.13a | 2.96b | 3.17a | 2.65c | 3.06a | 2.97b | 3.00a | 2.69c | ||
| ANOVA test | ΔSWS | ETc max | ETc min | ETc sum | WCI | ΔSWS | ETc max | ETc min | ETc sum | WCI |
| Water level (W) | ** | * | * | * | * | ** | * | ** | * | * |
| Salinity level (S) | ** | ns | ** | ** | ** | ** | ** | ** | * | * |
| W × S | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Treatments | 2018 | 2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | S0 | S1 | S2 | S3 | |||
| Plant height (cm) | Mean | Mean | ||||||||
| W1 | 24.40 | 24.20 | 23.10 | 23.10 | 23.70b | 24.60 | 24.90 | 24.20 | 24.60 | 24.58b |
| W2 | 26.30 | 28.30 | 26.20 | 24.20 | 26.25a | 25.90 | 28.50 | 26.50 | 24.70 | 26.40a |
| W3 | 28.50 | 29.40 | 27.50 | 25.20 | 27.65a | 26.80 | 29.50 | 27.50 | 25.10 | 27.23a |
| Mean | 26.40ab | 27.30a | 25.60ab | 24.17b | 25.77ab | 27.63a | 26.07ab | 24.80b | ||
| Aboveground biomass (g) | Mean | Mean | ||||||||
| W1 | 4.19 | 4.30 | 4.23 | 3.75 | 4.12 | 4.13 | 4.38 | 4.27 | 3.94 | 4.18c |
| W2 | 6.03 | 6.57 | 6.56 | 5.76 | 6.23 | 6.50 | 6.77 | 6.59 | 5.99 | 6.46b |
| W3 | 9.70 | 8.70 | 8.44 | 6.23 | 8.27 | 9.80 | 7.41 | 6.77 | 6.76 | 7.69a |
| Mean | 6.64a | 6.52a | 6.41a | 5.25b | 6.81a | 6.19ab | 5.88b | 5.56b | ||
| Root biomass (g) | Mean | Mean | ||||||||
| W1 | 2.46 | 2.35 | 2.31 | 2.21 | 2.33 | 2.53 | 2.44 | 2.41 | 2.23 | 2.40b |
| W2 | 2.65 | 2.61 | 2.58 | 2.48 | 2.58 | 2.75 | 2.60 | 2.56 | 2.54 | 2.61ab |
| W3 | 3.09 | 2.78 | 2.71 | 2.54 | 2.78 | 3.10 | 2.84 | 2.89 | 2.61 | 2.86a |
| Mean | 2.73a | 2.58ab | 2.53ab | 2.41b | 2.79a | 2.63ab | 2.62ab | 2.46b | ||
| Root/shoot ratio (%) | Mean | Mean | ||||||||
| W1 | 58.71 | 54.65 | 54.61 | 58.93 | 56.73a | 61.26 | 55.75 | 56.44 | 56.60 | 57.51a |
| W2 | 43.95 | 39.73 | 39.33 | 43.06 | 41.51b | 42.31 | 38.40 | 38.85 | 42.40 | 40.49b |
| W3 | 31.86 | 31.95 | 32.11 | 40.77 | 34.17c | 31.63 | 38.33 | 42.69 | 38.61 | 37.81c |
| Mean | 44.84b | 42.11c | 42.02c | 47.59a | 45.07ab | 44.16b | 45.99a | 45.87a | ||
| ANOVA test | Plant height | Aboveground biomass | Root biomass | Root/shoot ratio | Plant height | Aboveground biomass | Root biomass | Root/shoot ratio | ||
| Water level (W) | ** | *** | * | *** | * | *** | ns | *** | ||
| Salinity level (S) | * | *** | *** | ns | ns | ** | ** | ns | ||
| W × S | ns | *** | *** | ns | ns | *** | ** | *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.; Li, X.; Tian, D.; Xu, B.; Zhang, C.; Ren, J.; Chen, N. Evaluation of the Effects of Water and Salinity Stress on the Growth and Biochemistry of Alfalfa (Medicago sativa L.) at the Branching Stage. Sustainability 2022, 14, 10262. https://doi.org/10.3390/su141610262
Hou C, Li X, Tian D, Xu B, Zhang C, Ren J, Chen N. Evaluation of the Effects of Water and Salinity Stress on the Growth and Biochemistry of Alfalfa (Medicago sativa L.) at the Branching Stage. Sustainability. 2022; 14(16):10262. https://doi.org/10.3390/su141610262
Chicago/Turabian StyleHou, Chenli, Xianyue Li, Delong Tian, Bing Xu, Chen Zhang, Jie Ren, and Ning Chen. 2022. "Evaluation of the Effects of Water and Salinity Stress on the Growth and Biochemistry of Alfalfa (Medicago sativa L.) at the Branching Stage" Sustainability 14, no. 16: 10262. https://doi.org/10.3390/su141610262





