Mitigating Autogenous Shrinkage of Alkali-Activated Slag Mortar by Using Porous Fine Aggregates as Internal Curing Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Proportions
2.3. Test Methods
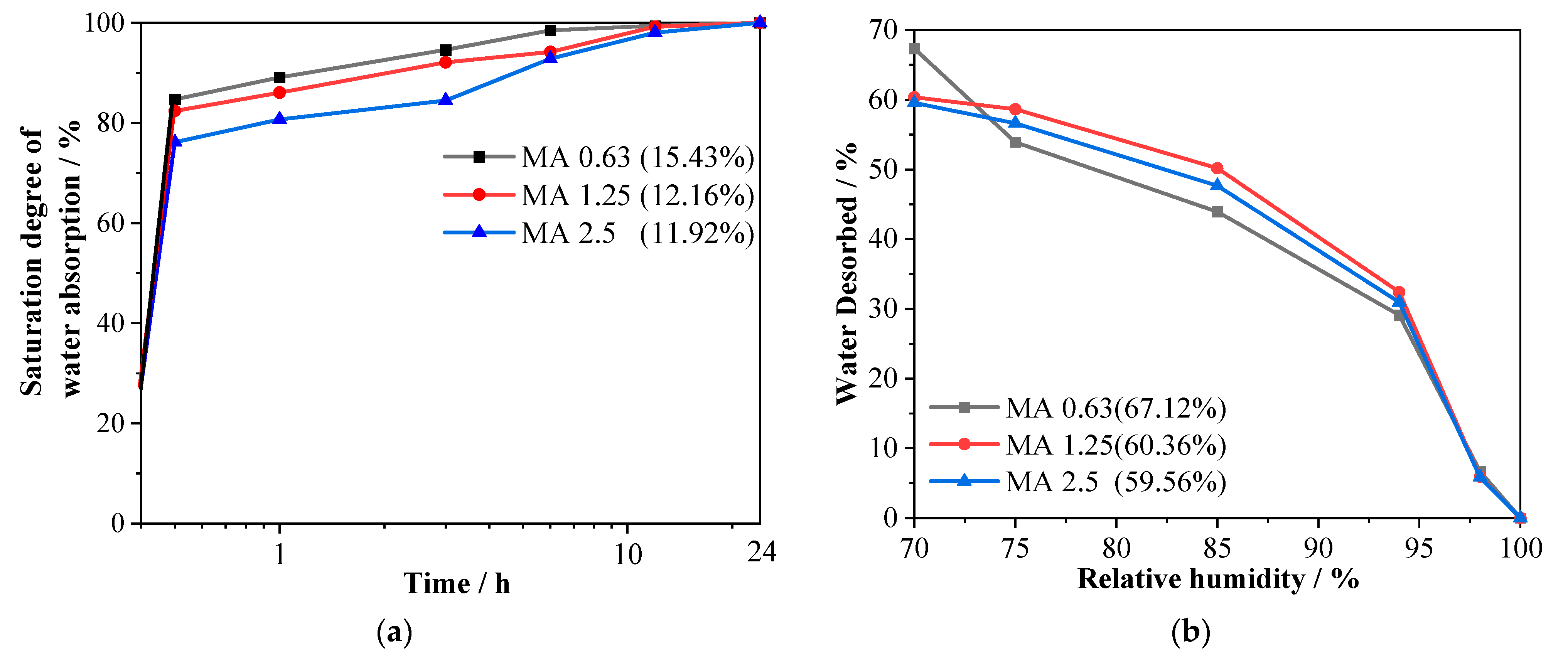
2.3.1. Absorption and Desorption Rates of RFAs
2.3.2. Flow
2.3.3. Internal Relative Humidity
2.3.4. Compressive Strength
2.3.5. Autogenous Shrinkage
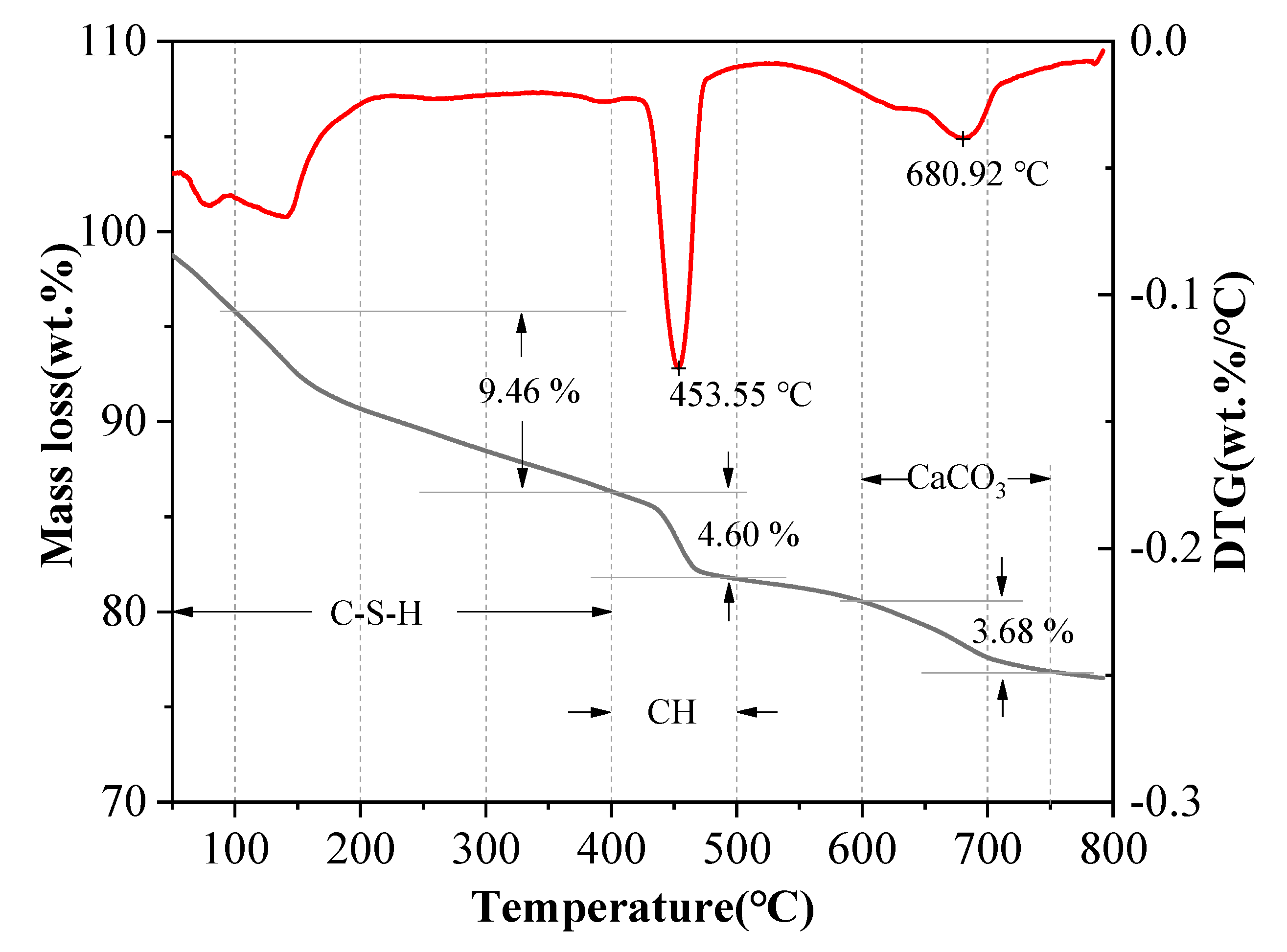
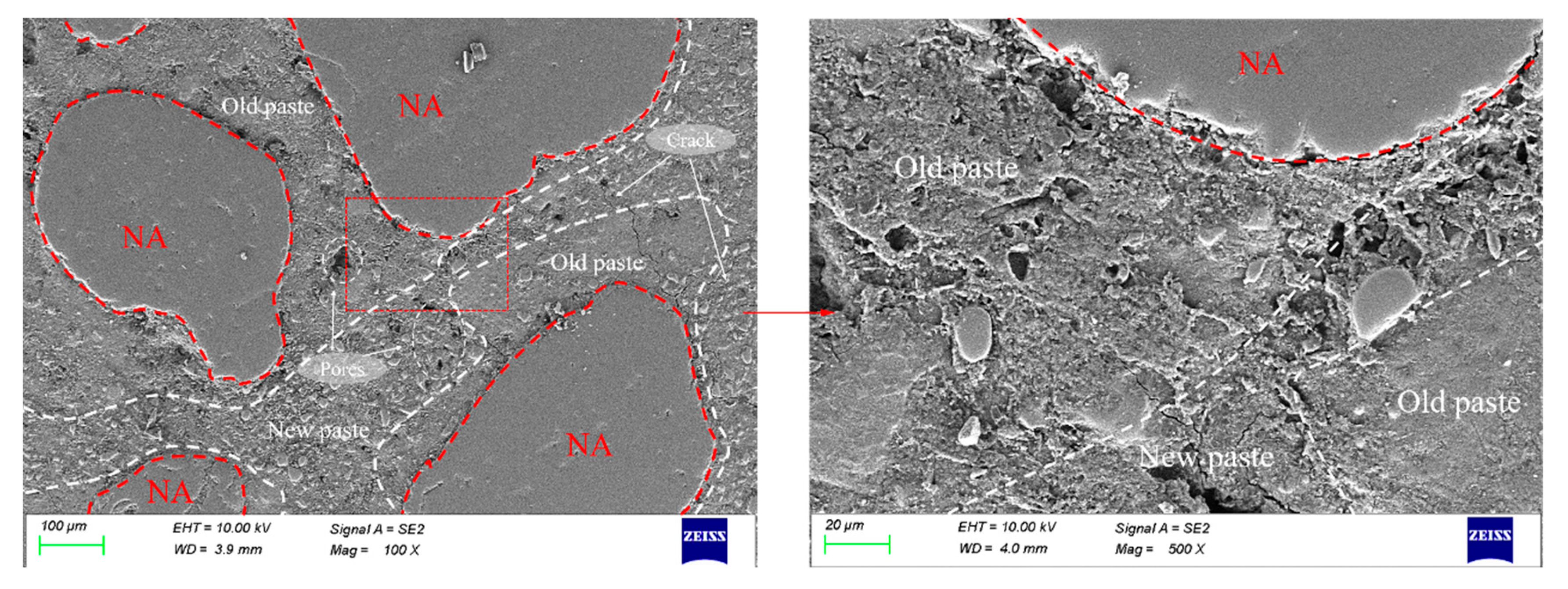
2.3.6. Pore Structure Testing and Interfacial Transition Zone (ITZ) Characterization
3. Results and Discussion
3.1. Absorption and Desorption Rates of RFAs
3.2. Flow
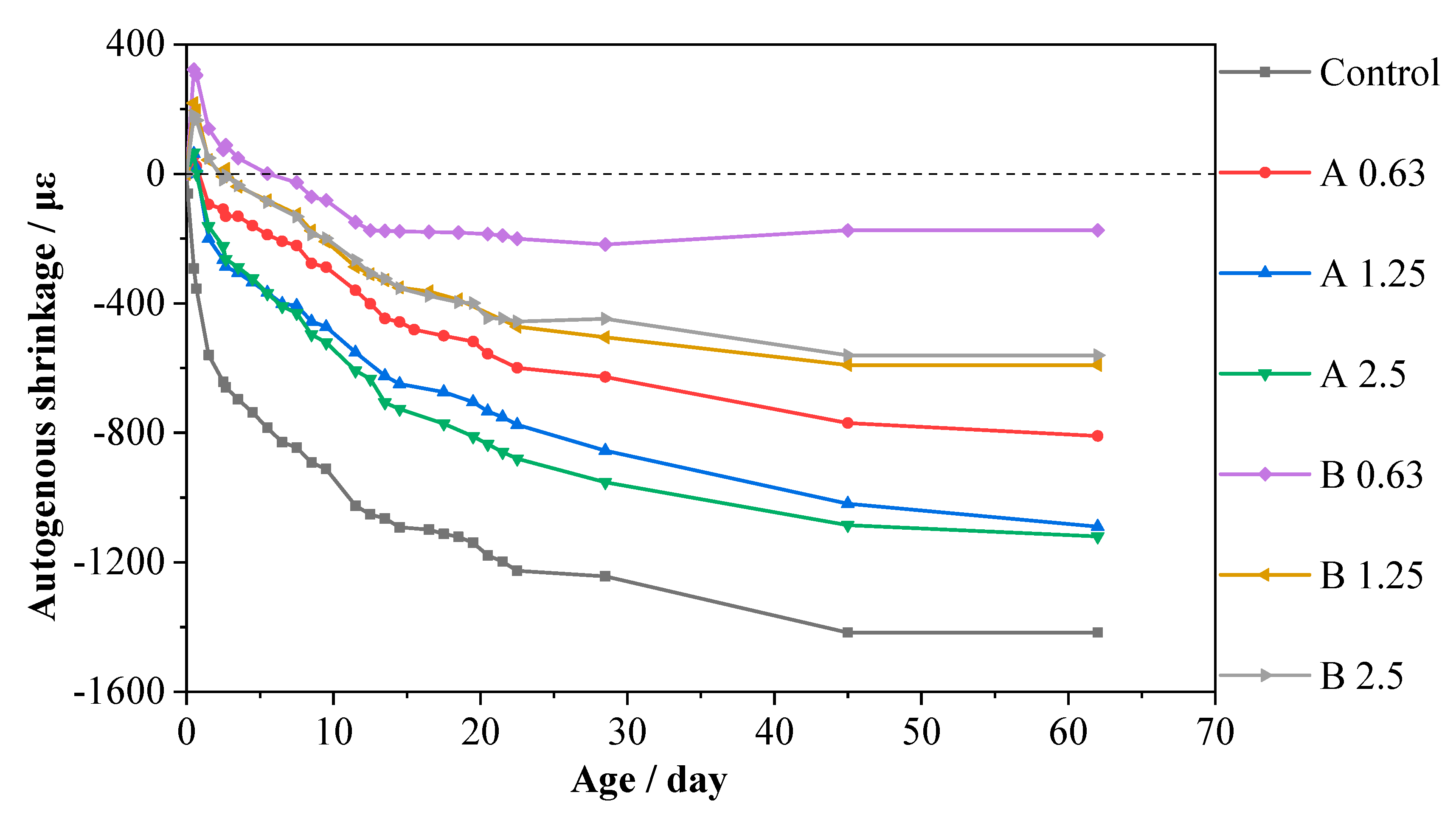
3.3. Autogenous Shrinkage
3.4. IRH of AAS Mortars
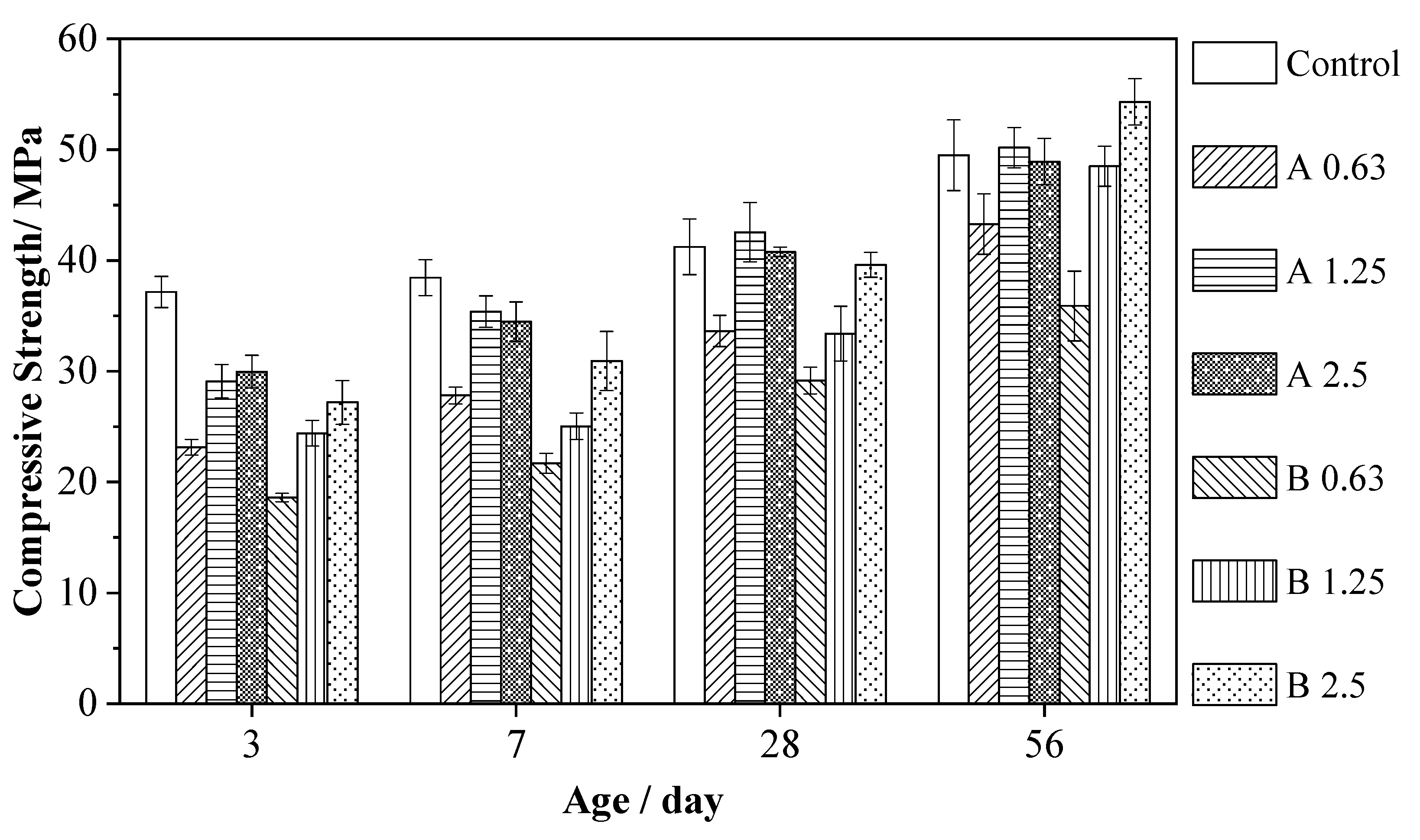
3.5. Compressive Strength
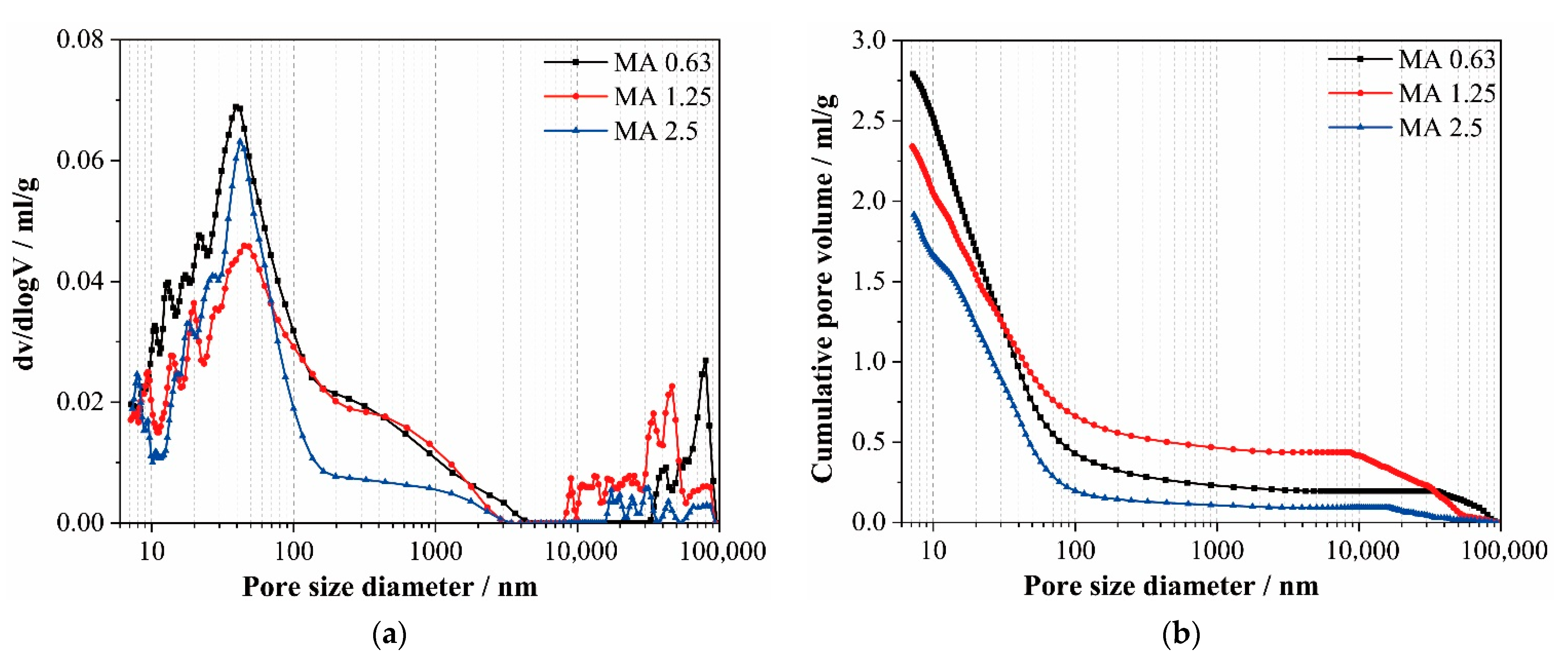
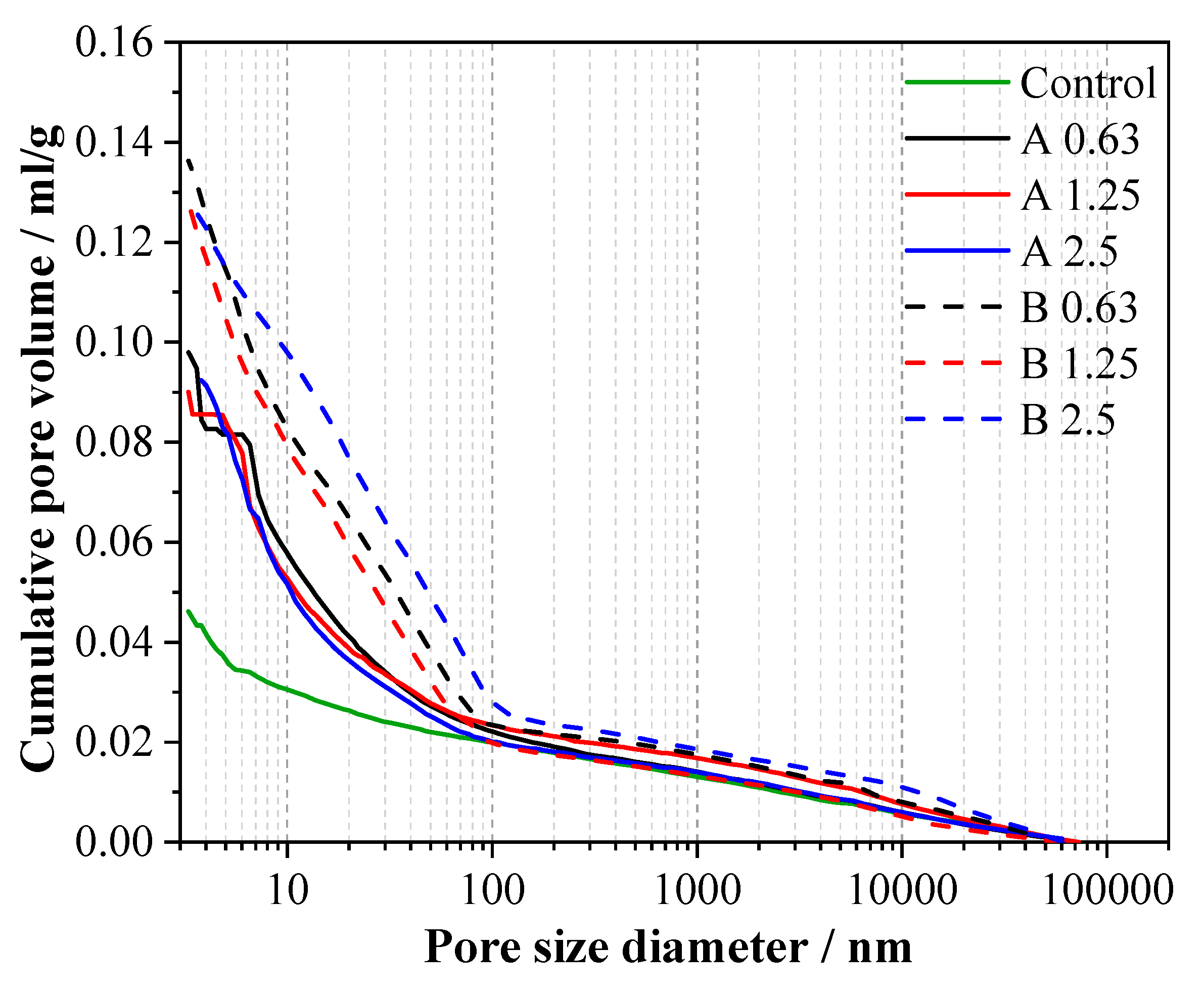
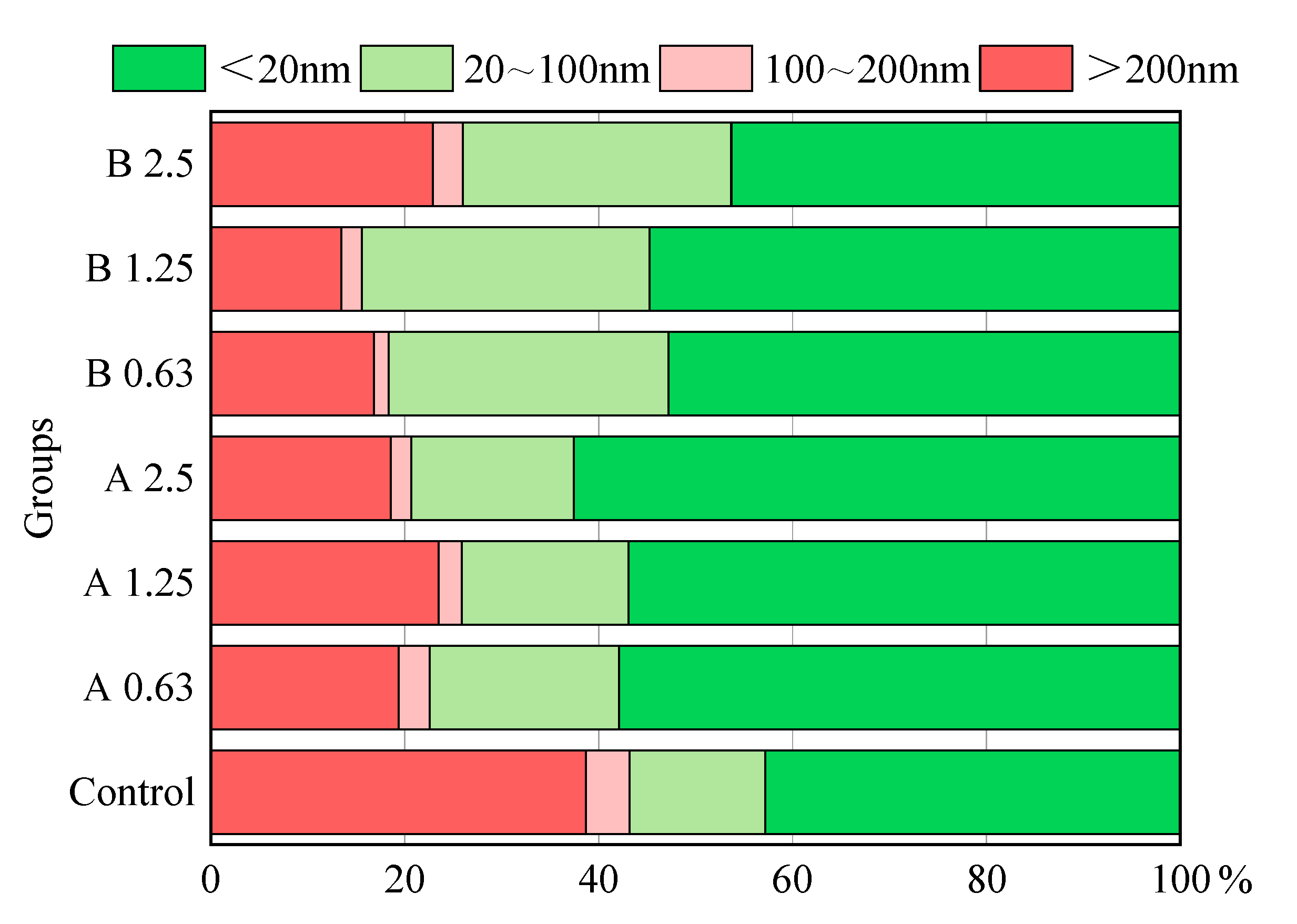
3.6. Pore Structure
4. Conclusions
- (1)
- MA is an effective internal curing agent to overcome the autogenous shrinkage of alkali-activated slag mortars. Its desorption ranged from 59.56 to 67.42%, depending on the sizes at a 70% relative humidity. The water carried by MAs can be released to be readily available when mixed with alkali-activated slag mixtures or exposed to environments with a higher relative humidity.
- (2)
- About 58.32 to 87.68% of the autogenous shrinkage of alkali-activated slag mortars could be reduced by incorporating saturated MAs. The effect became more significant as the MA particle size decreased, as smaller particles will introduce more additional water to the surrounding mortar. The internal curing water that was released helped to maintain the internal relative humidity at higher than 80% within the microstructure of alkali-activated slag mortars and significantly prolonged the critical time when the internal relative humidity started to decrease from 100%. Mortars incorporated with MAs with smaller sizes exhibited a superior mitigation of the autogenous shrinkage.
- (3)
- MAs are pozzolanic materials and continue to hydrate with an alkaline activator, which can compensate for the early compressive strength reductions. The pore structure of series A and B became more refined, and the percentages of finer pores increased versus the control. At 56 d, the compressive strengths of alkali-activated slag mortars with MAs were close to those of the reference mortar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, D.; Li, X.; Lv, Y.; Li, C.; Jiang, W.; Liu, Z.; Xu, J.; Zhou, Y.; Dan, J. Autogenous shrinkage and hydration property of alkali activated slag pastes containing superabsorbent polymer. Cem. Concr. Res. 2021, 149, 106581. [Google Scholar] [CrossRef]
- Chen, P.; Wang, J.; Wang, L.; Xu, Y. Perforated cenospheres: A reactive internal curing agent for alkali activated slag mortars. Cem. Concr. Compos. 2019, 104, 103351. [Google Scholar] [CrossRef]
- Lee, N.K.; Abate, S.Y.; Kim, H.-K. Use of recycled aggregates as internal curing agent for alkali-activated slag system. Constr. Build. Mater. 2018, 159, 286–296. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Hossain, M.M.; Karim, M.R.; Elahi, M.M.A.; Islam, M.N.; Zain, M.F.M. Long-term durability properties of alkali-activated binders containing slag, fly ash, palm oil fuel ash and rice husk ash. Constr. Build. Mater. 2020, 251, 119094. [Google Scholar] [CrossRef]
- Song, C.; Choi, Y.C.; Choi, C. Effect of internal curing by superabsorbent polymers—Internal relative humidity and autogenous shrinkage of alkali-activated slag mortars. Constr. Build. Mater. 2016, 123, 198–206. [Google Scholar] [CrossRef]
- Vafaei, B.; Farzanian, K.; Ghahremaninezhad, A. The influence of superabsorbent polymer on the properties of alkali-activated slag pastes. Constr. Build. Mater. 2020, 236, 117525. [Google Scholar] [CrossRef]
- Tu, W.; Fang, G.; Wang, X.; Zhang, M. Internal curing of alkali-activated fly ash-slag pastes using superabsorbent polymer. Cem. Concr. Res. 2019, 116, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liang, X.; Chen, Y.; Ye, G. Effect of metakaolin on the autogenous shrinkage of alkali-activated slag-fly ash paste. Constr. Build. Mater. 2021, 278, 122397. [Google Scholar] [CrossRef]
- Zhong, P.; Wyrzykowski, M.; Toroopovs, N.; Li, L.; Liu, J. Internal curing with superabsorbent polymers of different chemical structures. Cem. Concr. Res. 2019, 123, 105789. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Cheng, Y.; Huseien, G.F.; Shah, K.W. Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; Hu, X.; Liu, J.; Wu, Z.; Shi, C. Production of lightweight aggregates from bauxite tailings for the internal curing of high-strength mortars. Constr. Build. Mater. 2022, 341, 127800. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, P.; Zhang, Y.; Li, Z. Effect of superabsorbent polymer introduction on properties of alkali-activated slag mortar. Constr. Build. Mater. 2022, 340, 127541. [Google Scholar] [CrossRef]
- Kim, H.; Bentz, D. Internal Curing with Crushed Returned Concrete Aggregates for High Performance Concrete. In Proceedings of the NRMCA Concrete Technology Forum: Focus on Sustainable Development, Denver, CO, USA, 20–22 May 2008. [Google Scholar] [CrossRef]
- Van, V.-T.-A.; Rößler, C.; Bui, D.-D.; Ludwig, H.-M. Rice husk ash as both pozzolanic admixture and internal curing agent in ultra-high performance concrete. Cem. Concr. Compos. 2014, 53, 270–278. [Google Scholar] [CrossRef]
- Jongvisuttisun, P.; Kurtis, K.E. The role of hardwood pulp fibers in mitigation of early-age cracking. Cem. Concr. Compos. 2015, 57, 84–93. [Google Scholar] [CrossRef]
- Dixit, A.; Guota, S.; Pang, D.S.; Kua, H.W. Waste Valorisation using biochar for cement replacement and internal curing in ultra-high performance concrete. J. Clean. Prod. 2019, 238, 117876. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Qian, X.; Hollingsworth, J. Internal curing of high performance concrete using cenospheres. Cem. Concr. Res. 2017, 95, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Sakulich, A.R.; Bentz, D.P. Mitigation of autogenous shrinkage in alkali activated slag mortars by internal curing. Mater. Struct. 2012, 46, 1355–1367. [Google Scholar] [CrossRef]
- Rodríguez-Álvaro, R.; Seara-Paz, S.; González-Fonteboa, B.; Etxeberria, M. Study of different granular by-products as internal curing water reservoirs in concrete. J. Build. Eng. 2022, 45, 103623. [Google Scholar] [CrossRef]
- Gonzalez-Corominas, A.; Etxeberria, M. Effects of using recycled concrete aggregates on the shrinkage of high performance concrete. Constr. Build. Mater. 2016, 115, 32–41. [Google Scholar] [CrossRef]
- Khoshkenari, A.G.; Shafigh, P.; Moghimi, M.; Mahmud, H.B. The role of 0–2 mm fine recycled concrete aggregate on the compressive and splitting tensile strengths of recycled concrete aggregate concrete. Mater. Design. 2014, 64, 345–354. [Google Scholar] [CrossRef]
- Evangelista, L.; Brito, J. Mechanical behaviour of concrete made with fine recycled concrete aggregates. Cem. Concr. Compos. 2007, 29, 397–401. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xiao, J.; Zhong, P. Internal curing effect of saturated recycled fine aggregates in early-age mortar. Cem. Concr. Compos. 2020, 108, 103444. [Google Scholar] [CrossRef]
- Jesus, S.; Maia, C.; Farinha, C.B.; Brito, J.; Veiga, R. Rendering mortars with incorporation of very fine aggregates from construction and demolition waste. Constr. Build. Mater. 2019, 229, 116844. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Chen, P.; Pan, J.; Xu, Y.; Wang, H.; Shen, W.; Cao, K. Effect of Internal Curing by Super Absorbent Polymer on the Autogenous Shrinkage of Alkali-Activated Slag Mortars. Materials 2020, 13, 4318. [Google Scholar] [CrossRef]
- GB/T 18046; Ground Granulated Blast Furnace Slag Used for Cement and Concrete. China National Standardization Management Committee; Standards Press of China: Beijing, China, 2008.
- ASTM C128; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM E104; Standard Practice for Maintaining Constant Relative Humidity by Means of Aqueous Solutions. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM C1437-15; Standard Test Method for Flow of Hydraulic Cement Mortar. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM C349; Standard Test Method for Hydraulic-Cement Mortars (Using Portions of Prisms Broken in Flexure). ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- ASTM C1698; Standard Test Method for Autogenous Strain of Cement Paste and Mortar. ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- Zhutovsky, S.; Kovler, K.; Bentur, A. Efficiency of lightweight aggregates for internal curing of high strength concrete to eliminate autogenous shrinkage. Mater. Struct. 2002, 35, 97–101. [Google Scholar] [CrossRef]
- Chen, P.; He, S.; Wang, P.; Xu, Y.; Chen, Q. Ecological Upgrade of Normal-Strength Mortars by Using High Volume of GGBS. Adv. Civ. Eng. 2020, 7101469. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteriro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Savva, P.; Petrou, M.F. Highly absorptive normal weight aggregates for internal curing of concrete. Constr. Build. Mater. 2018, 179, 80–88. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Shi, C. A review on the use of LWA as an internal curing agent of highperformance cement-based materials. Constr. Build. Mater. 2019, 218, 385–393. [Google Scholar] [CrossRef]
- Wu, C.-R.; Zhu, Y.-G.; Zhang, X.-T.; Kou, S.-C. Improving the properties of recycled concrete aggregate with bio-deposition approach. Cem. Concr. Compos. 2018, 94, 248–254. [Google Scholar] [CrossRef]
- Zhao, Z.; Remond, S.; Damidot, D.; Xu, W. Influence of fine recycled concrete aggregates on the properties of mortars. Constr. Build. Mater. 2015, 81, 179–186. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, S.W.; Lee, K.M.; Choi, Y.C. Influence of internal curing on the pore size distribution of high strength concrete. Constr. Build. Mater. 2018, 192, 50–57. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Modification of the Bogue calculation. Adv. Cem. Res. 1989, 2, 73–77. [Google Scholar] [CrossRef]






| Density/ kg/m3 | BET Area/ m2/g | Chemical Composition/wt.% (XRF) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MaO | Fe2O3 | TiO2 | K2O | LOI | |||
| GGBS | 2800 | 1.535 | 43.7 | 26.5 | 18.2 | 4.9 | 1 | 1 | 0.8 | 3.9 |
| Mix/kg/m3 | Compressive Strength/MPa | ||||
|---|---|---|---|---|---|
| Cement | Water | Sand | 3d | 7d | 28d |
| 547 | 275 | 1430 | 23.18 | 30.08 | 35.12 |
| Sizes/mm | Specific Density/g/cm3 | Water Absorption/% | |
|---|---|---|---|
| MA 0.63 | 0.63–1.25 | 2.40 | 15.41 |
| MA 1.25 | 1.25–2.5 | 2.42 | 12.16 |
| MA 2.5 | 2.5–4.75 | 2.38 | 11.92 |
| Mixtures | GGBS | Activator | Sand | MA | Additional Water |
|---|---|---|---|---|---|
| Control | 616 | 308 | 1276 | 0 | 0 |
| A 0.63 | 616 | 308 | 396.02 | 800 | 123.20 |
| A 1.25 | 616 | 308 | 170.74 | 1013.16 | 123.20 |
| A 2.5 | 616 | 308 | 234.65 | 1032.69 | 123.20 |
| B 0.63 | 616 | 308 | 0 | 1160.03 | 178.76 |
| B 1.25 | 616 | 308 | 0 | 1169.67 | 142.23 |
| B 2.5 | 616 | 308 | 0 | 1150.38 | 137.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W.; Wang, L.; Chen, P.; Wang, H.; Cao, K. Mitigating Autogenous Shrinkage of Alkali-Activated Slag Mortar by Using Porous Fine Aggregates as Internal Curing Agents. Sustainability 2022, 14, 9823. https://doi.org/10.3390/su14169823
Shen W, Wang L, Chen P, Wang H, Cao K. Mitigating Autogenous Shrinkage of Alkali-Activated Slag Mortar by Using Porous Fine Aggregates as Internal Curing Agents. Sustainability. 2022; 14(16):9823. https://doi.org/10.3390/su14169823
Chicago/Turabian StyleShen, Wenfeng, Liang Wang, Peiyuan Chen, Hao Wang, and Ke Cao. 2022. "Mitigating Autogenous Shrinkage of Alkali-Activated Slag Mortar by Using Porous Fine Aggregates as Internal Curing Agents" Sustainability 14, no. 16: 9823. https://doi.org/10.3390/su14169823
APA StyleShen, W., Wang, L., Chen, P., Wang, H., & Cao, K. (2022). Mitigating Autogenous Shrinkage of Alkali-Activated Slag Mortar by Using Porous Fine Aggregates as Internal Curing Agents. Sustainability, 14(16), 9823. https://doi.org/10.3390/su14169823








