Achieving a Superhydrophobic, Moisture, Oil and Gas Barrier Film Using a Regenerated Cellulose–Calcium Carbonate Composite Derived from Paper Components or Waste
Abstract
:1. Introduction
2. Materials and Methods
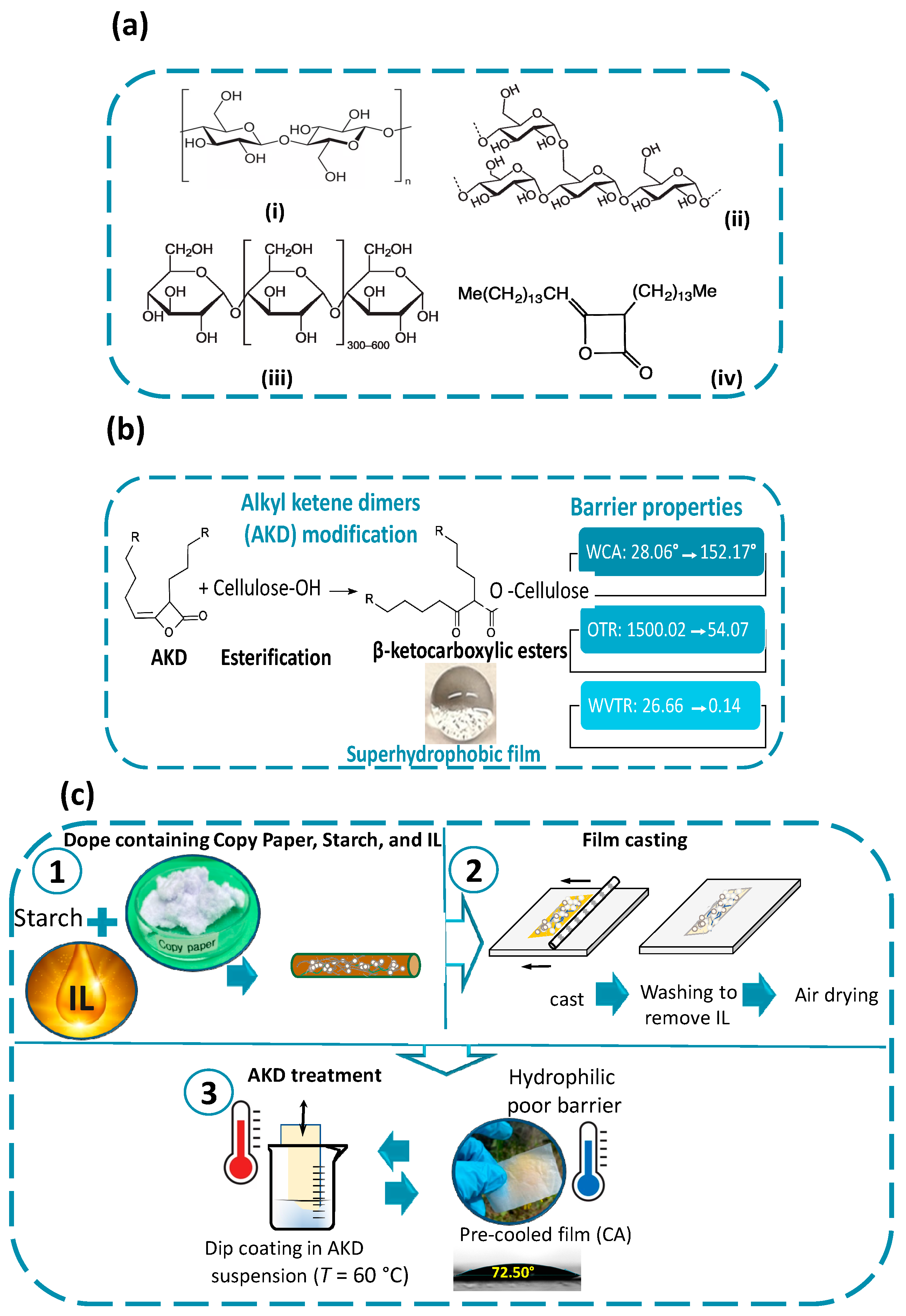
2.1. Dope Formulation and Preparation of Regenerated Cellulose Films
2.2. Surface Treatment of the Film Using Aqueous AKD Emulsion
2.3. Characterisation
2.3.1. Thickness, Density, Weight, and Porosity
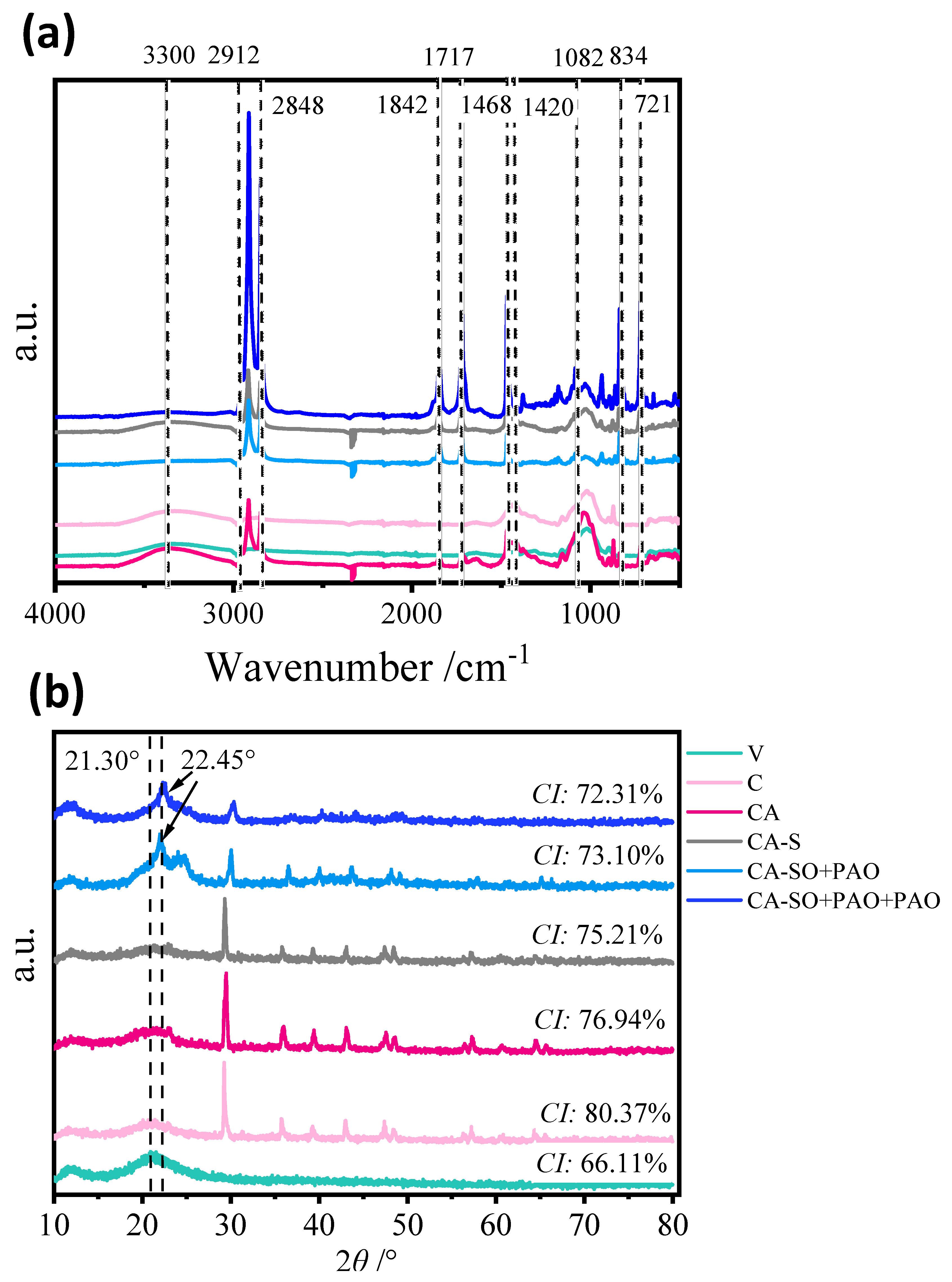
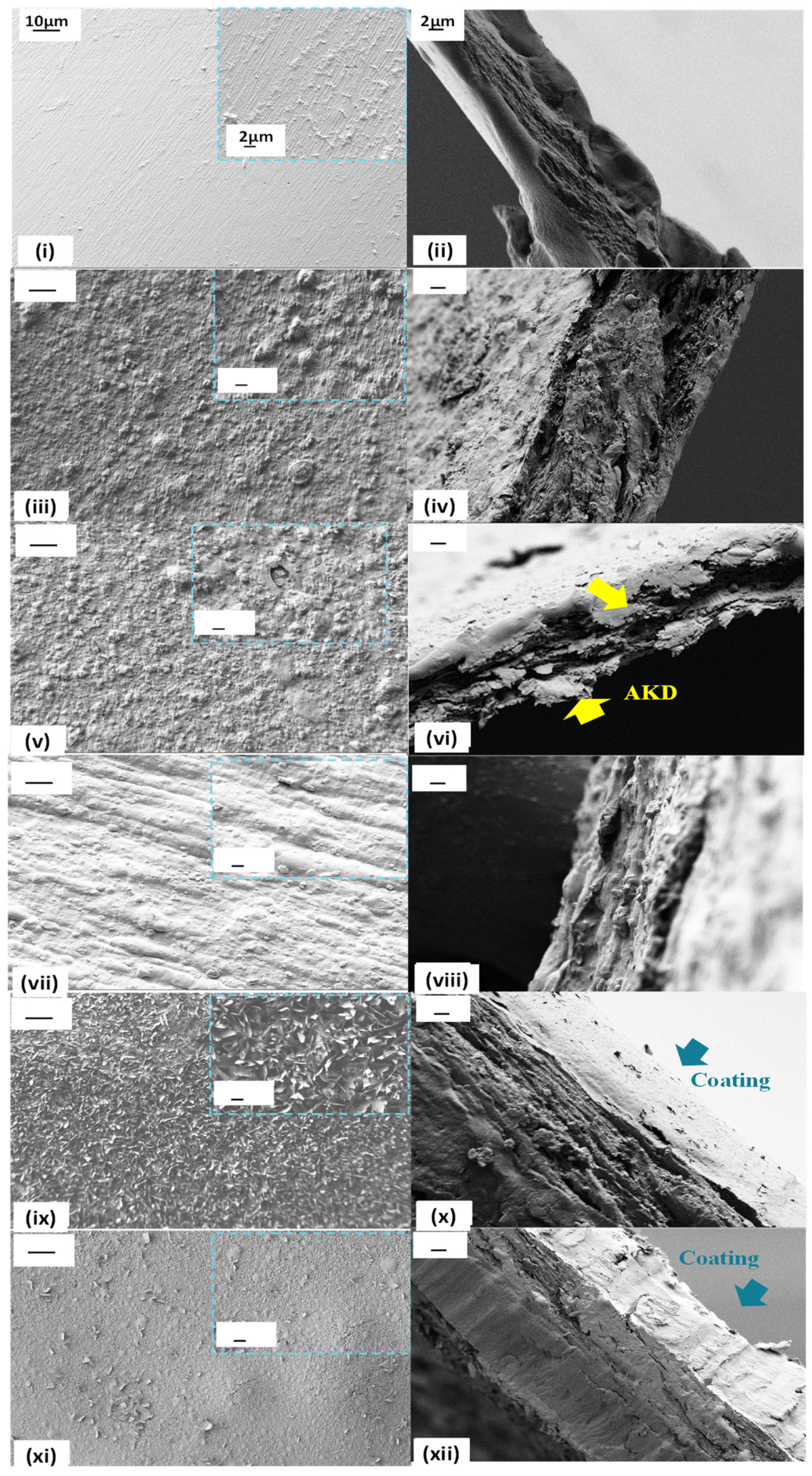
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR), X-ray Diffractometry (XRD), and Scanning Electron Microscopy (SEM)
2.3.3. Mechanical Analysis
2.3.4. Water Contact Angle (θWCA) and Roll-Off Angle (θRoA)
2.3.5. Water Vapour Transmission Rate (WVTR), and Oxygen Transmission Rate (O2TR)
2.3.6. Grease/Oil Penetration Rate
2.3.7. Abrasion Resistance Property
3. Results and Discussion
3.1. Characterisation of the Composite Films
3.2. Mechanical Properties of the Films
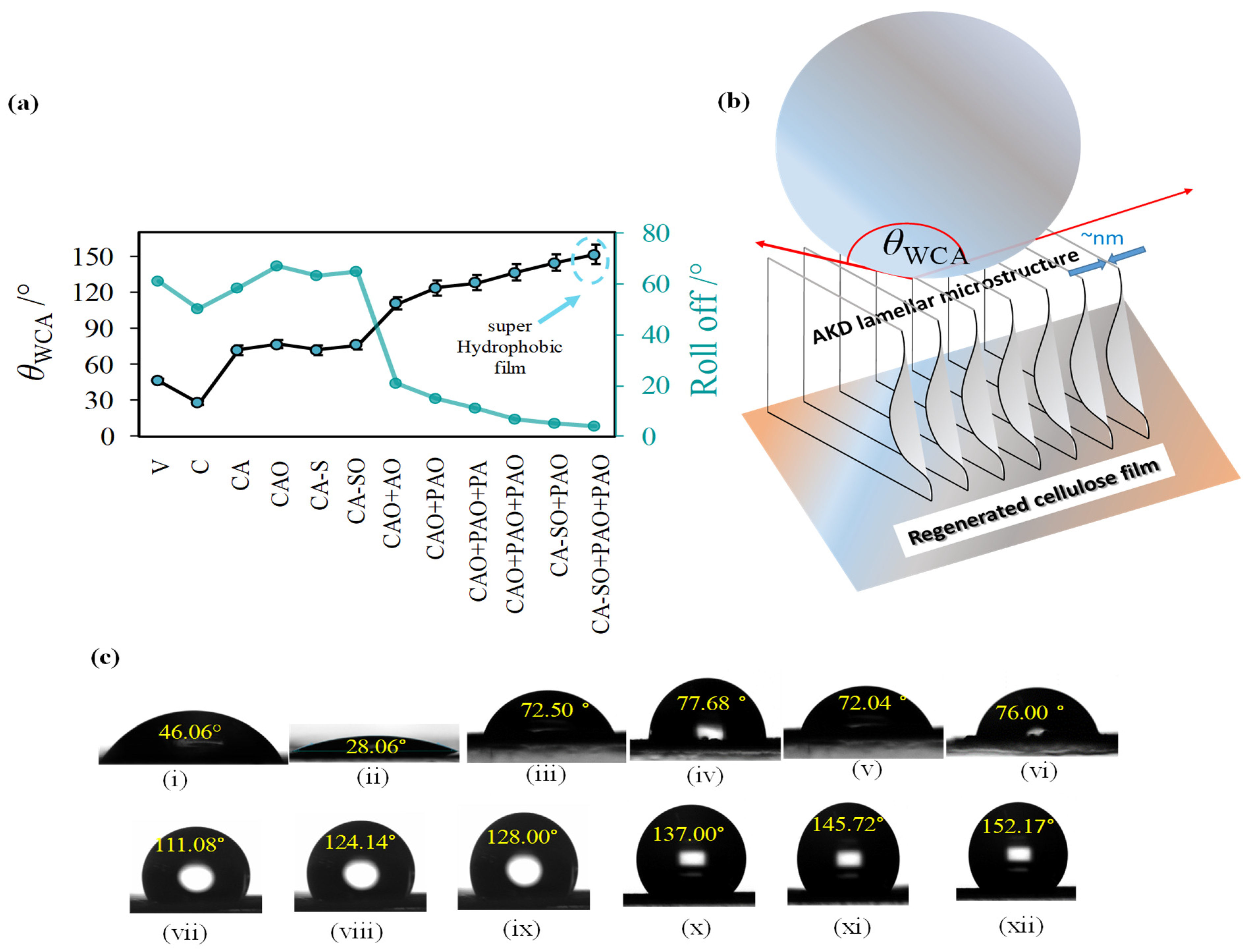
3.3. Changing from Hydrophilicity to Reach the Level of Superhydrophobicity
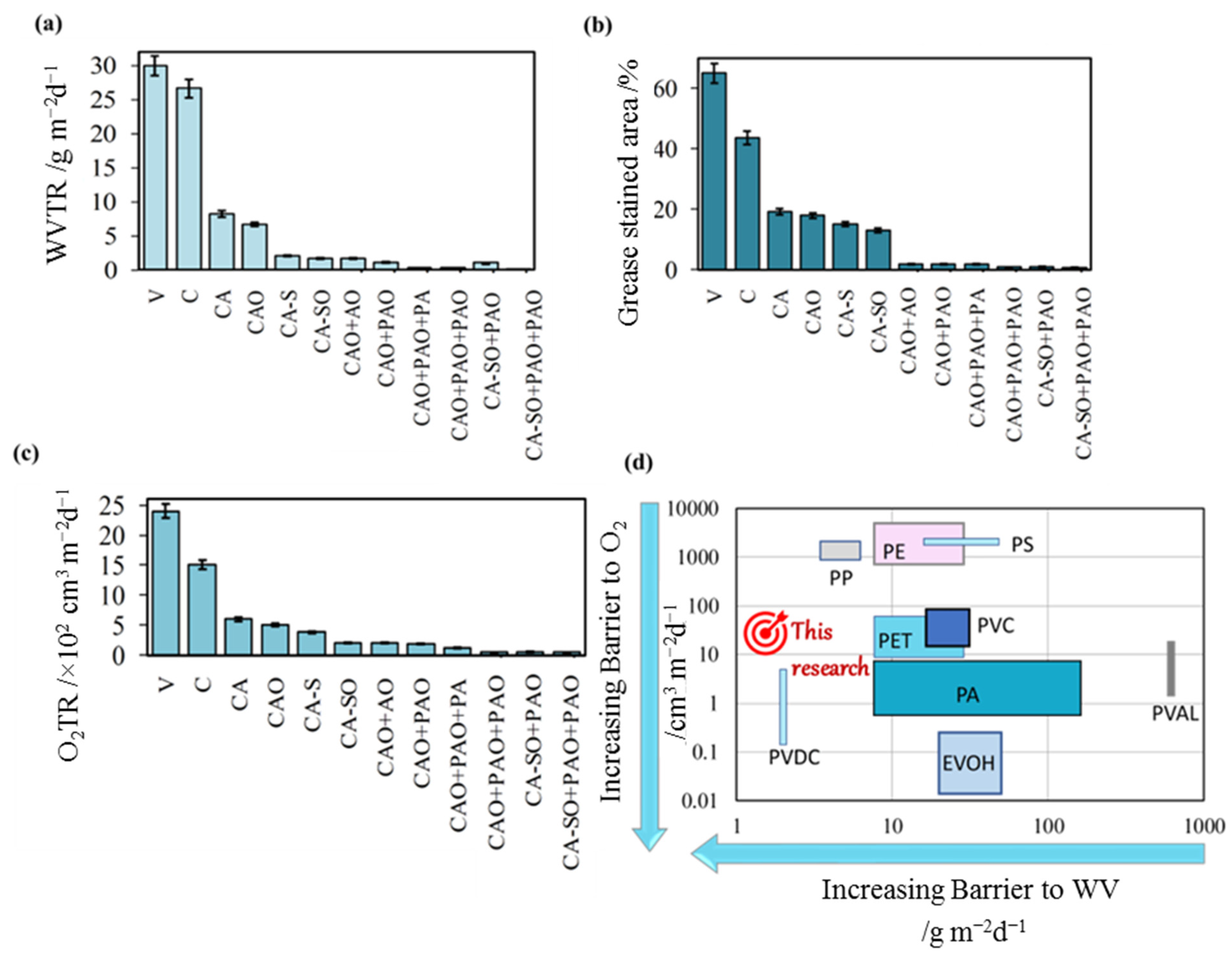
3.4. Barrier Properties of the Films
3.4.1. Water Vapour Transmission Rate
3.4.2. Grease/Oil Resistance
3.4.3. Oxygen Gas Permeation Properties
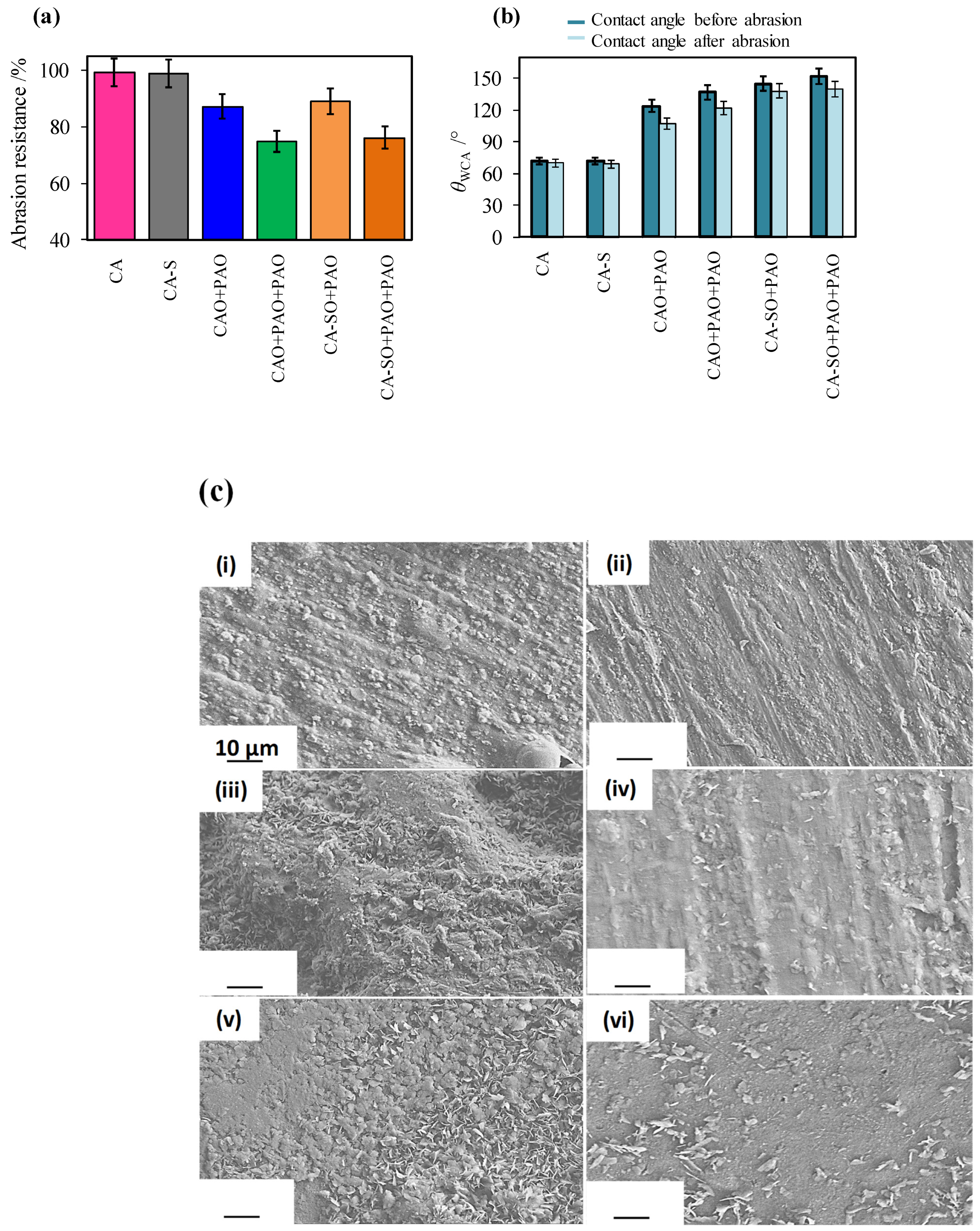
3.5. Reaching the Practical Barrier Property Target-Product Surface Robustness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdulkhani, A.; Marvast, E.H.; Ashori, A.; Karimi, A.N. Effects of dissolution of some lignocellulosic materials with ionic liquids as green solvents on mechanical and physical properties of composite films. Carbohydr. Polym. 2013, 95, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, B.; Hu, J.; Hou, H. Ionic liquid mediated technology for fabrication of cellulose film using gutta percha as an additive. Ind. Crops Prod. 2017, 108, 140–148. [Google Scholar] [CrossRef]
- Barać, N.; Barcelo, E.; Stojanović, D.; Milovanović, S.; Uskoković, P.; Gane, P.; Dimić-Mišić, K.; Imani, M.; Janacković, D. Modification of CaCO3 and CaCO3 pin-coated cellulose paper under supercritical carbon dioxide—Ethanol mixture for enhanced NO2 capture. Environ. Sci. Pollut. Res. 2021, 29, 11707–117171. [Google Scholar] [CrossRef]
- Gane, P.; Dimić-Mišić, K.; Barać, N.; Imani, M.; Janaćković, D.; Uskoković, P.; Barceló, E. Unveiling a recycling-sourced mineral-biocellulose fibre composite for use in combustion-generated NOx mitigation forming plant nutrient: Meeting sustainability development goals in the circular economy. Appl. Sci. 2020, 10, 3927. [Google Scholar] [CrossRef]
- Aimonen, K.; Imani, M.; Hartikainen, M.; Suhonen, S.; Vanhala, E.; Moreno, C.; Rojas, O.J.; Norppa, H.; Catalán, J. Surface functionalization and size modulate the formation of reactive oxygen species and genotoxic effects of cellulose nanofibrils. Part. Fibre Toxicol. 2022, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Adibi, A.; Valdesueiro, D.; Mok, J.; Behabtu, N.; Lenges, C.; Simon, L.; Mekonnen, T.H. Sustainable barrier paper coating based on alpha-1, 3 glucan and natural rubber latex. Carbohydr. Polym. 2022, 282, 119121. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef]
- Imani, M.; Ghasemian, A.; Dehghani-Firouzabadi, M.R.; Afra, E.; Gane, P.A.; Rojas, O.J. Nano-lignocellulose from recycled fibres in coatings from aqueous and ethanolic media: Effect of residual lignin on wetting and offset printing quality. Nord. Pulp Pap. Res. J. 2019, 34, 200–210. [Google Scholar] [CrossRef]
- Imani, M.; Ghasemian, A.; Dehghani-Firouzabadi, M.R.; Afra, E.; Borghei, M.; Johansson, L.S.; Gane, P.A.; Rojas, O.J. Coupling nanofibril lateral size and residual lignin to tailor the properties of lignocellulose films. Adv. Mater. Interfaces 2019, 6, 1900770. [Google Scholar] [CrossRef]
- Tarrés, Q.; Oliver-Ortega, H.; Ferreira, P.J.; Àngels Pèlach, M.; Mutjé, P.; Delgado-Aguilar, M. Towards a new generation of functional fiber-based packaging: Cellulose nanofibers for improved barrier, mechanical and surface properties. Cellulose 2018, 25, 683–695. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, C.; Cui, B.; Liu, P.; Wu, Z.; Zhao, H. Formation of high amylose corn starch/konjac glucomannan composite film with improved mechanical and barrier properties. Carbohydr. Polym. 2021, 251, 117039. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Zhou, Q.; Xu, Z.; Zhang, J.; Ji, X.; Zhang, J.; Nawaz, H.; Wang, J.; Peng, J. Cellulose-based films with ultraviolet shielding performance prepared directly from waste corrugated pulp. Polymers 2021, 13, 3359. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Mohammad, M. Recent advances in cellulose-based hydrophobic food packaging. Emerg. Mater. 2021, 5, 703–718. [Google Scholar] [CrossRef]
- Wu, R.-L.; Wang, X.-L.; Wang, Y.-Z.; Bian, X.-C.; Li, F. Cellulose/soy protein isolate blend films prepared via room-temperature ionic liquid. Ind. Eng. Chem. Res. 2009, 48, 7132–7136. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Kostić, M.; Imani, M.; Ivanovska, A.; Radojević, V.; Dimic-Mišić, K.; Barać, N.; Stojanović, D.; Janacković, D.; Uskoković, P.; Barceló, E.; et al. Extending waste paper, cellulose and filler use beyond recycling by entering the circular economy creating cellulose-CaCO3 composites reconstituted from ionic liquid. Cellulose 2022, 29, 5037–5059. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Liu, W.; Sun, F.; Jiang, L.; Meredith, J.C.; Deng, Y. Surface structure patterning for fabricating non-fluorinated superhydrophobic cellulosic membranes. ACS Appl. Polym. Mater. 2019, 1, 1220–1229. [Google Scholar] [CrossRef]
- Reverdy, C.; Belgacem, N.; Moghaddam, M.S.; Sundin, M.; Swerin, A.; Bras, J. One-step superhydrophobic coating using hydrophobized cellulose nanofibrils. Colloids Surf. A 2018, 544, 152–158. [Google Scholar] [CrossRef]
- Ganicz, T.; Rozga-Wijas, K. Siloxane-Starch-Based Hydrophobic Coating for Multiple Recyclable Cellulosic Materials. Materials 2021, 14, 4977. [Google Scholar] [CrossRef]
- MADE SAFE Ltd. Protolabs. Available online: https://www.madesafe.org/chemicalcallout-siloxanes-silanes/ (accessed on 9 April 2019).
- Lassen, C.; Hansen, C.L.; Mikkelsen, S.H.; Maag, J. Siloxanes—Consumption, Toxicity and Alternatives; No. 1031; Danish Ministry of the Environmental: Copenhagen, Denmark, 2005. [Google Scholar]
- Ryu, Y.S.; Lee, J.H.; Kim, S.H. Efficacy of alkyl ketene dimer modified microcrystalline cellulose in polypropylene matrix. Polymer 2020, 196, 122463. [Google Scholar] [CrossRef]
- Van Nguyen, S.; Lee, B.K. Polyvinyl alcohol/alkyl ketene dimer films with excellent water resistance and water vapor barrier properties. Mater. Lett. 2022, 307, 131045. [Google Scholar] [CrossRef]
- Yang, Q.; Saito, T.; Isogai, A. Facile fabrication of transparent cellulose films with high water repellency and gas barrier properties. Cellulose 2012, 19, 1913–1921. [Google Scholar] [CrossRef]
- Yang, Q.; Takeuchi, M.; Saito, T.; Isogai, A. Formation of nanosized islands of dialkyl β-ketoester bonds for efficient hydrophobization of a cellulose film surface. Langmuir 2014, 30, 8109–8118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Wang, Q.; Ji, X.; Yang, G.; Chen, J.; Fatehi, P. Strong, ductile and biodegradable polylactic acid/lignin-containing cellulose nanofibril composites with improved thermal and barrier properties. Ind. Crops Prod. 2021, 171, 113898. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, H.; Wang, Y.; Liang, N.; Wang, G. Intercalation of cetyl trimethylammonium ion into sericite in the solvent of dimethyl sulfoxide. Appl. Clay Sci. 2013, 74, 109–114. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, D.; Yang, T.; Liang, N.; Ding, H. The Stability of Intercalated Sericite by Cetyl Trimethylammonium Ion under Different Conditions and the Preparation of Sericite/Polymer Nanocomposites. Polymers 2019, 11, 900. [Google Scholar] [CrossRef] [Green Version]
- Gill, Y.Q.; Abid, U.; Song, M. High performance Nylon12/clay nanocomposites for potential packaging applications. J. Appl. Polym. Sci. 2020, 137, 49247. [Google Scholar] [CrossRef]
- Al-Attar, F.; Al-Samhan, M. Nano CaCO3 incorporation with polypropylene to reduce film water vapor permeability for packaging application. Asian J. Sci. Res. 2020, 13, 275–283. [Google Scholar] [CrossRef]
- Aframehr, W.M.; Molki, B.; Heidarian, P.; Behzad, T.; Sadeghi, M.; Bagheri, R. Effect of calcium carbonate nanoparticles on barrier properties and biodegradability of polylactic acid. Fibers Polym. 2017, 18, 2041–2048. [Google Scholar] [CrossRef]
- Parviainen, A.; Wahlström, R.; Liimatainen, U.; Liitiä, T.; Rovio, S.; Helminen, J.K.J.; Hyväkkö, U.; King, A.W.T.; Suurnäkki, A.; Kilpeläinen, I. Sustainability of cellulose dissolution and regeneration in 1, 5-diazabicyclo [4.3. 0] non-5-enium acetate: A batch simulation of the IONCELL-F process. RSC Adv. 2015, 5, 69728–69737. [Google Scholar] [CrossRef] [Green Version]
- Imani, M.; Dimić-Mišić, K.; Tavakoli, M.; Rojas, O.J.; Gane, P.A. Coupled effects of fibril width, residual and mechanically liberated lignin on the flow, viscoelasticity, and dewatering of cellulosic nanomaterials. Biomacromolecules 2020, 21, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, R.; Lahti, J.; Abitbol, T.; Swerin, A.; Kuusipalo, J.; Toivakka, M. Continuous processing of nanocellulose and polylactic acid into multilayer barrier coatings. ACS Appl. Mater. Interfaces 2019, 11, 11920–11927. [Google Scholar] [CrossRef] [PubMed]
- ASTM D 4060-10; Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber ASTM Int. ASTM: West Conshohocken, PA, USA, 2010; p. 5.
- Fedorov, P.P.; Luginina, A.A.; Kuznetsov, S.V.; Voronov, V.V.; Yapryntsev, A.D.; Lyapin, A.A.; Pynenkov, A.A.; Nishchev, K.N.; Chernova, E.V.; Petukhov, D.I.; et al. Hydrophobic up-conversion carboxylated nanocellulose/fluoride phosphor composite films modified with alkyl ketene dimer. Carbohydr. Polym. 2020, 250, 116866. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, M.; Li, J.; Wang, C.; Li, S. Structure Optimization of Cellulose Nanofibers/Poly (Lactic Acid) Composites by the Sizing of AKD. Polymers 2021, 13, 4119. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Amer, H.; Rosenau, T.; Zollfrank, C.; Dörrstein, J.; Jobst, C.; Zimmermann, T.; Keckes, J.; Veigel, S.; Gindl-Altmutter, W.; et al. Dry, hydrophobic microfibrillated cellulose powder obtained in a simple procedure using alkyl ketene dimer. Cellulose 2016, 23, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, W.; Xiang, H.; Wu, H.; Li, Z.; Zhou, H.; Huang, W. based flexible strain and pressure sensor with enhanced mechanical strength and super-hydrophobicity that can work under water. J. Mater. Chem. C 2022, 10, 3908–3918. [Google Scholar] [CrossRef]
- Swain, S.K.; Dash, S.; Kisku, S.K.; Singh, R.K. Thermal and oxygen barrier properties of chitosan bionanocomposites by reinforcement of calcium carbonate nanopowder. J. Mater. Sci. Technol. J. 2014, 30, 791–795. [Google Scholar] [CrossRef]
- Shen, Z.; Kwon, S.; Oh, K.; Abhari, A.R.; Lee, H.L. Facile fabrication of hydrophobic cellulosic paper with good barrier properties via PVA/AKD dispersion coating. Nord. Pulp Pap. Res. J. 2019, 34, 516–524. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, M.; Luo, T.; Zhu, P.; Cheng, F.; Zhang, Y.; Lin, Y. A comparative investigation of gelatinized and regenerated starch composites reinforced by microfibrillated cellulose. Food Chem. 2022, 373, 131470. [Google Scholar] [CrossRef]
- Yook, S.; Park, H.; Park, H.; Lee, S.-Y.; Kwon, J.; Youn, H.J. Barrier coatings with various types of cellulose nanofibrils and their barrier properties. Cellulose 2020, 27, 4509–4523. [Google Scholar] [CrossRef]
- Nguyen, H.-L.; Tran, T.H.; Hao, L.T.; Jeon, H.; Koo, J.M.; Shin, G.; Hwang, D.S.; Hwang, S.Y.; Park, J.; Oh, D.X. Biorenewable, transparent, and oxygen/moisture barrier nanocellulose/nanochitin-based coating on polypropylene for food packaging applications. Carbohydr. Polym. 2021, 271, 118421. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aguilar, M.; González, I.; Jiménez, A.M.; Tarrés, Q.; Quintana, G.; Mutjé, P. Cellulose nanofibers modified with alkyl ketene dimer for oil absorbent aerogels. Cellul. Chem. Technol. 2016, 50, 369–375. [Google Scholar]



| Sample Abbreviation | Dope Chemical Composition in IL | Post Film-Formed Treatment |
|---|---|---|
| V | 13 w/w% of virgin cellulose | - |
| C | 13 w/w% preground Copy Paper | - |
| CA | 13 w/w% preground Copy Paper/1 w/w% AKD | - |
| CAO | 13 w/w% preground Copy Paper/1 w/w% AKD | oven-dried |
| CA-S | 13 w/w% preground Copy Paper/0.5 w/w% AKD + 0.5 w/w% starch | - |
| CA-SO | 13 w/w% preground Copy Paper/0.5 w/w% AKD + 0.5 w/w% starch | oven-dried |
| CAO+AO | 13 w/w% preground Copy Paper/1 w/w% AKD | oven-dried, dip in hot AKD emulsion, oven-dried |
| CAO+PAO | 13 w/w% preground Copy Paper/1 w/w% AKD | oven-dried, pre-cooled, dip in hot AKD emulsion, oven-dried |
| CA-SO+PAO | 13 w/w% preground Copy Paper/0.5 w/w% AKD + 0.5 w/w% starch | oven-dried, pre-cooled, dip in hot AKD emulsion, oven-dried |
| CAO+PAO+PA | 13 w/w% preground Copy Paper/1 w/w% AKD | oven-dried, pre-cooled, first dip in hot AKD emulsion, oven-dried, pre-cooled, second dip in hot AKD emulsion (without final oven drying) |
| CAO+PAO+PAO | 13 w/w% preground Copy Paper/1 w/w% AKD | oven-dried, pre-cooled, first dip in hot AKD emulsion, oven-dried, pre-cooled, second dip in hot AKD emulsion, oven-dried |
| CA-SO+PAO+PAO | 13 w/w% preground Copy Paper/0.5 w/w% AKD + 0.5 w/w% starch | oven-dried, pre-cooled, first dip in hot AKD emulsion, oven-dried, pre-cooled, second dip in hot AKD emulsion, oven-dried |
| Sample label | Thickness/ µm | Density/ g cm−3 | Basis Weight/ g m−2 | Porosity/ % | Coated AKD/ g m−2 |
|---|---|---|---|---|---|
| V | 15.75 ± 5.72 | 0.62 ± 0.01 | 29.53 ± 0.86 | 57.53 | - |
| C | 34.83 ± 5.38 | 0.77 ± 0.08 | 34.89 ± 5.83 | 32.51 | - |
| CA | 22.01 ± 8.49 | 0.67 ± 0.08 | 33.98 ± 5.68 | 41.28 | - |
| CA-S | 59.00 ± 8.02 | 0.71 ± 0.03 | 42.36 ± 2.00 | 37.77 | - |
| CAO+AO | 93.80 ± 1.10 | 0.89 ± 0.01 | 49.04 ± 1.09 | 21.99 | 15.06 |
| CAO+PAO+PAO | 216.01 ± 7.23 | 1.08 ± 0.03 | 68.88 ± 1.87 | 5.34 | 34.90 |
| CA-SO+PAO | 129.33 ± 9.40 | 0.74 ± 0.02 | 52.97 ± 3.90 | 35.14 | 10.61 |
| CA-SO+PAO+PAO | 211.21 ± 9.25 | 1.20 ± 0.07 | 69.55 ± 4.55 | 5.16 | 27.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imani, M.; Dimic-Misic, K.; Kostic, M.; Barac, N.; Janackovic, D.; Uskokovic, P.; Ivanovska, A.; Lahti, J.; Barcelo, E.; Gane, P. Achieving a Superhydrophobic, Moisture, Oil and Gas Barrier Film Using a Regenerated Cellulose–Calcium Carbonate Composite Derived from Paper Components or Waste. Sustainability 2022, 14, 10425. https://doi.org/10.3390/su141610425
Imani M, Dimic-Misic K, Kostic M, Barac N, Janackovic D, Uskokovic P, Ivanovska A, Lahti J, Barcelo E, Gane P. Achieving a Superhydrophobic, Moisture, Oil and Gas Barrier Film Using a Regenerated Cellulose–Calcium Carbonate Composite Derived from Paper Components or Waste. Sustainability. 2022; 14(16):10425. https://doi.org/10.3390/su141610425
Chicago/Turabian StyleImani, Monireh, Katarina Dimic-Misic, Mirjana Kostic, Nemanja Barac, Djordje Janackovic, Petar Uskokovic, Aleksandra Ivanovska, Johanna Lahti, Ernest Barcelo, and Patrick Gane. 2022. "Achieving a Superhydrophobic, Moisture, Oil and Gas Barrier Film Using a Regenerated Cellulose–Calcium Carbonate Composite Derived from Paper Components or Waste" Sustainability 14, no. 16: 10425. https://doi.org/10.3390/su141610425
APA StyleImani, M., Dimic-Misic, K., Kostic, M., Barac, N., Janackovic, D., Uskokovic, P., Ivanovska, A., Lahti, J., Barcelo, E., & Gane, P. (2022). Achieving a Superhydrophobic, Moisture, Oil and Gas Barrier Film Using a Regenerated Cellulose–Calcium Carbonate Composite Derived from Paper Components or Waste. Sustainability, 14(16), 10425. https://doi.org/10.3390/su141610425